International Journal of Radiology and Radiation Oncology
Surveillance Computed tomography scan– Is there a role at five years in post curative resection of colorectal cancer?
Ishani Mukhopadhyay1*, Dariush Kamali2 and Venkatesh Shanmugam3
2BmedSci, BM, BS, MmedED, FRCS (Eng), Darlington Memorial Hospital, Woodlands Road, Darlington, DL3 6HX, UK
3University Hospital of North Tees and Hartlepool, Hardwick Road, Stockton-On-Tees, TS19 8PE, UK
Cite this as
Mukhopadhyay I, Kamali D, Shanmugam V (2020) Surveillance Computed tomography scan– Is there a role at five years in post curative resection of colorectal cancer? Int J Radiol Radiat Oncol 6(1): 022-026. DOI: 10.17352/ijrro.000041Objectives: There are significant worldwide variations in the use of Computed Tomography (CT) scan for postoperative surveillance of patients after curative treatment for colorectal cancer. The NICE (National Institute of Clinical Excellence) guidelines (CG131/NG151) recommends the use of 2 CT scans of chest, abdomen and pelvis in the first three years following curative resection.
Our hospital policy was to perform a third scan at five years prior to discharge from follow-up. This study aimed at determining the oncological benefit of the additional scan at 5-years post-surgery.
This current audit result will adds evidence to the planned introduction of stratified follow-up.
Method: Retrospective analysis of CT scans performed at five years post curative resection for colorectal adenocarcinoma in a single UK Trust, between December 2015 and December 2018.
Results: A total of 200 consecutive patients (133 male, 67 female; median age 73 years) were reviewed. No patients (0%) were found to have new colorectal recurrence at Year-5 scan. One patient underwent an expedited CT scan for symptoms and presence of suspicious findings on previous CT scans. The calculated sensitivity of CT scan for excluding colorectal recurrence was 100% with a specificity of 97.5%
Conclusion: The additional Year-5 CT scan beyond NICE recommended two scans did not demonstrate any significant clinical benefit in the detection of recurrence or metastatic colorectal cancer. In addition, CT scans expose patients to additional radiation risks and adds further burden to a resource-limited NHS.
Introduction
There are approximately 1.8 million new diagnosis of colorectal cancer (CRC) annually. The five year survival rate is >90% if diagnosed at an early stage [1]. Early diagnosis with absent metastasis carries a a relatively good prognosis. It is well known that surgical resection is the definitive treatment option for operable colorectal cancers, with the addition of adjuvant or neo-adjuvant chemo and/or radiotherapy to achieve remission for advanced tumour with or without nodal involvement [2].
Unfortunately 30 to 40% of colorectal cancers recur within the first five years of surgical resection; the first two years being the most crucial time [3]. Regular follow-up is therefore necessary to detect early recurrence and offers potential opportunity to salvage it. Combination of the follow-up tools help with this early detection of recurrence. This includes clinical review of the patients along with Carcino-embryonic antigen (CEA) monitoring and regular imaging to detect recurrence in addition to the endoscopic surveillance at the end of one year and four years post-surgery. Computed Tomography (CT) has been considered a sensitive mean to image recurrence within the limits of the scan for the size of the recurrent lesions [4]. However, there are significant worldwide variation in the follow-up protocol for these patients, particularly the CT scan surveillance (Figure 1) [5]. While the NICE (National Institute for Health and Clinical Excellence - UK) guidelines (CG131/NG151) recommends the use of 2 CT scans over the first three years following curative treatment, the American Society of Colon and Rectal Surgeons (ASCRS) recommends the use of annual CT imaging for five years [6,7].
Several NHS (National Health Service) Trusts within the UK continue to pursue more intensive follow-up protocols deviating from the national recommendation [8]. This study was performed in a Trust where a further CT scan at Year-5 was routinely performed prior to the patient discharge from the protocol follow-up. We therefore aimed to study the oncological benefit of performing a CT scan at 5 years post curative resection of colorectal cancer (CRC) in addition to the standard national recommendation.
Method
Retrospective analysis of all consecutive patients aged over 18 years diagnosed with colorectal cancer and underwent a curative resection at a single UK trust, were included for the study. The CTscan at 5 years following curative resection of CRC between December 2015 and December 2018 were analysed. The CT image acquisition was made through imaging request and reporting systems (ICE). CT scans performed between 4.5 and 5.5 years following primary resection of colorectal cancers were included. This is to cover any logistical variations from 5-years follow-up.
Information collected on patient demographics included age, sex, tumour site, TNM staging and neo-adjuvant treatment details (down-staging treatment). Histology results (tumour grade, completeness of resection and any adverse features) were also recorded. Patients with a diagnosis other than adenocarcinoma and with metastasis (TxNxM1) on initial diagnosis were excluded.
The Year-5 CT scan was categorised into positive and negative findings. For patients with positive diagnosis of recurrence or metastasis on the CT, the former CT scans (performed at Year 1 and Year 2) were analysed to establish earlier negative result. New positive findings on Year-5 CT scans were subsequently categorised into benign and malignant pathology. Malignancy-related findings were then analysed to determine any relation to the previously treated colorectal cancer. Clinic letters were also reviewed for presence of symptom when Year-5 CT scans showed positive malignant findings.
Statistical calculations were performed to predict the sensitivity, specificity, positive (PPV) and negative (NPV) predictive value of CT scan.
Results
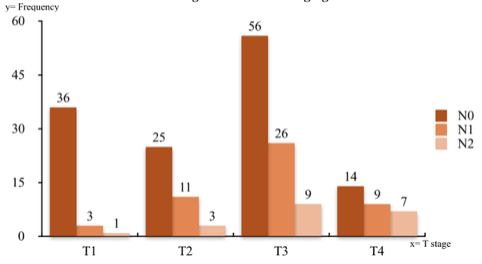
A total of 200 patients underwent Year-5 CT scan during the study period. Median age was 73 years (range 31–94), with 133 male to 67 female. Out of the total 200 cases, 114 (57%) were colonic and 86 (43%) rectal tumour. Majority of the patients were between the ages 61 and 80 years (62%) which generally reflects the trend in colorectal cancer prevalence (Tables 1,2). Also, the left sided cancers were common (75.5%). Twenty eight percent of cancers were radiologically staged at T3 N0 M0 followed by T1 N0 M0 (18%) (Figure 1). A total of 69 (34.5%) patients received adjuvant chemotherapy and/or radiotherapy and 38 (19%) received neo-adjuvant therapy.
Out of the 200 CT scans reviewed, 194 (97%) were reported showing no evidence of colorectal recurrence or metastasis. Six CT scans (3%) were reported to be suspicious for CRC malignancy. Further investigations revealed true colorectal malignancy in only 1 (true positive= 0.5%) patient; one patient’s scan showed features suggestive of hepatocellular carcinoma that was later confirmed histologically. Two scans (1%) reported recurrence of non-CRC malignancy; two (1%) scans reported incidental findings of new malignancies. Liver cysts and renal calculi were the commonest benign findings (7.5%).
The calculated sensitivity of CT scan to exclude colorectal recurrence was 100% with a specificity of 97.5%. The positive predictive value (PPV) and negative predictive value (NPV) were 16.67% and 100%, respectively. This confirms the reliability of the CT scan and adds value to the decision making in the follow-up protocol.
Discussion
Over the past decade, several studies have been conducted to establish the optimal and safe followup protocol for colorectal cancer resections. Traditionally, it was believed that more intensive strategies would aim to detect recurrence at an earlier stage particularly asymptomatic patients, thus allowing timely intervention for salvage and a better survival benefit [9,10]. However, a systematic review conducted by Jeffrey, et al. found that this was not associated with improved survival rate [4].
The FACS trial conducted in the UK in 2014 analysed the surgical treatment of recurrence and associated mortality in a four arm protocol [11]; (1) minimal follow-up with 1 CT scan at 12-18 months post curative resection, (2) follow-up with CEA only, (3) follow-up with CT scan only and (4) combination of CEA and CT imaging. Their conclusion was that the Protocols 2-4 were superior to protocol 1. The trial also concluded that there are no advantages of combination of both modalities over individual test. However, this study explored two extreme follow-up strategies where one arm received only 1 CT scan throughout the follow-up process, while the other arms received at least a form of monitoring for 5 years which included 6 monthly CEA or CT or both over initial 2 years and then annual for 3 more years. The COLOFOL randomised-controlled trial performed by Wille-Jørgensen, et al. between 2006 to 2015 established that follow-up with CEA and CT scan at Year 1 and Year 3 post curative resection for stage II and III colorectal cancer as opposed to 6-monthly review for 3 years did not increase 5-year mortality. Both studies reinforce the key observation that earlier detection and resection of recurrence has not reflected on the survival benefit [12].
A prospective study performed by Walter, et al. in 2011 followed 207 patients over 5 years after curative resection of colorectal adenocarcinoma [13]. The study showed a higher mortality rate (36%) within five years. Not all patients were offered CT scan at the completion of five years post-surgery. There were no new colorectal recurrences detected in 81 patients who underwent CT scan of chest, abdomen and pelvis at five years. However, it is difficult to establish their criteria for requesting the 5-year CT scan.
From our study, only one (0.5%) of the 200 patients was found to have colorectal recurrence at year-5 CT scan. This patient was diagnosed with T2 N0 M0 rectal cancer for which they underwent an initial TEMS procedure followed by completion laparoscopic APER considering the T2 disease and the patient’s preferred choice. A CT scan performed at one year post resection showed radiological evidence of pre-sacral soft tissue thickening and was concluded in the Multidisciplinary Team meeting as post-surgical change. However, further scans were advised annually for two years as a safety net. The immediate subsequent annual surveillance scan showed static appearance of the soft tissue thickening. However, the patient became symptomatic with pelvic pain 15-months after the year 3 scan. Therefore the Year-5 CT scan was expedited to be performed at that stage which demonstrated pelvic recurrence with increased size of the pre-sacral thickening (46x71x46mm). CEA level remained normal throughout suggesting a non-secreting tumour. For all technical reasons, this patient would not come under the recurrence on 5-year CT scan.
Radiation risk from CT scan
A CT scan of chest, abdomen and pelvis (CT CAP) exposes a patient to 17 mSv (millisieverts) ionizing radiation; considered to be equivalent to five years natural background radiation [14]. Studies over the past two decades have highlighted the risks of radiation-induced carcinogenesis. Although there is limited availability of experimental data. The Bier report (latest published Bier VII) analysed data from a cohort of Japanese atomic bomb survivors at Hiroshima and Nagasaki to derive risk models and calculated lifetime attributable risk (LAR; additional cancer risk beyond baseline cancer risk) [15]. While a single CT scan does not necessarily expose a patient to a significant amount of radiation, multiple exposure can increase the cumulative risk significantly. Furthermore, exposed organs and their individual sensitivity to radiation leading to carcinogenesis differs. The effective dose (mSv) considered in the studies includes these factors along with the direct absorbed radiation dose [16]. Age and sex have been determined to be two important human factors in risk calculations. The younger the age at time of exposure, the higher the risk to subsequent development of radiation-induced cancer. This is likely due to increased radiosensitivity at a younger age and also longer time available to allow development of cancer [17]. The female sex was found to be more susceptible. A study by Smith-Bindman, et al. estimated that undergoing a CT Abdomen and Pelvis at the age of 20, a female would carry a predicted risk of 1 in 470 of developing radiation-induced cancer; while the predicted risk is 1 in 620 for a male individual. The risks are significantly lower when the radiation exposure happens at the age of 60 years (females 1 in 1320; male 1 in 1250) [18]. Majority of our study population were elderly and 33.5% were female gender.
Cost-effectiveness
In addition to this, our hospital estimated current cost for a CT scan of Chest, abdomen and pelvis is £121.95. This equates to nearly £25,000 spent over three years on this additional surveillance CT scans included in this study with no significant impact on patient survival but increase the potential risk of radiation related side effects. There have been several studies to prove that follow-up protocol as opposed to no follow-up is more cost effective, however there is no study yet to investigate the cost effectiveness of CT scanning at different stages of follow-up [19,20].
The result of this study is in keeping with national guidelines (NICE) and shows no role for surveillance CT scan five years post curative resection for Colorectal cancer especially in asymptomatic patients. There are some drawbacks in our study. This was a retrospective study with its own disadvantages. However, we feel that the number of patients included were adequate enough for a meaningful conclusion and no patients were lost to follow-up. The statistics for the study was simple due to the fact that the primary aim is to identify the benefit of the year-5 scan with no comparison group. We also reiterated the significant risk associated with repeated radiation exposure from CT scan and the cost implications for a public health service. Our study also adds to the already existing evidence of the published data in favour of not much benefit in performing additional scan at five years. There was no control group in the study to establish the risk involved with the absence of additional scanning. It can be argued that there may be minimal, yet potential risk of missing detection of asymptomatic CRC recurrence in the absence of CT imaging at later stages of follow-up. The PRAISE study (Personalized Risk-Adaptive Surveillance Strategies in Cancer) by Bansal, et al. may provide more information on the optimal follow-up strategy [21].
Conclusion
While regular annual CT scans were preferred traditionally, several studies have shown no inferiority in less intensive follow-up. From our study, we found no significant clinical benefit in performing additional CT scan five years post curative resection for colorectal cancer. Our hospital policy has now been updated to perform regular CT scans at Year 1 and Year 2 post curative resection for asymptomatic CRC. This change in the CT scan policy have been adopted to incorporate the new national drive for stratified follow-up on histologically good prognostic tumour group. Our aim is to revisit this new strategy by another audit after an appropriate duration to make sure the patient safety is not compromised.
- Cancer Research UK (2020) Bowel Cancer Statistics. Link: https://bit.ly/3lrpILZ
- Mayo Clinic (2020) Colon cancer. Link: https://mayocl.in/3df4iyA
- Godhi S, Godhi A, Bhat R, Saluja S (2017) Colorectal cancer: Postoperative Follow-up and surveillance. Indian J Surg 79: 234-237. Link: https://bit.ly/3iGpIWN
- Jeffrey M, Hickey BE, Hider PN, See AM (2019) Follow-up strategies for patients treated for nonmetastatic colorectal cancer. Cochrane Database of Syst Rev 9: CD002200. Link: https://bit.ly/2SHnM5z
- Liu SL, Cheung WY (2019) Role of surveillance imaging and endoscopy in colorectal cancer follow-up: Quality over quantity? World J Gastroenterol 25: 59-58. Link: https://bit.ly/3jOQttm
- NICE UK (2020) Colorectal cancer NICE guideline [NG151]. Link: https://bit.ly/3lrg8bY
- Steele SR, Chang GJ, Hendren S, Weiser M, Irani J, et al. (2015) Practice Guideline for the Surveillance of Patients After Curative Treatment of Colon and Rectal Cancer. Dis Colon & Rectum 58: 713-725. Link: https://bit.ly/30Mbpts
- Rutter MD, East J, Rees CJ, Cripps N, Docherty J, et al. (2020) British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut 69: 201-223. Link: https://bit.ly/2SJ5aC8
- Rodrigues RV, Pereira da Silva J, Rosa I, Santos I, Pereira N, et al. (2017) Intensive Follow-Up After Curative Surgery for Colorectal Cancer. Acta Med Port 30: 633-641. Link: https://bit.ly/3jL0Mib
- Renehan AG, Egger M, Saunder MP, O'Dwyer ST (2002) Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ 324: 813-820. Link: https://bit.ly/3llvyyu
- Primrose JN, Perera R, Gray A, Rose P, Fuller A, et al. (2014) Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 311: 263-270. Link: https://bit.ly/36OGujV
- Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, et al. (2018) Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer–Specific Mortality in Patients With Stage II or III Colorectal Cancer. JAMA 319: 2095-2103. Link: https://bit.ly/2GURfGs
- Walter CJ, Al-Allak A, Borley N, Goodman A, Wheeler JM (2013) Fifth-year surveillance computed tomography scanning after potentially curative resections for colorectal cancer. Surgeon 11: 25-29. Link: https://bit.ly/2SHsrEC
- RadiologyInfo.org (2019) Radiation Dose in X-Ray and CT Exams. Link: https://bit.ly/3iGIixP
- Committee to assess health risks from exposure to low levels of ionizing radiation National Research Council. Health risks from exposure to low levels of ionizing radiation BEIR VII Phase 2. Washington. National Academies Press 2005. Link: https://bit.ly/2GQhfmF
- Fisher DR, Fahey FH (2017) Appropriate Use of Effective Dose in Radiation Protection and Risk Assessment. Health Phys 113: 102-109. Link: https://bit.ly/2I5Hjus
- Brenner DJ, Hall EJ (2007) Computed Tomography — An Increasing Source of Radiation Exposure. N Engl J Med 357: 2277-2284. Link: https://bit.ly/36UvqBQ
- Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, et al. (2009) Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 169: 2078-2086. Link: https://bit.ly/3nurRZ8
- Renehan AG, O'Dwyer ST, Whynes DK (2004) Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ 328: 81. Link: https://bit.ly/3iShc7f
- Mant D, Gray A, Pugh S, Campbell H, George S, et al. (2017) A randomised controlled trial to assess the cost-effectiveness of intensive versus no scheduled follow-up in patients who have undergone resection for colorectal cancer with curative intent. Health Technology Assessment 21: 1-86. Link: https://bit.ly/2SC8EGI
- National Cancer Institute. DCCPS Grants. Link: https://bit.ly/30QngXD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
