International Journal of Radiology and Radiation Oncology
Direct and Bystander Effect on Cervix Cancer Cells (SiHa) Exposed to High Dose-Rate Gamma Radiation Sourced from Ir192 Used in Brachytherapy
Megha Anilkumar Kulkarni1, Safa Abdul Syed Basheerudeen1, Sidonia Vallas Xavier2, Murugan Appaswamy2, Thayalan Kuppusamy2 and Perumal Venkatachalam1*
2Department of Radiology and Medical Physics, Dr. Kamakshi memorial Hospital, no1 Radial Road, Pallikaranai, Chennai-600100, India
Cite this as
Kulkarni MA, Syed Basheerudeen SA, Xavier SV, Appaswamy M, Kuppusamy T, et al. (2015) Direct and Bystander Effect on Cervix Cancer Cells (SiHa) Exposed to High Dose-Rate Gamma Radiation Sourced from Ir192 Used in Brachytherapy. Int J Radiol Radiat Oncol 1(1): 007-013. DOI: 10.17352/ijrro.000004Introduction: Brachytherapy is a preferred choice of radiotherapy in the treatment of sensitive tissues cancer like intestine and gonad. The treatment is expensive because of the frequent replacement of radionuclide sources. A better understanding of cell killing and the cellular responses at different dose rates, might aid in tumor cell killing with fewer doses thereby enhancing a better prognosis.
Methods: The cervix cancer cell line was irradiated with doses ranging from 2Gy-10 Gy at three different dose-rates as used in brachytherapy along with unexposed sample as control. The biological effects of different doses and dose rate of the cells was assessed by measuring its cytotoxicity, genotoxicity and clonogenic ability of exposed cells. The bystander effect was examined by co-culturing the exposed tumor cells with the unexposed normal blood lymphocyte and vice-versa.
Results: A significant and dose dependent changes in cell viability (trypan blue exclusion), genotoxicity (Micronucleus assay) and colony forming ability (Clonogenic assay) was observed in the cells exposed to different doses of radiation (p<0.0001); however, the changes were dose-rate independent. Furthermore, the bystander study results show an enhanced cell killing in the tumor cells which suggest a beneficial bystander effects.
Conclusion: The observed elevated bystander response in the tumour cells compared to that of normal blood lymphocytes suggest that if it happens under in-vivo situation, could results in has a therapeutic gain.
Introduction
Cancer is the disease of uncontrolled cell proliferation leading to tumour formation in localized and or many other parts of the body [1] .The various modalities used in the treatment of cancer are surgery, radiation, chemicals and antibodies [2], either alone or in combination depending upon the stage as well as regions involved [3]. The radiotherapy minimizes un-intended exposure of radiation to normal cells/tissue and cause death of the cancer cell more effectively. During radiotherapy, radiation was administered to patient either with external beam, (teletherapy) or with internal radiation (brachytherapy) and/or combination. The energy deposited in the biological system is discrete yet in random manner and, biological effect occurs as a consequence of transfer of energy to the atoms or molecules within the cells [4]. Brachytherapy is a technique in which radioactive sources are placed as interstitial implants within body cavities (intracavitary therapy), or onto epithelial surfaces (surface moulds) [5-7]. Of the various radio-isotopes, Ir192 is one of a most widely used radionuclide in brachytherapy [8] but the major limitation with the radioisotopes is their half-life, which is only 72 days [9] which means the source has to be changed frequently, which turns out to be expensive for therapeutic purpose. Many studies [5-7] have been conducted to investigate the dose rate effects of radionuclide which emits radiation at low dose rates (LDR), meagre studies have been reported on the radionuclide which are used to deliver at HDR as in brachytherapy.
Clinically the Ir192 HDR brachytherapy source is used approximately from four to six months period and during this period the dose rate for a particular application varies from 12Gy/hr to 150Gy/hr (eg. intercavitary applications for ca cervix patient). During this period, though the time of exposure increases with the decrease in activity, cell killing and its effects after irradiation at these dose rates has not been reported; thus a better understanding of the cellular response to radiation may improve therapy [4,9]. This can be achieved better under in-vitro exposures of relevant cell culture model and then relate to the in-vivo situation. Hence, the aim of present study was to investigate the dose rate effect of the Ir192 HDR brachytherapy sourced on the SiHa cell lines of cervix tissue origin. Furthermore, bystander effect of the irradiated SiHa cells on the unexposed normal cells (PBL) and vice versa also was examined to examine the non-targeted of the exposed tumour cells.
Materials and Methods
Procurement and maintenance of cell lines
Cervix cancer cell line (SiHa), used for the study, was obtained from ATCC, (New Jersey U.S.A) and maintained as monolayer cultures in the laboratory. SiHa (p16-p25) cells was grown in plastic tissue culture flasks using Dulbecco’s Modified Eagles Medium (DMEM) (GIBCO, Grand Island, New York, USA), supplemented with 10% fetal bovine serum (FBS) (GIBCO, Grand Island, New York, USA) and antibiotics (Penicillin 50IU/ml, Streptomycin 35µg/ml and Gentamycin 2.5µg/ml) (GIBCO, Grand Island, New York, USA) in a humidified incubator at 370C with 5% CO2.
Irradiation setup
Approximately 1×105 cells placed in cryovial (1X 1 cm) were exposed to different doses of gamma rays (2-10Gy) from an Ir192 source at various dose rates (58.8, 50.0 and 40.0Gy/hour, at 37 ± 10C). An aliquot of cells without exposure to any radiation was used as control. The exposed and unexposed cells were further used for cell viability assay, genotoxicity assay, bystander experiments and Clonogenic assay.
The model used for irradiating the blood samples was a thermocole box with a cavity that had been made to fit in a 15 ml glass test tube attached with four long plastic tubes. The plastic tubes carried the radiation source in the form of wire, which were at equidistant points, perpendicular to the mould and were attached to the brachytherapy machine. Inside the test tube, the 1ml vial was placed which contained the SiHa cell in suspension (1×105 cells). Then the cells were irradiated at room temperature and transported to the laboratory in an icebox and were placed at room temperature for 1 hour before initiating experiments.
Peripheral blood lymphocytes(PBL)
About 6 ml of human peripheral blood was collected in a heparinised sterile container from healthy male volunteers aged 25 years with informed consent and clearance from the Institutional Ethics committee. Approximately 1 ml of blood was exposed to different doses of HDR gamma rays (2 and 4 Gy) at a dose rate of 40Gy/hr. Irradiation was carried out as similar to that of described for SiHa cells. The exposed and unexposed cells were further used for bystander experiments. For the experiments PBL were grown in RPMI-1640 medium (GIBCO) supplemented with 20% FBS (GIBCO) and antibiotics (Penicillin 50IU/ml, Streptomycin 35µg/ml and Gentamycin 2.5 µg/ml) (GIBCO) and maintained in humified 370C incubator with 5% CO2.
Radiation exposure and co-culture of cells
The co-culture methodology described by Geraschenko and Howell (2003) was adopted in the present study. The SiHa cells grown as monolayer in T25 flask were trypsinised, made as single cells in suspension and irradiated using HDR gamma Ir192 radiation at different doses. Briefly about 1×105 irradiated cells were seeded in into commercially available trans well culture inserts (Thincert, Greiner Bio one, Germany) with a permeable membrane (pore size 0.4µm).At the same time, an equal number of cells (1×105 ) were plated into six-well plates. Following exposure, radiation-treated cells in the transwell culture inserts (directly exposed) were placed into the wells of a six-well plate containing untreated (bystander) cells and co-cultured at 37ºC for 24 hour.
Quantification of DNA damages and bystander effect using micronucleus assay (MN)
Twenty-four hours after co-culture, the cells (SiHa) were detached using trypsin-EDTA (GIBCO, Grand Island, New York, USA), collected after centrifuge (1000 rpm/ 5 minutes) and counted using a haemocytometer. About 1x105 cells were seeded into P-60 dishes in their respective media (DMEM supplemented with 10% fetal bovine serum) and incubated at 37ºC in a 5% CO2 incubator. To arrest cells at the cytokinesis stage, cytochalasin-B (3 µg/ml) (Sigma, Bellefonte, USA) was added to the medium two hours after seeding the cells. At the end of 48 hours of incubation, the cells were washed with phosphate buffer saline (PBS) and fixed with ice cold methanol and air dried. Then the cells were stained with diamino-phenyle-indole (DAPI) (Vysis Inc, Downers Grove, USA) and were analyzed using a fluorescence microscope (40X) with an appropriate filter. A total of one thousand binucleated cells were scored and the MN frequency was calculated.
For PBL, Cytochalasin B (3 µg/ml) (Sigma, Bellefonte, USA) was added at 44th hr to arrest cells at cytokinesis stage. After 72 hour of incubation the cells were centrifuged (800rpm/8min) and washed using ice cold hypotonic solution KCl (0.075M) and fixed in Carnoys fixative (3:1).The cells were cast on clean glass slides and stained using Giemsa stain and analysed for the presence of MN using a light microscope (40X). A total of one thousand binucleated cells were scored and the MN frequency was calculated [11].
Cell viability assay
Trypan blue assay [10], was performed to check the cell viability followed by radiation exposure. Equal volume of cells and trypan blue were mixed (10µl) and incubated for 3 minutes; 10µl of mixture was loaded on haemocytometer and the number of dead cells was scored. The count was repeated twice and the average of both counts was used to calculate the per cent viability.
Clonogenic assay
To study the colony forming ability [13], of the exposed cells, about 250 to 10,000 cells (depended upon the dose) were seeded onto p60 petri-plates with 3ml of complete media in triplicates, and incubated at 37ºC for 10-12 days with regular media change. After 10-12 days of culture, the cells were washed with PBS and fixed with methanol. Then they were stained with 2% crystal violet, and the numbers of colonies were counted. The experiment was performed in triplicates and an average was taken to calculate the plating efficiency and survival fraction.
Statistical analysis
The mean values of DNA damages, cell viability and colony forming ability between the controls and exposed cells were compared using “t” test one using Graph pad INSTAT version 3.0 software.
Results
Viability of SiHa cells exposed to HDR gamma radiation using trypan blue exclusion assay
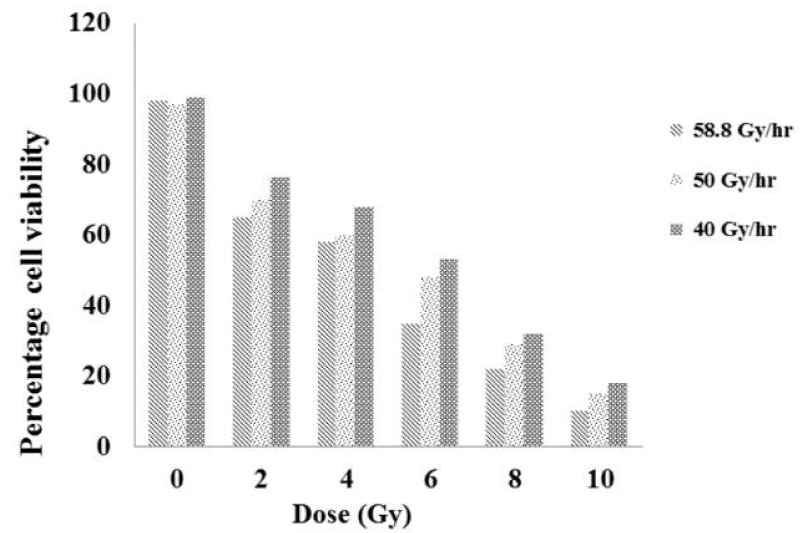
The viability of the SiHa cells immediately after exposure to radiation was quantified using try pan blue exclusion assay and the results are shown in Figure 1. The obtained results shows that the dose and cell viability are inversely proportional to each other; that as the dose increased, the cell viability shows an significant decrease (p<0.0001). Among the dose rates employed, a trend of difference in the cell viability was observed; the difference is appreciable between 40 and 58.8 Gy/hr when compared to that of either 40 and 50 Gy/hr or 50 and 58.8 Gy/hr. However, there were no statistical differences in the cell viability among the dose-rated studied (p>0.5).
Micronucleus (MN) assay
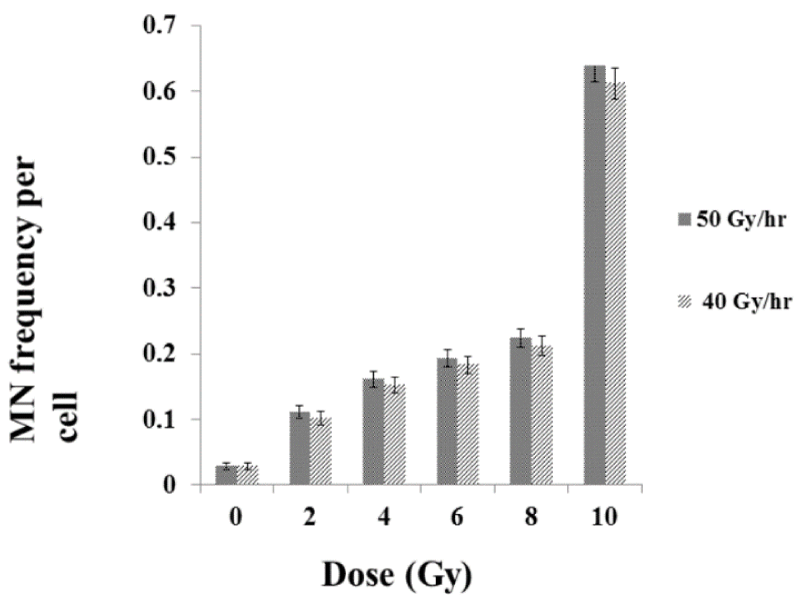
We did not observe any statistical differences in the SiHa cell viability immediately after exposures to radiation among the different dose-rates studied, hence, the amount of DNA damages were measured using MN assay (40Gy/hr and 50Gy/hr). The MN assay is a measure of residual damages in the exposed cells and an indicator of genotoxic effect of radiation. There was as dose dependent extremely significant increases (p<0.0001) in the yield of MN frequency at various doses. However, there was no significant difference in the yield of MN frequency in the different dose rate studied (p>0.5) (Figure 2).
Nuclear division index (NDI)
To study the cellular response upon the DNA damage, the cell proliferation kinetics was examined in the exposed cell population by calculating the nuclear division index (NDI). Tables 1, 2 show the number of cells with different nucleus obtained for different doses for the 50Gy/hr and 40Gy/hr dose-rates respectively. The observed NDI suggest that a decrease in the rate of cell proliferation upon exposure to various doses (p<0.001), however, there was no statistical difference observed in the NDI at various dose rates (p>0.5).
Clonogenic assay
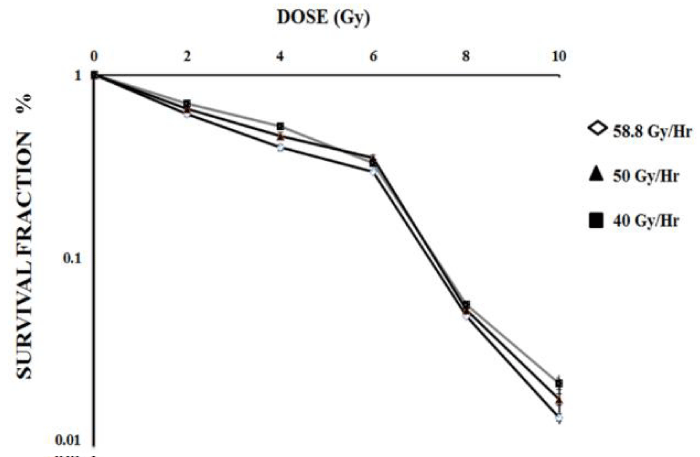
Colony forming ability of the exposed population is considered as a relevant end point in cancer radiotherapy; therefore, the reproductive integrity of the SiHa cells exposed to three different dose-rates of gamma radiation was examined. Consistent with the results of cytotoxicity and genotoxicity, the survival fraction of SiHa cells exposed at various doses of gamma HDR radiation was found to be inversely proportional (p<0.0001) and did not show any statistical differences among the dose rates studied (p>0.5) (Figure 3).
SiHa cells exposed to HDR gamma radiation induced a bystander response in unexposed SiHa cells and peripheral blood lymphocytes
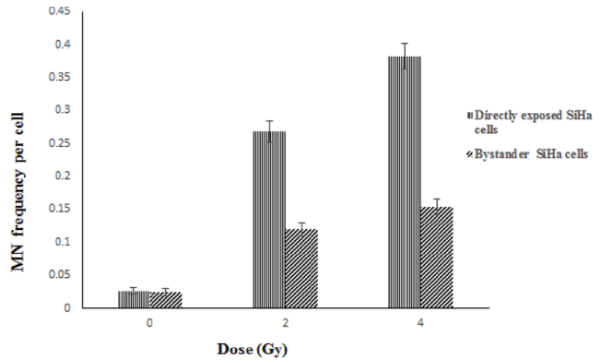
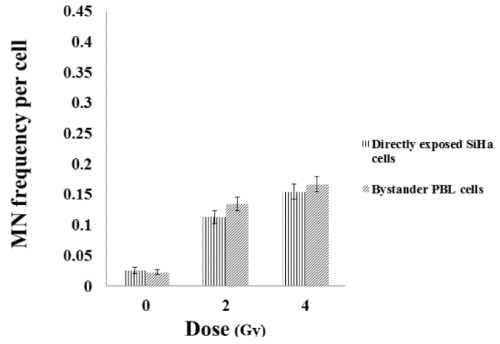
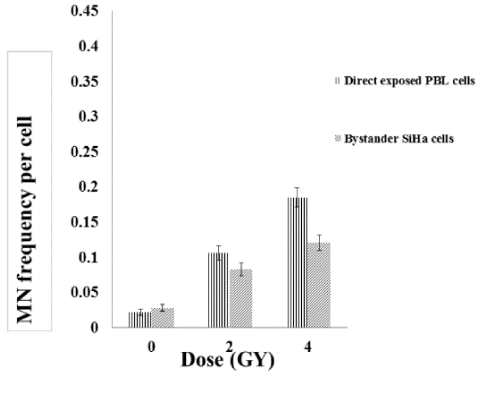
To study the non-targeted effects of radiation, SiHa cells exposed to radiation was co-cultured with unexposed (bystander cells) SiHa and PBL cells and vice versa. While, the directly exposed SiHa cells showed a dose dependent increase in the MN frequency which is statistically significant (p<0.001), the bystander SiHa and PBL cells that are co-cultured with the radiation exposed SiHa cells also showed a significant increase in the DNA damages (Figures 4, 5). It was also observed that un-exposed SiHa cells when co-cultured with exposed PBL cells also showed an enhanced significant (p<0.001), bystander effect when compared to that of unexposed control cells (Figure 6), suggesting that the SiHa cells when exposed to HDR gamma radiation induced a bystander response as well as responded to the bystander signals received from normal cells.
Discussion
Radiotherapy and chemotherapy are used for cancer treatment depending upon the stages of the cancer [3]. The modalities being in practice to deliver radiation are teletherapy and brachytherapy [4]. In the present study we investigated the dose rate effect of the Ir192 gamma radiation used in brachytherapy on the SiHa cell lines of cervix tissue origin. Brachytherapy is the preferred choice of treatment of the cervix cancer [16]. Therefore, SiHa cells, a cervical cancer cell line was selected for the study [9]. The radiation effect on the cell line was assessed for cytotoxic effect, genotoxicity and colony forming ability. To assess cytotoxicity ‘dye exclusion method’ was performed as it is more convenient, simple, in-expensive and rapid method to check cell viability in response to cytotoxic hazards. The exclusion test is based on ability of viable cells to permeate the dye [17]. Micronucleus assay was performed to check the genotoxic effect on the cells caused after exposure, due to its robust nature and ease in scoring the MN within the binucleated cells [18], it is extensively used as bio-dosimetric tool to measure the amount of DNA damage after radiation exposure [19], and to predict the patient sensitivity to radiation [18]. It is also used to determine the patients response to radiotherapy [20]. Colony forming ability was used to measure the survival fraction of the cells after radiation exposure as the intrinsic radiosensitivity of a tumour is an important determinant of its response to radiotherapy [21]. Thus clonogenic assay is considered as a ‘gold standard’ method for measuring the radiosensitivity status [22]. Although the bystander response phenomenon has been observed widely in many cellular models exposed to a variety of radiation types, in course of time, few studies have failed to show these responses and it became evident that not all cell types produce bystander signals or not all cell types respond to these signals [36]. Thus the available literature implies that the radiation-induced bystander effects are highly variable, dependent on the individual donors or cell lines tested or depend upon adopted methodology or LET. Hence, we studied the HDR gamma radiation sourced from Ir192 induced bystander response in human cervical cancer (SiHa) cells and peripheral blood lymphocytes (PBL), to measure the bystander response.
The cell viability assessed in SiHa cells exposed to various doses of gamma radiation showed significantly higher cell death when compared to its unexposed control cells. Furthermore, the exposed cells also showed enhanced DNA damages measured by MN assay and altered cellular perturbations in terms of reduced proliferation as well as reproductive ability measured by clonogenic assay in a dose dependent manner. All these events are the typical cellular response upon exposure radiation; thus when the incident photons deposit its energy on the DNA it induces a spectrum of DNA damages. Hydrolysis of water molecules releases free radicals which in turn induces strand break which in turn can cause DNA damages, these damages are expressed as MN. An enhanced MN frequency observed at higher doses can be attributed to the single and dual track travelled by the electrons after irradiation [14]. At lower doses due to single electron path, electrons hit the nuclear content only once, whereas at higher doses due to dual path track, electrons hit the nuclear content more than once, which results in dose dependent increase in DNA damages. Not only such a dose dependent increase in DNA damages was seen but also cells with more than two MN support the evidence indeed at higher doses the damages are induced by the traversal of both single as well as double electron track events. Similar reports have been reported earlier that at high doses radiation induces high as well as complex aberrations measured by chromosomal aberrations [23] and micronuclei [24]. In consistent with MN, the results obtained for NDI (Tables 1, 2) showed a decreased rate of cell proliferation upon exposure to radiation and the delay was high in cells exposed to high dose. This observed effect may be attributed due to the stress condition produced in cell at higher doses [25,26], and due to the activation of other pathways in response to DNA damage. The stress condition leads to activation of various checkpoints proteins that senses the DNA damage and depending upon the cell cycle stages (G0/G1, S and G2M) various repair pathways are activated [27,28]. At higher doses, because of accumulation of complex damages, the cell need more time to complete the repair of those damages and thus cell cycle is delayed, resulting in decrease in NDI as dose increases. Further as the SiHa cells were asynchronously growing, the cells could have got arrested at any phase within the cell cycle. Even though, we did not measure the stage at which the cells were arrested, radiation is a well-known agent which act on the cells in any stage within the cell cycle [25,29]. Therefore reduced NDI in exposed cells are the another hall mark of radiation exposure that can interfere and alter the rate of cell cycle kinetics in the exposed populations.
The results obtained with the clonogenic assay, showed decreases in colony forming ability in a dose dependent manner similar to that of cell viability measured by trypan blue assay immediately upon exposure to radiation. An enhanced cell death compared to that of control was due to exposed cell populations which showed more complex DNA damages as measured by MN frequency, the mis-repaired damages which are expressed in terms of MN hinder the cell proliferation and halt its reproductive integrity completely [30,31]. Moreover, the decreases in colony forming ability as the dose increased can also be related to the type of damages that occur due to radiation exposure; sub-lethal damages that occur due to radiation exposure are repaired rapidly but damages at higher doses are elevated and complex when compared to that at lower doses, thereby, requiring more time to get repaired, consequentially, there is exponentially less survival fraction at higher doses [26-28]. The shape of the clonogenic dose-response curves shows typical linear quadratic pattern upon exposure to gamma radiation sourced from Ir192, which is consistent with the published literature [28-30,32]. It has been reported that as the dose rate reduces, the survival curve becomes shallower and its shoulder tends to disappear (i.e. the survival curve becomes an exponential function of dose) [28-30,32], thus the shape of the dose-response curve was influenced by the type of DNA damages induced by the radiation, total dose and dose-rates delivered along with the cellular radiosensitivity. The linear component obtained at lower doses attributed due to repair of sub lethal and potentially lethal damages as the time gap between inductions of two strand breaks within the short duration as well as distance is low and the damages induced predominantly by the traversal of single electron track can be repaired. On the other hand at higher doses, the induced damages are complex and formed by the traversal of both single and double track events; the complex damages can get fixed and arrest the cell division irreversibly in a dose squared dependent manner.
Further it was observed that, there was no statistically significant difference in the cell viability, genotoxicity and colony forming ability of the cell exposed at three different dose rates (58.8Gy/hr, 0.50Gy/hr and 0.40Gy/hr). Such a difference was more prominent, if the differences among the dose rates were in the order of 0.01- 1Gy/min, and below this dose-rate range the survival curve changes a little in response to dose rate [33,34]. The results obtained in the present study had some difference in the frequencies of MN as well as the clonogenic ability at higher doses among the different dose-rates studies, were not significant. This difference seen may be due to the cell lines used for the study or type of irradiation used or the dose rates studied.
Brachytherapy reduces doses on the surrounding cell compared to teletherapy due to its nature of tumour site directed therapy and reduced therapy time [33]. Therefore to investigate the non-targeted effects of HDR gamma radiation sourced from Ir192 upon the unexposed tumor and normal cells, co-culture methodology was employed and the bystander effect was measured using MN assay. The majority of studies on bystander response are limited to X-radiation and high LET radiations [34], or medium transfer from gamma irradiated cultures at low dose rates [35]. The porous membrane present between the two populations of cells allows co-culture of cells from different origins by providing a conditioned niche for the proper growth and identity of cell type and permits to study cell to cell interaction through indirect signaling. Because the PET membrane (pore size 0.4μm) can be easily examined microscopically, cell viability was monitored with light microscopy. The results obtained in the present study demonstrate for the first time that HDR gamma radiation sourced from Ir-192 could induce bystander response in tumour cells (SiHa) as well as normal cells (PBL). This was evident by a statistically significant increase in the MN frequency in bystander SiHa cells and PBL upon co-cultured with radiation exposed SiHa cells and vice versa (Figures 4, 5). The bystander response has been also studied in relation to ultraviolet radiation, heat and chemotherapeutic agents. There is a huge debate that elevated damages in the unexposed cells due to radiation and chemicals have advantages in cancer therapy or limitations in the treatment outcome. In the positive sense, an enhanced cell killing of the tumor cells due to the bystander effects could reduce exposure of patients to higher doses of radiation and its associated side effects. Alternatively, the therapeutic agents inducing bystander responses in surrounding normal cells has deleterious consequences that can accelerate the side effects or late consequences like genomic instability and second malignancy [37]. The results obtained support the earlier view that the observed bystander effect can have therapeutic gain in human cervical cancer cell line, SiHa. Though, the bystander effects was seen in tumour (SiHa) and normal cells (PBL), the magnitude of bystander effect was more in SiHa cells than compared to PBL. Thus the measured bystander effect by MN assay showed 2-2.5 folds increased in bystander SiHa cells; whereas the bystander effect in SiHa and blood co-cultured vice versa was increased only by 1-1.2 folds. These results indicate that the bystander effect observed in SiHa lead to enhanced tumour cell killing and better prognosis. Bystander effect due to blood in SiHa and vice versa was comparatively less which, may not affect the surrounding normal tissues. This phenomenon is observed due to radiation damage wherein, free radicals are produced which lead to the induction of DNA damages by various mediators such as COX-2, ROS and NOS production, cytokines and other immune mediators [35], these free radicals can travel few micron distance, thereby, affecting the nearby tumour cell to greater extent than the cells that are following in the blood vessels or far away cell at different site [37].
Conclusion
The SiHa cells exposed to HDR gamma radiation sourced from Ir192 showed an dose dependent significant difference in the biological effects studied by cell viability, DNA damages and colony forming ability among the different doses studied, However, the different dose rates studied did not show any significant increase in biological effects. Further, the observed elevated bystander response in the tumour cells compared to that of normal blood lymphocytes suggest that the bystander effect observed in SiHa lead to enhanced tumour cell killing with fewer doses that might further enhance a better prognosis, provided if it happens under in-vivo therapeutic conditions.
We sincerely acknowledge the financial assistance from Department of Science and Technology, Government of India (SR-SO/HS-127/2012).
- Ashkenazi A, Dixit VM (1999) Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 11: 255-260.
- Douglous H, Robert A (2000) The Hallmarks of Cancer. Cell 100: 57-70.
- Bachireddy P, Rakhra K, Flesher DW (2012) Immunology in clinical review series: focus on cancer: multiple roles for immune system in oncogene addiction. Clin Exp Immunol 167: 188-194.
- Margon JJ, Scalliet P, Limbergen EV (2003) Radiobiology of Brachytherapy and the Dose rate effect. In: The GEC ESTRO Handbook of Brachytherapy. Estropp Inc., Belgium
- Bagdonas S, Dahle J, Kaalhus O (1999) Cooperative inactivation of cells in microcolonies treated with UVA radiation. Radiat Res 152: 174-179 .
- Dahle J, Bagdonas S, Kaalhus O, Olsen G, Steen HB, et al. (2000) The bystander effect in photodynamic inactivation of cells. Biochim Biophys Acta 1475: 273-280 .
- DeVeaux LC, Durtschi LS, Case JG, Wells DP (2006) Bystander effects in unicellular organisms. Mutat Res 597: 78-86.
- Jin C1, Wu S, Lu X, Liu Q, Qi M, et al. (2011) Induction of the bystander effect in Chinese hamster V79 cells by actinomycin D. Toxicol Lett 202: 178-185.
- Guiseppe SA, Sorbello L, Syadam G (2005) Cytotoxicity and cell growth assays. In: Cell Biology, Four-Volume Set: A laboratory handbook (Julio E. Celis,eds.) Elsevier., Denmark .
- Mothersill C, Stamato TD, Perez ML, Cummins R, Mooney R, (2000) Involvement of energy metabolism in the production of 'bystander effects' by radiation. Br J Cancer 82: 1740-1746.
- Fenech M (2007) Cytokinesis- block micronucleus cytome assay. Nat protoc 2: 1084-1104 .
- Belloni P, Latini P, Palitti F (2011) Radiation-induced bystander effect in healthy G(0) human lymphocytes: Biological and clinical significance. Mutat Res 713: 32-38 .
- Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C (2006) Clonogenic assay of cells in vitro. Nat protoc 1: 2315-2319.
- Fenech M (2010) The lymphocyte cytokinesis-block micronucleus cytome assay and its application in radiation biodosimetry. Health Phys 98: 234-243 .
- Hall JE, Brenner DJ (1991) The dose-rate effect revised: Radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys 21: 1403-1414.
- Adachi H, Matsumoto K, Kunishio K, Furuta T, Ohmoto T (1997) New method for the evaluation of the response of cultured cell lines to continuous low-dose-rate irradiation from encapsulated iridium-192 seeds with hyperthermia. Neurol Med Chir 37: 320-326.
- Gerashchenko BI, Howell RW (2004) Proliferative response of bystander cells adjacent to cells with incorporated radioactivity. Cytometry A 60: 155-164 .
- Azzam EI, de Toledo SM, Little JB (2004) Stress signaling from irradiated to non-irradiated cells. Curr Cancer Drug Targets 4: 53-64 .
- Eastham AM, Atkinson J, West CM (2001) Relationship between clonogenic cell survival, DNA damage and chromosomal radiosensitivity in nine human cervix carcinoma cell lines. Int J Radiat Biol 77: 295-302.
- Kiltie AE, Orton CJ, Ryan AJ, Roberts SA, Marples B, et al. (1997) A correlation between residual DNA double-strand break and clonogenic measurements of radiosensitivity in fibroblasts from preradiotherapy cervix cancer patient. Int J Radiat Oncol Biol Phys 39: 1137-1144 .
- Nagasawa H, Little JB (1999) Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: evidence for a bystander effect. Radiat Res 152: 552-557.
- Belyakov OV, Prise KM, Trott KR, Michael BD (1999) Delayed lethality, apoptosis and micronucleus formation in human fibroblasts irradiated with X-rays or alpha-particles. Int J Radiat Bio Phys 75: 985-993 .
- Qin JC, Li L, Yang ZJ, Yao DS, Li F, et al. (2011) Clinicopathologic analysis of small cell neuroendocrine carcinoma of the cervix. Zhonghua Fu Chan Ke Za Zhi 46: 360-363 .
- Sancer A, Lindsey BLA, Boltz KV (2004) Molecular mechanism of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 73: 39-85 .
- Singh S, Datta NR, Krishnani N, Lal P, Kumar S (2005) Radiation therapy induced micronuclei in cervix cancer-does it have a predictive value for local disease control? Gynecol Oncol 97: 764 -771 .
- Sara GE, Hall J (2003) Use of the cytokinesis-block micronucleus assay to measure radiation-induced chromosome damage in lymphoblastoid cell lines. Mutat Res 535: 1-13.
- Beford JS, Hall EJ (1963) Survival of HeLa cells in-vitro and exposed to protracted gamma radiation. Intl J of Radiat Biol 7: 377-383.
- Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, et al. (2005) Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene 24: 7257-7265.
- Bedford JS, Mitchell JB (1973) Dose-rate effects in synchronous mammalian cells in culture. Radiat Res 54: 316-327.
- Belli JA, Dicus GJ, Bonte FJ (1967) Radiation response of mammalian tumor cells: Repair of sub-lethal damage in-vivo. J Natl Cancer Inst 38: 673-682.
- Ang KK, Thames HD Jr, van der Kogel AJ, van der Schueren E (1987) Is the rate of repair of radiation induced sub lethal damage in rat spinal cord dependent on the size of dose per fraction? Int J Radiat Oncol Biol Phys 13: 557-562.
- Camphausen KA, Lawrence RC (2008) Principles of Radiation Therapy. In:Cancer Management: A Multidisciplinary Approach, (Daniel G Haller,eds.), Publishers of Journal Oncology
- Hagelstrom RT, Askin KF, Williams AJ, Ramaiah L, Desaintes C, (2008) DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals. Oncogene 27: 6761-6769.
- Müller WU, Rode A (2002) The micronucleus assay in human lymphocytes after high radiation doses (5-15 Gy). Mutat Res 502: 47-51.
- Ashby J, Tinwell H (2001) Continuing ability of the rodent bone marrow micronucleus assay to act as a predictor of the possible germ cell mutagenicity of chemicals. Mutat Res 478: 211-213 .
- Mothersill C, Seymour C (1997) Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol 71:421-427 .
- Mothersill C, Seymour C (2001) Radiation-induced bystander effects: past history and future directions. Radiat Res 155: 759-767.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
