International Journal of Pharmaceutical Sciences and Developmental Research
A review of microfluidic impedance sensors for pathogen detection
Chen Li, Mu Yuan* and Zhian Li
Cite this as
Li C, Yuan M, Li Z (2022) A review of microfluidic impedance sensors for pathogen detection. Int J Pharm Sci Dev Res 8(1): 046-056. DOI: 10.17352/ijpsdr.000042Copyright License
© 2022 Li C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The development of rapid, sensitive and specific methods for the detection of foodborne pathogens is important to ensure food safety. Currently, detection methods such as counting methods, immunoassays, and biosensors have been developed for detecting foodborne pathogenic bacteria, and impedance sensors combined with microfluidic technology have received extensive attention. This paper outlines the advances and applications of microfluidic impedance biosensors for the detection of foodborne pathogens. And reviews the current advances in microfluidic impedance sensors based on transducer materials and detection techniques, including detection technology based on interdigitated microarrays electrode, electrophoresis technology, nanotechnology, etc. Finally, the challenges and development trends of current microfluidic impedance sensors are discussed.
Introduction
Recently, diseases caused by foodborne pathogens pose a serious threat to public health and food safety and constitute a major obstacle to global socio-economic development [1,2]. Among them, bacteria are the most common foodborne pathogens [3]. Among these bacteria, Salmonella, Listeria, Escherichia coli O157:H7, Staphylococcus aureus, and Bacillus cereus are the main foodborne pathogen bacteria [4]. Therefore, the rapid detection of foodborne pathogenic bacteria and the development of detection technology are of great significance.
In the past few decades, great efforts have been made in the rapid detection of foodborne pathogens, and various detection techniques have been studied. In addition to conventional bacterial culture counts, there are a variety of other methods, such as nucleic acid-based, immunology-based, and biosensors (eg, optical, electrochemical, and mass spectrometry-based biosensors) for rapid detection of foodborne pathogens [5,6].
Many detection strategies, such as electrochemical, fluorescence, chemiluminescence, and colorimetric methods, have been developed for establishing point-of-care biosensors [7]. Impedance biosensors are one of the effective ways to detect foodborne pathogens based on biosensors because of their portability, speed, sensitivity, and suitability for on-site detection [8-10]. Recently, microfluidic technology has been deeply studied [11]. This technology can integrate enrichment, capture, detection, analysis, and other processes into the chip to improve detection performance [12]. It combines with biosensing to provide high-throughput analysis and integrated micro-distribution, realizing the advantage of intelligent instant diagnosis in the detection of bacteria [13-15]. The combination of microfluidic technology and impedance biosensors provides a broader perspective for the detection of pathogens, especially in the field of biosensors, where the development of new technologies (such as nanotechnology, etc.) provides strong technical support to promote the pathogen enrichment process and improve impedance signals and improves detection sensitivity. This paper briefly introduces the principle of microfluidic impedance detection and reviews the progress and application of impedance sensors based on microfluidic technology in the detection of foodborne pathogens, especially recent development. Finally, the problem and development trend of the current microfluidic impedance sensors are discussed.
Microfluidic impedance detection technology and principle
Microfluidic impedance biosensors are instruments based on microfluidic systems that convert biological concentrations into electrochemical impedance signals for identification and detection. They are widely considered to be promising analytical tools for on-site inspection due to their fast response time, high sensitivity, and simple operation, and facilitate the development of miniaturized and automated instrumentation. The specificity and effectiveness of biomarker ligands to capture target bacteria, the signal-transformation level of the bio-impedance of the transducer material, and the auxiliary enrichment ability are all important factors in determining the sensitivity of the microfluidic impedance sensor. Various contaminants in the actual sample may act as inhibitors, resulting in false negative results. There are many factors hindering the realization of truly “one-step” detection on a chip, sufficient reaction time is undoubtedly the important one, which significantly affects the collision opportunity between molecules, thereby affecting the binding between molecules, including the labels and targets, as well as the capture antibody and the labeled complex, and finally affecting the detection sensitivity. Therefore, how to control the reaction time has attracted much attention for on-chip detection [16]. A large number of research literature show that the use of IDAM-based detection technology, dielectrophoresis technology, and nanotechnology can improve the capture rate of bacteria and improve the impedance signal level to improve the detection sensitivity of microfluidic impedance sensors.
Principle of microfluidic impedance detection
The principle of impedance detection of microfluidic impedance biosensors is that based on a microfluidic system, a specific complex formed by the biometric molecule with an analyte at the surface of the conductive (or semiconductor) transducer, directly or indirectly alters the electron transfer capability of the recognition surface. Then establish a relationship between the change in electron transfer capability and the concentration of the analyte to achieve the purpose of detection.
The electrical impedance (Z) is defined as the ratio of the voltage increment change to the current change, V(t)/I(t). According to this definition, the impedance Z is the quotient of the voltage-time function V(t) and the resulting current-time function I(t):
Where V0 and I0 are the maximum voltage and current signals, f is the frequency, t is the time, ϕ is the phase shift between the voltage-time and the current time function, and Y is the complex conductance or admittance [17]. At the same time, the impedance is a complex value, which can be expressed as two parts: real part ZRe and imaginary part ZIm:
Where j is the plural sign, j2=-1, ZRe is the real impedance, and ZIm is the imaginary impedance.
Therefore, the results of impedance measurement can be described by two different forms of electrochemical impedance spectroscopy EIS: a Nyquist diagram with the real part as the X axis and the imaginary part as the Y axis. Each point of the Nyquist diagram corresponds to a frequency in the impedance, with low frequencies on the right and high frequencies on the left. Another form of EIS is the Bode diagram with the logarithm of the frequency as the X axis, the absolute value of the impedance, and the phase angle as the Y axis. The Bode diagram can show the relationship between the frequency and the impedance value and the phase angle.
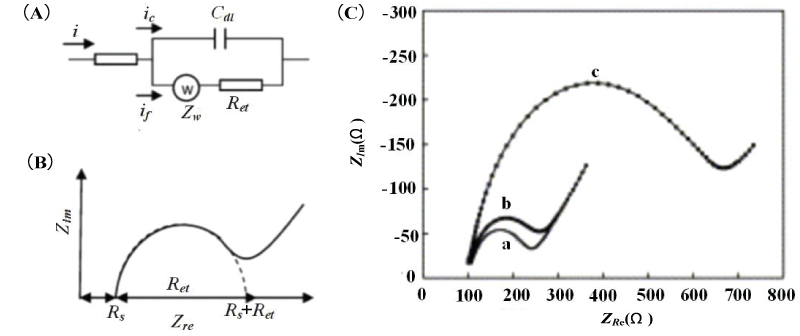
In order to express the characterization of surfaces, layers, or membranes after binding of immobilized biomolecules and bacteria, EIS is typically analyzed by using an equivalent circuit. And it contributes to analyzing the impedance changes of the electrolyte and the medium on the electrode, so as to determine the main factors of the system impedance change. From an electrical point of view, a simple equivalent circuit in series with a resistor and capacitor is sufficient to indicate the behavior of the impedance test system when the two electrodes are immersed in a conductive medium. The equivalent circuit model is composed of resistance, capacitance, and other components. Commonly used electrical components generally include electrolyte solution resistance, electric double layer capacitance, electron transfer resistance, Warburg impedance, etc. Each component represents one or more electrode processes and electrochemical properties.
The Randles equivalent circuit model is usually used to fit the Nyquist impedance spectrum. When performing Faraday impedance measurement in a solution containing a redox system. In Randles equivalent circuit, the electrolyte resistance Rs and Warburg impedance Zw represent the properties of the bulk solution and the diffusion characteristics of the redox couple, which are not affected by the surface reaction of the electrode;
Double layer capacitance Cdl and electron transfer resistance Ret characterize the dielectric and insulation properties of the electrode/electrolyte interface (Figure 1A). The Faraday AC impedance spectrum Nyquist the diagram is composed of a semicircle that intersects the coordinate axis and a straight line after it (Figure 1B). When the voltage changes in a high-frequency band, the impedance spectrum is a semicircle, corresponding to the electron transfer process in an electrochemical system. The electron transfer resistance Ret is equal to the semicircle’s diameter. And the intercept of the semicircle on the real axis corresponds to the electrolyte resistance Rs. When the voltage change is in a low-frequency band, the impedance spectrum is a straight line, corresponding to the ion diffusion process of the electrolyte solution. Yang, et al. [18] used AC impedance spectroscopy to study the impedance change mechanism of Salmonella typhimurium when adsorbed on a gold electrode in the environment containing 0.01M Fe(CN)63-/4- redox probe, using Randles equivalent circuit to fit the Nyquist impedance spectrum. The obtained results are shown in Figure 1 (C). Compared with the two cases of bare electrodes and immersing the electrodes in phosphate buffer solution (curves a and b), When the electrodes are immersed in the Salmonella typhimurium solution (curve c), the diameter of the semicircle representing the electron transfer resistance Ret is increased from 0.3 kΩ to 0.9 kΩ, and the double-layer capacitance Cdl is reduced. This not only indicates that the adsorption of Salmonella typhimurium on the electrode leads to an increase in impedance but also shows that the mechanism of impedance change is mainly caused by changes in electron transfer resistance and electric double layer capacitance.
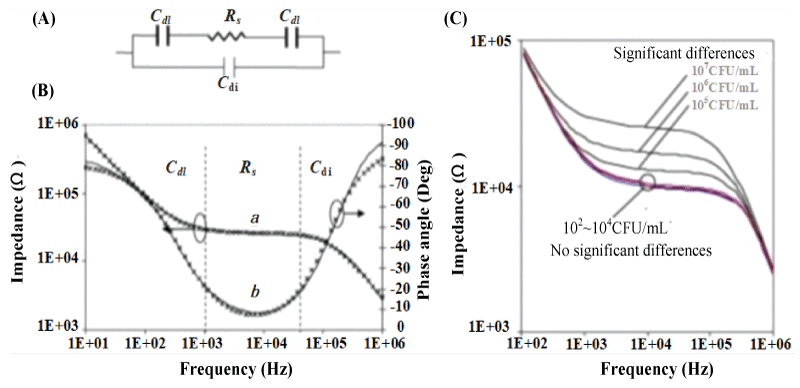
Varshney, et al. [19] used the microfluidic impedance method to detect E. coli O157: H7, and used an equivalent circuit to fit the electrochemical impedance Bode diagram to analyze the impedance change mechanism. In the equivalent circuit model, two double-layer capacitors Cdl and electrolyte resistor Rs are connected in series and connected in parallel with the dielectric capacitor Cdi (Figure 2A). The double layer capacitance Cdl represents the influence of the ion type on the electrode surface capacitance; the electrolyte resistance Rs represents the change in the conductivity of the electrolyte solution; Cdi represents the dielectric capacitance of the electrolyte solution. The test results are shown in Figure 2 (B). In the low-frequency band (10 Hz ~ 1 kHz), the change of impedance signal is dominated by the double layer capacitor Cdl. In the middle frequency band (1 kHz ~ 50 kHz), the impedance signal is dominated by the electrolyte resistance Rs. In the high-frequency range (50 kHz ~ 1 MHz), the impedance signal is dominated by the dielectric capacitor Cdi. It can also be concluded that in the detection range, the impedance increases with the increase of the concentration of E. coli (Figure 2C). When the E. coli is adsorbed on the electrode, the electrolyte resistance Rs increases, and the increase of Rs becomes the main reason for the change in impedance.
Analysis of influencing factors of cavitation bubble collapse by micro-jet
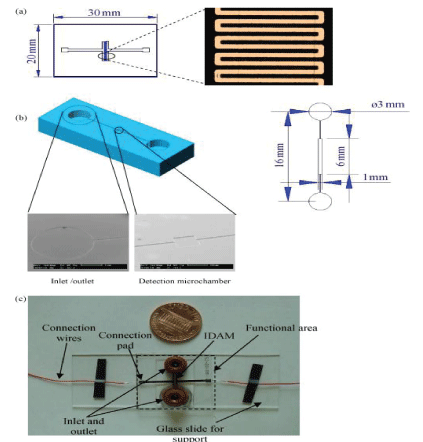
Gomez, et al. [28] created the first integrated silicon-based microfluidic IDAM chip for microbial metabolic impedance detection. They developed a flow cell embedded in platinum IDAM to detect the metabolic activity of a few live bacterial cells. Varshney [29] integrated the microfluidic flow cell with the embedded gold IDAM into an impedance biosensor to quickly detect pathogens in the ground beef sample. They combined the IDAM chip on the microchannel made by PDMS. The entire flow cell consisted of a detection microcavity and an inlet and outlet microchannel (Figure 3). Bacterial cells in the active layer above the microelectrodes were collected using a detection microchamber having a size of 6 mm×0.5 mm×0.02 mm, a volume of 60 nL namely. The target bacteria were isolated and concentrated by immunomagnetic separation technique. The device was used to detect E.coli O157:H7 in pure culture and beef samples. The detection limits were as low as 1.6×102 and 1.2×103 CFU/mL, respectively, and the detection time was less than 30 min. Among the microfluidic impedance sensors, the detection technology using IDAM as the transducer material is the most common. IDAM is also made of many materials, such as gold, ITO, Pt, Ti, Rh, etc. For example, Dasditer [26] used a gold-finger array microelectrode immobilized antibody to detect Salmonella typhimurium. in a microfluidic chip. Chen, et al. [30] detected Listeria based on the microfluidic impedance of ITO interdigitated array microelectrodes. Examples of pathogens detected by microfluidic impedance sensors of different IDAM materials are shown in Table 1.
Although IDAM research has broad prospects, IDAM still has some shortcomings. Due to the less repeatable detection of the IDAM chip, the detection cost is increased. In addition, the effective integration of IDAM on microfluidic chips also faces challenges. It is urgent to use more advanced processing technology and advanced materials to further develop the production of IDAM microfluidic impedance biosensors.
Dielectrophoresis
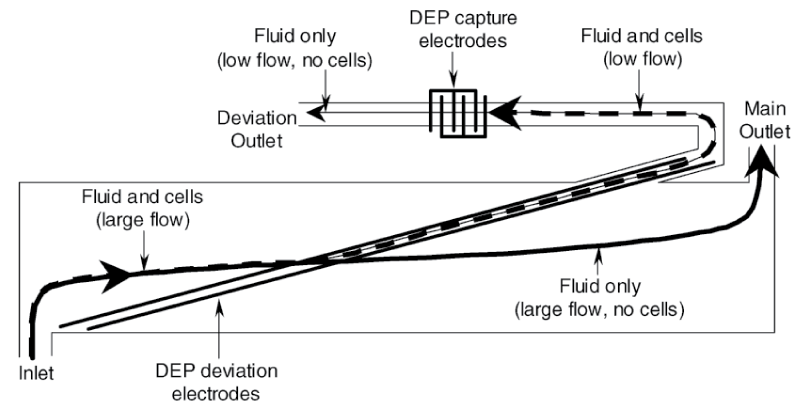
DEP is the electrokinetic movement of the dielectric material in a non-uniform electric field. The dielectrophoresis used for impedance measurement is called DEPIM, the dielectrophoretic impedance technique. It is one of the effective techniques for microfluidic impedance detection of pathogens because of its ability to highly enrich target cells while achieving low detection limits and high-throughput detection [39,40]. Gomez, et al. [31] developed a microfluidic impedance sensing device based on DEP technology. The design concept is to use DEP to transfer bacterial cells from the main channel to a small channel, allowing the cells to enter a measurement chamber with a volume of 400 μL (Figure 4). The impedance growth curves of Listeria cells using DEP technology and not using DEP were compared. Impedance metabolic signals grow exponentially at approximately 1 hour during DEP action, while samples containing similar concentrations of DEP-free cells require approximately 7.5 hours to produce a detectable impedance signal. The detection time has been significantly reduced, and 8.0×104 CFU/mL Listeria can be detected within 2 hours.
Dastider, et al. [33] used two sets of IDA. The agminated E.coli is concentrated by pDEP to the center of the microchannel towards the detection zone, the volume of the detection zone being significantly smaller than the volume of the flow channel, and wherein the polyclonal anti-E. coli antibody is not specifically immobilized on the sensing electrode array. The impedance results showed that the detection limit of E.coli O157:H7 bacterial cells was 3×102 CFU/mL. Kim [34] used high pDEP force to aggregate E.coli and pass the sensing electrode. The trapped bacteria form a bridge on the electrode gap, thereby reducing the impedance of the sensing electrode. After detection, E.coli cells were released by shutting off the DEP force. The efficacy of the system was confirmed using four different concentrations of E.coli with a detection limit of 300 CFU/mL.
Páezavilés, et al. [41] reported a number of studies on the detection of pathogenic bacteria in combination with dielectrophoresis on microfluidic chips and impedance methods. In addition, there are also studies on the separation and concentration of bacteria by dielectrophoresis under the action of a magnetic field and sound waves [42-44]. DEP technology can effectively separate pathogens from microfluidics, but high DEP collection efficiency can only be obtained at low sample flow rates. So there is still a need for pre-sorting steps using filtration or immunomagnetic separation methods to increase sample throughput.
Nanotechnology
The function of nanomaterials serves two purposes: to improve the response characteristics of the transducer and the immobilization matrix of the bioreceptor [45]. Nanomaterials have been used to improve pathogen enrichment capture efficiency and amplified the detection signal to achieve lower detection limits and high sensitivity due to their high specific surface area, good electronic properties and electrocatalytic activity, and good biocompatibility and adsorption due to nanometer size and specific physicochemical properties [46-48]. Nanomaterials currently used in microfluidic impedance sensors include nanoparticles, nanotubes, nanoporous membranes, and nanometer two-dimensional materials.
Nanotechnology
Nanoparticles are used to enhance electron transfer and capture more surface loading due to superior conductivity and ultra-high surface area to improve bio-impedance signal conversion levels. Typical are gold nanoparticles AuNPs. For example, Kang, et al. [49] used a double-layered gold nanoparticle and chitosan to prepare a microfluidic impedance sensor for the detection of Bacillus cereus. The use of double-layer gold nanoparticles increased the amount of antibody immobilized and retained the antibody activity. The detection sensitivity of the device is 5.0×101~5.0×104 CFU/mL, the detection limit is 10.0 CFU/mL, and the stable impedance response is maintained for a certain period of time. Michael, et al. [50] developed an impedance immunosensor based on a porous volume microfluidic detection element and a silver-enhanced gold nanoparticle probe. The porous method is used to increase the rate of pathogen capture, and the silver particles are used to enhance the impedance response. The device has a detection limit of 10 CFU/mL.
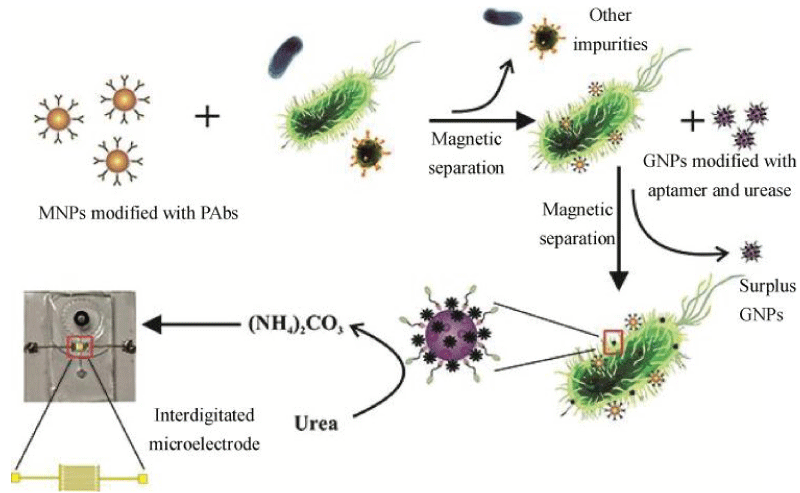
In addition to being used as a marker and improving the impedance signal level, the magnetic nanoparticles can form an immunomagnetic bead IMB with the target antibody, and the IMB binds to the target bacteria to form a beaded bacterial complex. The composite is oriented and moved under the action of an external magnetic field and is finally absorbed and retained in the magnetic field so that the target bacteria can be easily separated from the food substrate and the sample background. This method is called immunomagnetic separation technology (IMS). The immunomagnetic separation technology can effectively eliminate the background interference in the sample, and achieve the purification and enrichment of the sample, so as to shorten the detection time and improve the sensitivity [51]. Damira, et al. [52] used magnetic nanoparticles with a diameter of 30 nm in combination with functionalized Listeria monocytogenes antibodies to form immunomagnetic nanoparticles (MNPs). Impedance measurements indicate that 104 and 105 CFU/mL of Listeria monocytogenes were detected in samples of lettuce, milk, and ground beef. Recently, drawing on the experience of Chen [30] and Wang [53] using nanoparticle immunomagnetic separation technology and urease to amplification signal, Yao, et al. [38] skillfully combined magnetic nanoparticles (MNPs) for bacterial separation, urease for biosignal amplification, and microfluidic chips for impedance measurement for rapid, sensitive, and continuous flow detection of E.coli O157: H7.As shown in Figure 5, after streptavidin-modified MNPs bind to biotinylated polyclonal antibodies (PAbs) to form immune MNPs, the target bacteria are first isolated from the background by MNPs to form MNP-bacteria complexes. Then, MNP-bacteria was conjugated with E.coli O157:H7 modified with urease and gold nanoparticles (GNPs) to form an MNP-bacteria-GNP-urease complex. Finally, the complex is used to catalyze the hydrolysis of urea to ammonium carbonate, resulting in a decrease in impedance. the concentration of E.coli O157:H7 was determined by measuring impedance online and using impedance normalization analysis. A good linear relationship between relative impedance change and bacterial concentration was obtained at a low detection limit of 12 CFU/mL.
The application of nano-magnetic beads in the field of microbial detection has been relatively mature. The efficiency of nano-magnetic beads in capturing pathogenic bacteria can reach 60%~100%, and the interference of non-specific adsorption can be reduced. Especially under the combined action of nanomagnetic beads, bioligands and functional modifiers, the detection limit of microfluidic impedance can be lower, but it is necessary to pay attention to the interference of excess magnetic beads or dense magnetic beads for impedance response.
Nanoporous membrane
The nanoporous alumina membrane is simple and inexpensive to manufacture, and its application in the microfluidic impedance sensor is because it allows a large number of target molecules to be adsorbed on the nanopore wall by covalent bonding, which can significantly improve the detection sensitivity. Jiang [54] used the sensor system of the smartphone as a platform to realize high sensitivity and rapid on-site detection of E.coli in water based on microfluidic bacteria preconcentration and electrical impedance spectroscopy. The device filtered out macromolecular particles through a large number of nano-aluminum pore membranes with a diameter of 16 micrometers, and the detected bacteria were left in the microfluidic detection chamber through the filtration membrane. The process that detected bacteria passed through the pore membranes corresponded to the preconcentration of the bacteria. Subsequently, the pre-concentrated bacterial cells distributed around the interdigitated electrodes are subjected to impedance sensing. The smartphone sensing platform obtained the impedance spectrum through the scanning frequency of 2 kHz to 100 kHz. The E.coli concentration can be obtained by fitting the measurement result to the calibration curve and the corresponding formula by the mobile phone program. The detection limit of bacterial detection of this device is 10 CFU/mL, and the detection concentration range is 10 CFU/mL~103 CFU/mL.
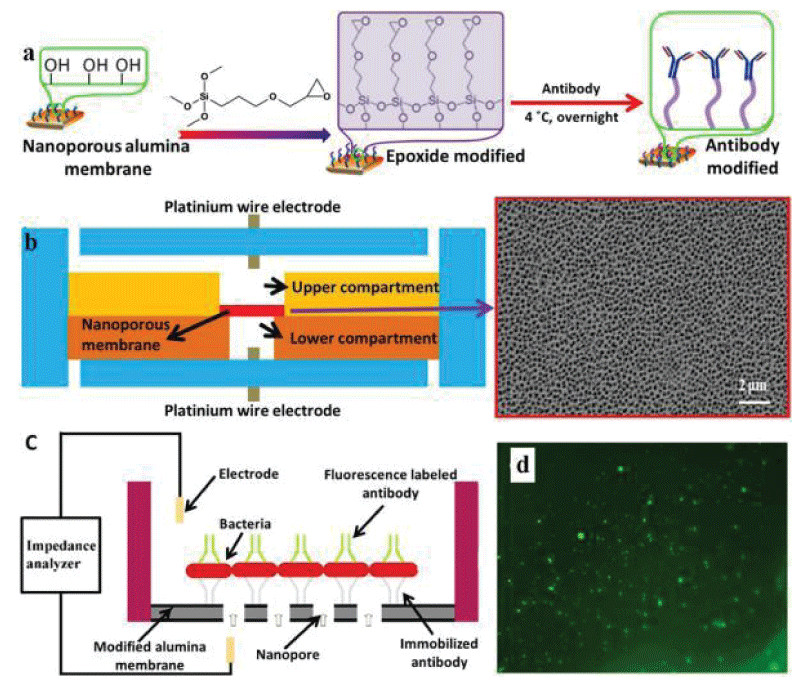
Tan, et al. [55] efficiently detected S.aureus and E.coli O157:H7 using an antibody-immobilized nanoporous alumina membrane integrated into the device. The antibody was covalently immobilized on a nanoporous alumina membrane by trimethoxysilane (GPTMS) (Figure 6a). The film has a diameter of 13 mm and a thickness of 60 μm, the film was integrated between two PDMS layers treated by oxygen plasma, and the platinum wire electrode was used for impedance sensing (Figure 6b). The sample containing the bacteria was loaded into the upper compartment, and the antibody bound on the nanoporous alumina membrane was able to capture the pathogen, causing electrolyte current to clog through the membrane and thus causing an increase in impedance (Figure 6c). The microfluidic immunosensor device quickly detected bacteria within 2 hours with a detection limit of 102 CFU/mL, which shows better sensitivity than conventional microelectrode-based impedance sensors. Tian, et al. [56,57] slightly improved the above device and installed two nanoporous membranes to simultaneously detect E.coli O157:H7 and Staphylococcus aureus at a concentration of 102 CFU/mL.
Immobilization of antibodies on nanoporous alumina membrane. The membranes were first treated with 10% hydrogen peroxide (H2O2) to remove any contaminants and generate a reactive hydroxyl group on the surface. After drying, toluene solution with 1% GPMS was applied overnight to functionalize the surface with epoxide groups. Next, the antibody was immobilized on the surface through the reaction of the amine groups on the antibody with the epoxy groups on the surface of the membrane. (b) Schematic illustration of the PDMS microfluidic device integrated with nanoporous alumina membrane and SEM image of the porous membrane. (c) The mechanism of impedance sensing via antibody immobilized on nanoporous alumina membrane. The pathogen will anchor to complimentary antibodies on the modified nanoporous alumina membrane, once the sample with target bacteria loads into the upper compartment. When bacteria are captured on the membrane, the nanopores will be blocked, and subsequently, the electrolyte current through the membrane will decrease and can be observed in the impedance spectrum. (d) Fluorescence image of S. aureus captured on the antibody-modified membrane with a concentration of 1 × 105 CFU/ml.
The application of nanoporous membranes has the advantages of high sensitivity and fast on-site detection, but the disadvantages of nanoporous membranes are that their own preparation process is complex, and the success rate of generating nanoporous layers is low, affecting the stability of the sensor. In general, membrane-assisted sample enrichment in microfluidic systems is still in an early stage, so additional efforts are needed to investigate new concepts that can be practically applied to design such miniaturized sensing devices.
Nano two-dimensional material
Graphene and molybdenum disulfide (MoS2) are novel nano-two-dimensional materials that have generated significant interest in designing electrochemical devices for biosensing applications. Nano-two-dimensional materials, whether as electrode materials or chemical modification, bring higher sensitivity to microfluidic impedance sensors. For graphene, since all the carbon atoms in the graphene layer are located on the surface, intermolecular interactions and electron transfer are very advantageous. These properties make graphene material with high electrical conductivity, good catalytic activity of electrochemical reaction, and high specific surface area [58]. For example, Pandey, et al. [59] utilized graphene for excellent electron transfer and bacterial capture to specifically detect E.coli. The graphene nanostructures directly bind to the interdigitated microelectrodes to capture bacteria and amplify the impedance signal, resulting in a detection limit of 10 CFU/mL. Chandra, et al. [60] based on graphene-coated copper oxide-cysteine for detection of Escherichia coli. After 40 days, the impedance response of the sensor remained at 90.2% of the initial value, indicating the electrocatalytic activity and stability of the graphene material.
Recently, synergistic effects based on graphene and other nanomaterials have been extensively studied, and it has been found that the combination of graphene and other nanostructured materials further enhances electron transfer and bacterial capture efficiency. Ma, et al. [61] proposed a glassy carbon electrode modified with graphene oxide and gold nanoparticles as an impedance biosensor for Salmonella detection, whose limitation of detection reached 3 CFU/mL and the bacterial recovery rate was close to 100% in 10~1000 CFU/mL pork samples. In addition, Romano, et al. [62] reported that graphene oxide-carbon nanotube (GO-CNTs) nanocomposites have a maximum electroactive surface area that can control porosity and provide a larger surface for immobilized biomolecules. Carbon nanotubes themselves have a high surface area and strong electrical conductivity for the development of electrochemical immunosensors for the detection of pathogens, achieving an excellent detection sensitivity of 13 CFU/mL [63]. Recently, Chandan, et al. [64] used graphene oxide (GO) nanosheets encapsulating multi-walled carbon nanotubes (cMWCNTs) to modify ITO microelectrodes to detect Salmonella typhimurium without labeling. The measured impedance signal is much higher compared to microfluidic chips based solely on cMWCNT/ITO or GO/ITO modifications. In summary, the application of two-dimensional graphene-based nanomaterials in microfluidic impedance devices has made great progress, greatly improving the sensitivity of pathogen detection. In particular, the synergistic effect of graphene and its nanomaterials makes nanotechnology more advantageous in efficiently capturing pathogens and amplifying impedance signals, but the latest technology in the field cannot be used in the field or point-of-use applications.
MoS2 has a higher surface area than graphene, and the presence of the MoS2 band gap can increase the detection sensitivity by a factor of two. Chandan, et al. [64] described an effective microfluidic chip for the detection of Salmonella typhimurium with a detection limit of 1.56 CFU/mL and a detection range of 10-107 CFU/mL. They used MoS2 nanosheets functionalized by stripped CTAB as transducer materials. The positively charged CTAB-MoS2-NS formed a film on the surface of the ITO electrode by electrostatic interaction, capturing the target bacteria and changing the impedance signal.
In general, the traditional advantages of impedance biosensors, such as rapidity, ease of manufacture, and field suitability, can be further enhanced with the help of two-dimensional nanomaterials. In addition, nanomaterials and microfluidic chips are both micro-technologies, minimizing the sensitivity and specificity of devices for electrochemical biosensors, and giving them great potential for assessing food safety on site. However, the mass production technology of nano two-dimensional materials is immature and the cost is high, which is a problem that needs to be solved in the application of microfluidic impedance sensors.
Microfluidic impedance detection technology and principle
Microfluidic impedance biosensors concentrate the entire process of pathogenic bacteria samples on the microfluidic chip from injecting, mixing, and detecting to measuring, reducing detection time, saving detection costs, and improving analysis efficiency. Detection technology based on IDAM can take advantage of the high sensitivity of IDAM and fast impedance measurement, but it needs to solve the problem of less repeatable detection. DEP technology can achieve efficient isolation and capture pathogenic bacteria at low throughput so which improves detection sensitivity, but the capture rate of pathogenic bacteria needs to be improved at high throughput. Nanotechnology and the union of multiple nanotechnologies can reduce the detection limit of foodborne bacterial to single digits, but there are still some defects: if the nanoparticles are too dense the results will be affected, and the success rate of nanoporous membrane production is not high and the mass production technology of two-dimensional nanomaterials is immature. These defects have limited the field application of microfluidic impedance sensors [65-72].
At present, the construction of microfluidic impedance biosensors is still in the stage of continuous exploration. In future research, we need to continuously learn from optical and other electrochemical-based methods and technologies, and we need to rely on the development of the latest nanomaterials and technologies suitable for impedance detection. So that it can be used to quickly detect foodborne pathogens in the field in real-time. In addition, in order to meet the low price requirements of microfluidic devices for pathogen detection, future research can shift from highly complex manufacturing technologies to polymers or paper devices that can meet the needs of end users. Such as polymers and paper-based chips replacing silicon and glass, and screen-printed electrodes or semiconductor nanomaterials replacing metal electrodes.
The authors acknowledge the financial support provided by the National Key Research and Development Program of China (2018YFC0809200, the National Natural Science Foundation of China (No.51905515), and the Public Welfare Technology Project of Zhejiang Province (LGF19G030004).
Funding
The authors acknowledge the financial support provided by the National Key Research and Development Program of China (2018YFC0809200), National Natural Science Foundation of China (No.51905515) and Public Welfare Technology Project of Zhejiang Province (LGF19G030004).
Availability of data and material
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributions
All authors contributed to the study's conception and design. Material preparation, data collection, and analysis were performed by Mu Yuan, Chen Li, and Qingdao Xu. The first draft of the manuscript was written by Mu Yuan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript
- Newman KL, Leon JS, Rebolledo PA, Scallan E. The impact of socioeconomic status on foodborne illness in high-income countries: a systematic review. Epidemiol Infect. 2015 Sep;143(12):2473-85. doi: 10.1017/S0950268814003847. Epub 2015 Jan 20. PMID: 25600652; PMCID: PMC4508232.
- WHO. WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007-2015. Geneva: World Health Organization, 2015;254-311.
- Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect. 2015 Oct;143(13):2795-804. doi: 10.1017/S0950268814003185. Epub 2015 Jan 30. PMID: 25633631; PMCID: PMC9151020.
- Wu YN, Liu XM, Chen Q, Liu H, Dai Y, Zhou YJ, Wen J, Tang ZZ, Chen Y. Surveillance for foodborne disease outbreaks in China, 2003 to 2008. Food Control. 2018 Feb;84:382-388. doi: 10.1016/j.foodcont.2017.08.010. Epub 2017 Aug 14. PMID: 32288325; PMCID: PMC7125948.
- Zhao X, Lan Q K, Chen R. Rapid detection of salmonella in edible fungi by microdrop digital PCR. Journal of food and biotechnology,2017;36(03):315-321.
- Zhao X, Lin CW, Wang J, Oh DH. Advances in rapid detection methods for foodborne pathogens. J Microbiol Biotechnol. 2014 Mar 28;24(3):297-312. doi: 10.4014/jmb.1310.10013. PMID: 24375418.
- Quynh H N, Moon I K. Using Nanomaterials in Colorimetric Toxin Detection. Bio Chip Journal. 2021;15:123-134.
- Li SQ, Wang XS, Yang K. Electrochemical disease sensor based on two-dimensional nanomaterials. Chinese Science Bulletin, 2016;61(11):1222-1232.
- Zhang D, Chen SY, Qin LF. Study on electrochemical impedance spectroscopy biosensors for detection of E.coli O157:H7. Journal of sensing technology. 2005;(01):5-9.
- Wang Y, Ye Z, Ying Y. New trends in impedimetric biosensors for the detection of foodborne pathogenic bacteria. Sensors (Basel). 2012;12(3):3449-71. doi: 10.3390/s120303449. Epub 2012 Mar 12. PMID: 22737018; PMCID: PMC3376556.
- Kant K, Shahbazi MA, Dave VP, Ngo TA, Chidambara VA, Than LQ, Bang DD, Wolff A. Microfluidic devices for sample preparation and rapid detection of foodborne pathogens. Biotechnol Adv. 2018 Jul-Aug;36(4):1003-1024. doi: 10.1016/j.biotechadv.2018.03.002. Epub 2018 Mar 10. PMID: 29534915.
- Wang M, Zhao C, Miao X, Zhao Y, Rufo J, Liu YJ, Huang TJ, Zheng Y. Plasmofluidics: Merging Light and Fluids at the Micro-/Nanoscale. Small. 2015 Sep 16;11(35):4423-44. doi: 10.1002/smll.201500970. Epub 2015 Jul 3. PMID: 26140612; PMCID: PMC4856436.
- Kumar S, Kumar S, Ali MA, Anand P, Agrawal VV, John R, Maji S, Malhotra BD. Microfluidic-integrated biosensors: prospects for point-of-care diagnostics. Biotechnol J. 2013 Nov;8(11):1267-79. doi: 10.1002/biot.201200386. Epub 2013 Sep 6. PMID: 24019250.
- Lei K F. Microfluidic systems for diagnostic applications: a review. J Lab Autom. 2012;17(5):330-347.
- Tasoglu S, Gurkan UA, Wang S, Demirci U. Manipulating biological agents and cells in micro-scale volumes for applications in medicine. Chem Soc Rev. 2013 Jul 7;42(13):5788-808. doi: 10.1039/c3cs60042d. PMID: 23575660; PMCID: PMC3865707.
- Testa G, Persichetti G, Bernini R. Planar Optofluidic Integration of Ring Resonator and Microfluidic Channels. Micromachines (Basel). 2022 Jun 28;13(7):1028. doi: 10.3390/mi13071028. PMID: 35888845; PMCID: PMC9315487.
- Lisdat F, Schäfer D. The use of electrochemical impedance spectroscopy for biosensing . Analytical and Bioanalytical Chemistry. 2008;391(5):1555-1567.
- Yang L, Bashir R. Electrical/electrochemical impedance for rapid detection of foodborne pathogenic bacteria. Biotechnol Adv. 2008 Mar-Apr;26(2):135-50. doi: 10.1016/j.biotechadv.2007.10.003. Epub 2007 Nov 12. PMID: 18155870.
- Varshney M, Li Y, Srinivasan B. A label-free, microfluidics and interdigitated array microelectrode-based impedance biosensor in combination with nanoparticles immunoseparation for detection of Escherichia coli O157:H7 in food samples. Sensors and Actuators B Chemical. 2007;128(1):99-107.
- Yang L, Ruan C, Li Y. Detection of viable Salmonella typhimurium by impedance measurement of electrode capacitance and medium resistance. Biosens Bioelectron. 2003 Dec 30;19(5):495-502. doi: 10.1016/s0956-5663(03)00229-x. PMID: 14623474.
- Jiaxing W, Sibin G, Zhejun T. A context-aware recommendation system for improving manufacturing process modeling [J]. Journal of Intelligent Manufacturing. 2021; 2021:1-22.
- Dapeng T, Shuting C, Junguan B. An embedded lightweight GUI component library and ergonomics optimization method for industry process monitoring. Frontiers of Information Technology & Electronic Engineering. 2018; 19: 604-625.
- Ruan C, Yang L, Li Y. Immunobiosensor chips for detection of Escherichia coil O157:H7 using electrochemical impedance spectroscopy. Anal Chem. 2002 Sep 15;74(18):4814-20. doi: 10.1021/ac025647b. PMID: 12349988.
- Wang R, Lum J, Callaway Z, Lin J, Bottje W, Li Y. A Label-Free Impedance Immunosensor Using Screen-Printed Interdigitated Electrodes and Magnetic Nanobeads for the Detection of E. coli O157:H7. Biosensors (Basel). 2015 Dec 15;5(4):791-803. doi: 10.3390/bios5040791. PMID: 26694478; PMCID: PMC4697145.
- Shuting C, Dapeng T. A SA-ANN-Based Modeling Method for Human Cognition Mechanism and the PSACO Cognition Algorithm. Complexity. 2018; 2018:1-21.
- Ghosh Dastider S, Barizuddin S, Yuksek NS. Efficient and Rapid Detection of Salmonella Using Microfluidic Impedance Based Sensing . Journal of Sensors. 2015; 2015:1-8.
- Wang T, Li Lin, Yin Z. Investigation on the flow field regulation characteristics of the right-angled channel by impinging disturbance method. Proceedings of the Institution of Mechanical Engineers, Part C: Journal of Mechanical Engineering Science. 2022. In Press.
- Gómez R, Bashir R, Bhunia A K. Microscale electronic detection of bacterial metabolism. Sensors and Actuators B Chemical.2002;86(2):198-208.
- Varshney M, Li Y. Interdigitated array microelectrodes based impedance biosensors for detection of bacterial cells. Biosens Bioelectron. 2009 Jun 15;24(10):2951-60. doi: 10.1016/j.bios.2008.10.001. Epub 2008 Oct 17. PMID: 19041235.
- Sitkov N, Zimina T, Kolobov A, Sevostyanov E, Trushlyakova V, Luchinin V, Krasichkov A, Markelov O, Galagudza M, Kaplun D. Study of the Fabrication Technology of Hybrid Microfluidic Biochips for Label-Free Detection of Proteins. Micromachines (Basel). 2021 Dec 24;13(1):20. doi: 10.3390/mi13010020. PMID: 35056185; PMCID: PMC8779695.
- Gomez-Sjoberg R, Morisette D T, Bashir R. Impedance microbiology-on-a-chip: microfluidic bioprocessor for rapid detection of bacterial metabolism. Journal of Microelectromechanical Systems.2005;14(4):829-838.
- Mannoor MS, Zhang S, Link AJ, McAlpine MC. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc Natl Acad Sci U S A. 2010 Nov 9;107(45):19207-12. doi: 10.1073/pnas.1008768107. Epub 2010 Oct 18. PMID: 20956332; PMCID: PMC2984209.
- Wang D, Chen Q, Huo H. Efficient separation and quantitative detection of Listeria monocytogenes based on screen-printed interdigitated electrode, urease and magnetic nanoparticles . Food Control. 2017;73:555-561.
- Dastider S, Barizuddin S, Dweik M, et al. A micromachined impedance biosensor for accurate and rapid detection of E.coli O157:H7 . Rsc Advances. 2013;3(48):26297-26306.
- Nair MP, Teo AJT, Li KHH. Acoustic Biosensors and Microfluidic Devices in the Decennium: Principles and Applications. Micromachines (Basel). 2021 Dec 26;13(1):24. doi: 10.3390/mi13010024. PMID: 35056189; PMCID: PMC8779171.
- Gómez R, Bashir R, Bhunia A K. Microscale electronic detection of bacterial metabolism. Sensors and Actuators B Chemical. 2015;86(2):198-208.
- Oliveira T R, Martucci D H, Faria R C. Simple disposable microfluidic device for Salmonella typhimurium detection by magneto-immunoassay . Sensors and Actuators B: Chemical. 2018;255:684-691.
- Yao L, Wang L, Huang F. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E.coli O157:H7 . Sensors and Actuators B: Chemical.2018;259:1013-1021.
- Fernandez RE, Rohani A, Farmehini V, Swami NS. Review: Microbial analysis in dielectrophoretic microfluidic systems. Anal Chim Acta. 2017 May 8;966:11-33. doi: 10.1016/j.aca.2017.02.024. Epub 2017 Mar 6. PMID: 28372723; PMCID: PMC5424535.
- Liu YS, Walter TM, Chang WJ, Lim KS, Yang L, Lee SW, Aronson A, Bashir R. Electrical detection of germination of viable model Bacillus anthracis spores in microfluidic biochips. Lab Chip. 2007 May;7(5):603-10. doi: 10.1039/b702408h. Epub 2007 Apr 5. PMID: 17476379.
- Páezavilés C, Juanolafeliu E, Puntervillagrasa J, et al. Combined Dielectrophoresis and Impedance Systems for Bacteria Analysis in Microfluidic On-Chip Platforms . Sensors. 2016;16(9):1514.
- Ngamsom B, Lopez-Martinez MJ, Raymond JC, Broyer P, Patel P, Pamme N. On-chip acoustophoretic isolation of microflora including S. typhimurium from raw chicken, beef and blood samples. J Microbiol Methods. 2016 Apr;123:79-86. doi: 10.1016/j.mimet.2016.01.016. Epub 2016 Feb 4. PMID: 26835844.
- Ngamsom B, Esfahani MM, Phurimsak C, Lopez-Martinez MJ, Raymond JC, Broyer P, Patel P, Pamme N. Multiplex sorting of foodborne pathogens by on-chip free-flow magnetophoresis. Anal Chim Acta. 2016 Apr 28;918:69-76. doi: 10.1016/j.aca.2016.03.014. Epub 2016 Mar 18. PMID: 27046212.
- Guo PL, Tang M, Hong SL, Yu X, Pang DW, Zhang ZL. Combination of dynamic magnetophoretic separation and stationary magnetic trap for highly sensitive and selective detection of Salmonella typhimurium in complex matrix. Biosens Bioelectron. 2015 Dec 15;74:628-36. doi: 10.1016/j.bios.2015.07.019. Epub 2015 Jul 14. PMID: 26201979.
- Vasireddi R, Gardais A, Chavas LMG. Manufacturing of Ultra-Thin X-ray-Compatible COC Microfluidic Devices for Optimal In Situ Macromolecular Crystallography Experiments. Micromachines (Basel). 2022 Aug 22;13(8):1365. doi: 10.3390/mi13081365. PMID: 36014287; PMCID: PMC9416059.
- Zeng Y, Zhu Z, Du D. Nanomaterial-based electrochemical biosensors for food safety . Journal of Electroanalytical Chemistry. 2016;781:147-154.
- Huang J, Yang G, Meng W, Wu L, Zhu A, Jiao X. An electrochemical impedimetric immunosensor for label-free detection of Campylobacter jejuni in diarrhea patients' stool based on O-carboxymethylchitosan surface modified Fe3O4 nanoparticles. Biosens Bioelectron. 2010 Jan 15;25(5):1204-11. doi: 10.1016/j.bios.2009.10.036. Epub 2009 Nov 4. PMID: 19932018.
- Lu H, Zhang L, Tan D. A collaborative assembly for low-voltage electrical apparatuses [J]. Frontiers of Information Technology & Electronic Engineering, 2021. In Press.
- Kang X, Pang G, Chen Q. Fabrication of Bacillus cereus electrochemical immunosensor based on double-layer gold nanoparticles and chitosan . Sensors and Actuators B Chemical. 2013;177(1):1010-1016.
- Xiong Q, Cui X, Saini J K, et al. Development of an immunomagnetic separation method for efficient enrichment of Escherichia coli O157:H7 . Food Control.2014;37(1):41-45.
- Wiederoder MS, Misri I, DeVoe DL. Impedimetric Immunosensing in a Porous Volumetric Microfluidic Detector. Sens Actuators B Chem. 2016 Oct 29;234:493-497. doi: 10.1016/j.snb.2016.05.015. Epub 2016 May 6. PMID: 27721569; PMCID: PMC5053616.
- Kanayeva DA, Wang R, Rhoads D, Erf GF, Slavik MF, Tung S, Li Y. Efficient separation and sensitive detection of Listeria monocytogenes using an impedance immunosensor based on magnetic nanoparticles, a microfluidic chip, and an interdigitated microelectrode. J Food Prot. 2012 Nov;75(11):1951-9. doi: 10.4315/0362-028X.JFP-11-516. PMID: 23127703.
- Wang L, Huang F, Cai G, Yao L, Zhang H, Lin J. An Electrochemical Aptasensor Using Coaxial Capillary with Magnetic Nanoparticle, Urease Catalysis and PCB Electrode for Rapid and Sensitive Detection of Escherichia coli O157:H7. Nanotheranostics. 2017 Oct 9;1(4):403-414. doi: 10.7150/ntno.22079. PMID: 29071202; PMCID: PMC5647763.
- Sun X, Li B, Li W, Ren X, Su N, Li R, Li J, Huang Q. A Resistance-Based Microfluidic Chip for Deterministic Single Cell Trapping Followed by Immunofluorescence Staining. Micromachines (Basel). 2022 Aug 7;13(8):1272. doi: 10.3390/mi13081272. PMID: 36014194; PMCID: PMC9416254.
- Tan F, Leung P HM, Liu ZB. A PDMS microfluidic impedance immunosensor for E.coli O157:H7 and Staphylococcus aureus detection via antibody-immobilized nanoporous membrane [J]. Sensors and Actuators B Chemical. 2011; 159(1):328-335.
- Tian F, Jing L, Shi J. A polymeric microfluidic device integrated with nanoporous alumina membranes for simultaneous detection of multiple foodborne pathogens. Sensors and Actuators B Chemical. 2016; 225:312-318.
- Ji S M, Weng XX, Tan D. Analytical method of softness abrasive two-phase flow field based on 2D model of LSM. Acta Physica Sinica. 2012; 61(1): 010205.
- Hasanzadeh M, Shadjou N, Mokhtarzadeh A, Ramezani M. Two dimension (2-D) graphene-based nanomaterials as signal amplification elements in electrochemical microfluidic immune-devices: Recent advances. Mater Sci Eng C Mater Biol Appl. 2016 Nov 1;68:482-493. doi: 10.1016/j.msec.2016.06.023. Epub 2016 Jun 8. PMID: 27524045.
- Pandey A, Gurbuz Y, Ozguz V, Niazi JH, Qureshi A. Graphene-interfaced electrical biosensor for label-free and sensitive detection of foodborne pathogenic E. coli O157:H7. Biosens Bioelectron. 2017 May 15;91:225-231. doi: 10.1016/j.bios.2016.12.041. Epub 2016 Dec 16. PMID: 28012318.
- Pandey C M, Tiwari I, Singh V N. Highly sensitive electrochemical immunosensor based on graphene-wrapped copper oxide-cysteine hierarchical structure for detection of pathogenic bacteria. Sensors and Actuators B Chemical. 2017; 238:1060-1069.
- Ma X, Jiang Y, Jia F, Yu Y, Chen J, Wang Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. J Microbiol Methods. 2014 Mar;98:94-8. doi: 10.1016/j.mimet.2014.01.003. Epub 2014 Jan 17. PMID: 24445115.
- Romano MS, Li N, Antiohos D, Razal JM, Nattestad A, Beirne S, Fang S, Chen Y, Jalili R, Wallace GG, Baughman R, Chen J. Carbon nanotube - reduced graphene oxide composites for thermal energy harvesting applications. Adv Mater. 2013 Dec 3;25(45):6602-6. doi: 10.1002/adma.201303295. Epub 2013 Oct 25. PMID: 24167027.
- Bhardwaj J, Devarakonda S, Kumar S. Development of a paper-based electrochemical immunosensor using an antibody-single walled carbon nanotubes bio-conjugate modified electrode for label-free detection of foodborne pathogens. Sensors and Actuators B: Chemical. 2017; 253:115-123.
- Singh C, Ali M A, Reddy V. Biofunctionalized Graphene Oxide Wrapped Carbon Nanotubes Enabled Microfluidic Immunochip for Bacterial Cells Detection. Sensors and Actuators B Chemical. 2017; 255:2495-2503.
- Singh C, Ali M A, Kumar V. Functionalized MoS2 nanosheets assembled microfluidic immunosensor for highly sensitive detection of food pathogen. Sensors and Actuators B: Chemical. 2018; 259:1090-1098.
- Liming L, Vamiq M M, Guangyu H. Classification of Soft Tissue Sarcoma Specimens with Raman Spectroscopy as Smart Sensing Technology. Cyborg and Bionic Systems. 2021; 2021: 100-108.
- Fukuda T. Cyborg and Bionic Systems: Signposting the Future. Cyborg and Bionic Systems. 2020; 2020:109-112.
- Wang H, Jiacheng K, Xin Z. Pt/CNT Micro-Nanorobots Driven by Glucose Catalytic Decomposition. Cyborg and Bionic Systems. 2021; 2021:22-24.
- Yin Z, Ni Y, Tan D. Numerical modelling and experimental investigation of a two-phase sink vortex and its fluid-solid vibration characteristics. Journal of Zhejiang University-SCIENCE A, 2022. In Press.
- Shuihua Z, Yankun Y, Mianzhen Q. A modal analysis of vibration response of a cracked fluid-filled cylindrical shell. Applied Mathematical Modelling. 2020; 91:341-344.
- Yangyu W, Yongle Z, Dapeng T. Key Technologies and Development Trends in Advanced Intelligent Sawing Equipments. Chinese Journal of Mechanical Engineering. 2021; 34(03):12-18.
- Linbin Z, Huangpei L, Dapeng T. Adaptive quantum genetic algorithm for task sequence planning of complex assembly systems. Electronics Letters. 2018; 54:870-872.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
