International Journal of Pharmaceutical Sciences and Developmental Research
Preparation of itraconazole nanoparticles and its topical nanogel: Physicochemical properties and stability studies
Kamlesh Amrut Wadile, Pradum Pundlikrao Ige* and Raju Onkar Sonawane
Cite this as
Wadile KA, Ige PP, Sonawane RO (2019) Preparation of itraconazole nanoparticles and its topical nanogel: Physicochemical properties and stability studies. Int J Pharm Sci Dev Res 5(1): 001-008. DOI: 10.17352/ijpsdr.000020The need of present study hypothesized due to drug has low bioavailability (55%) because of low aques solubility and first pass effect. Nanoparticles were prepared by solvent diffusion method by using different stabilizers such as Tween80, Pluronic F127, Sodium lauryl sulfate, span 80 and Polyvinyl alcohol. The prepared nanoparticles were characterized through particle size, polydispersity index (PDI), zeta potential, drug content, in vitro drug release study, infra-red spectroscopy, X-ray powder diffractometry and scanning electron microscopy. The optimized batch of Itraconazole nanoparticles (NF2), had particle size 167.2nm, polydispersity index 0.208, zeta potential (-17.8mV), drug content 94.8% and % cumulative drug release 86.15% ±0.5%. These prepared nanoparticles has transformed into topical nanogel. Different batches of topical nanogel were prepared like NG1, NG2 and NG3 by using Carbopol 940. All batches of topical nanogel were characterized through pH, homogeneity, spreadability, viscosity, drug content and in vitro drug release study. Based on good homogeneity, pH 6.8, viscosity (160.2 poise), spreadability (2.20 cm), drug content (77%) and cumulative drug release 85.24 ±0.9 %., NG2 batch was subjected to in vitro antifungal activity. In conclusion, prepared topical nanogel of itraconazole was stable and could be used with promising potential for fungal infection.
Introduction
Topical formulations are dosage forms used for localized effect at the site of application by penetrating into the skin layers of organism. Nanoparticles explore the controlled release profiles for many drugs in topical route and exhibiting low toxicity which is produced by the drug. In targeted delivery, drug is targeted to their selected site to produce therapeutic effect. It is also limits the access of drug to the non targeted or non-selective site and ultimately increases the desired effect of drug. Recently, nanoparticles are used in topical products because they have ability to target the skin, to cure skin related diseases. Corticosteroids, antifungal, antiviral, antibiotics, antiseptics, local anesthetics and antineoplastics are the example of the drugs delivered through topical route [1-4].
Numerous antifungal creams and ointments available to treat dermatological infections. However, fungal infections fail to cure. Topical preparations may produce red rashes, skin irritation, erythematic reactions and redness of skin. Creams had chemical and physical stability problem that leads to separation of water and oil phases. Drugs used to treat fungal infections like Itraconazole, Miconazole and Ketoconazole have been administered orally but they produces side effects, hepatic, systemic side effect in longer duration of treatment. Therefore, there is need to bypass the drugs to avoid hepatic, systemic side effect and gastric disorders [5-7].
The prefered method of preparation of nanoparticles is depends on the nature and physiochemical parameters of drug and polymer. Some methods are available for preparation of nanoparticles, emulsion solvent evaporation method, double emulsion and solvent evaporation method, salting out method, emulsions diffusion method, solvent displacement or precipitation method, dialysis method, emulsion polymerization method, mini-emulsion polymerization method, micro-emulsion polymerization method and interfacial polymerization method. The method used for preparation of nanoparticles of poorly water soluble drugs is solvent diffusion method. The process is based on water miscibility with organic solvents. In this, dissolved drug is trasformed into solid spheres due to diffusion of drug from organic phase to the continuous phase. The emulsion is emulsified by using emulsifier (Pluronic F127, Sodium lauryl sulfate, etc.) to form the nanospheres due to solvent diffusion. Advantages of this technique are high entrapment efficiency (70%), batch to batch reproducibility, simplicity and narrow particle size distribution. Its limitation is chances of leakage of water soluble drug into aqueous phase during emulsification leads to reduction in encapsulation efficiency and high quantity of water is required [8-15].
The nanoparticulate system had problem of particle growth. The nanoparticles have higher surface energy, this leads to formation of aggregates. The stabilizers avoid the formation of particle growth by providing ionic barrier to the surface of the particles. The amount and type of the stabilizer decides the stability of the nanoparticulate system. Stabilizers that are used poloxamers, polysorbates, povidones, lecithins and cellulose derivatives. The work is based on the topical application. Topical nanogel was prepared by using Carbopol 940 by some researchers [16-24].
Itraconazole is BCS class II drug with half-life 21 h, protein binding (99.8%) and absolute bioavailability (55%) due to its low aqueous solubility. The mechanism of action is that, triazole contains the azole ring in their structure, which have free nitrogen atom. This nitrogen atom compete oxygen atom; this causes cytochrome P450 enzyme inhibition, which is essential for synthesis of ergosterol in the fungi cell wall. The cytochrome P-450 is important for Cl4 demethylation of lanosterol which helpful for synthesis of ergosterol. The prevention of synthesis of the ergosterol loss membrane fluidity of cell. The accumulation of phospholipids and the unsaturated fatty acid is there in the fungal cell, cause cell death. The bioavailability of Itraconazole is affected by the gastric acidity. It reduces to 40% when administered in fasting condition. The Itraconazole therapy had some adverse effect. In short term Itraconazole therapy, produces nausea, headache, and abdominal pain and in long treatment these side effects are increases to chronic [25-27].
The topical nanogel was prepared to avoid the side effects of the Itraconazole after oral administration like hepatic and gastrointestinal side effect. This formulation can rectify the limitations of creams that include separation of emulsion and stability problems. Drug loaded nanoparticles were characterized through particle size, zeta potential, polydispersity index (PDI), in vitro drug release, IR spectroscopy, X-ray powder diffraction (XRPD) and surface topography. The prepared nanoparticles was dispersed into gel and it was characterized through pH, homogeneity, spread ability, viscosity, drug content, in vitro drug release and in vitro antifungal activity The novelty of work is that, neither nanoparticles and nor nanogel of itraconazole is reported, therefore first time the prepared nanogel is desired to produce better therapeutic effect in treatment of fungal infection.
Experimental
Materials
Itraconazole was obtained as gift sample from IPCA Laboratories Limited (Bombay, India). Pluronic F127 and Carbopol 940 were purchased from Sigma Aldrich Chemicals Pvt. Ltd. (Banglore, India) and Molychem (Bombay, India) respectively. Span 80, sodium lauryl sulfate, polyvinyl alcohol and triethanolamine were purchased from Loba Chemical Pvt. Ltd. (Bombay, India). Tween80, dichloromethane and methanol were purchased from Rankem Pvt. Ltd. (Bombay, India). Mannitol and Saboraud dextrose agar was purchased from Merck Pvt. Ltd. (Bombay, India).
Methods
Solubility study
An unknown quantity of itraconazole was added and dissolved to in each medium such as water, methanol, 0.1 N HCl (pH 1.2), phosphate buffer solution (PBS) (pH 6.8) and PBS (pH 7.4) to obtain saturated solutions. The solutions were kept at ambient temperature for 24 h in an Orbital shaking incubator (Remi instruments Ltd., Bombay, India) at 37 ± 2°C. The equilibrated samples were filtered and filtrates were collected and diluted properly with respective medium. For quantification of itraconazole in filtrate and it was scanned in range of 200 to 400 nm (λmax 263 nm) using UV spectrophotometer (Shimadzu 1700, Japan).
Preparation of Itraconazole nanoparticles
Itraconazole nanoemulsion was prepared by using solvent diffusion method. Itraconazole (150 mg) was dissolved in dichloromethane (9 mL). This drug solution was poured into 0.2% w/w stabilizer solution (66 mL) and subjected for stirring at high speed homogenizer using Ultra Turrax T25 (IKA, Ultra turrax T25, USA) at 8000 rpm for 5 min. The emulsion was obtained and it was homogenized for 5 cycles at pressure 200 bar using high pressure homogenizer (Panda 2K, Niro Soavi Ltd, Italy). Purified water (75 mL) was added into previously prepared emulsion and again homogenized with 200 bar pressure and 5 cycles for diffusion of solvent and convert into solid particles. Mannitol (cryoprotectant) (3% w/v) was added to prepared nanosuspension and it was then kept in deep freezer at -70 ºC for 24 h before lyophilization. The freeze drying was done using lab freeze dryer (Benchtop freeze dryer, Virtis ltd, USA) at -70 °C for 48 h.
Preparation of topical nanogel formulation
The topical nanogel was prepared by using Carbopol 940 as gelling agent. The required amount itraconazole nanoparticles were added to water to get 1% drug in gel. Carbopol 940 was dispersed to previously prepared itraconazole nanoparticles and stirred using magnetic stirrer at 500 rpm. Stirring was continued till complete dispersion of the Carbopol 940 to obtain homogeneous gel. Triethanolamine (0.05%) was added to adjust the pH of gel.
Physicochemical properties of itraconazole nanoparticles
Determination of mean particle size, polydispersity index (PDI) and zeta potential: The characterization of all batches of nanocrystals for mean particle size, polydispersity index (PDI) and zeta potential was done by photon correlation spectroscopy using Zetasizer (Nano ZS 90, Malvern Instruments, Worcestershire, UK). Before the particle size measurements, each sample was diluted with purified water IP to produce suitable scattering intensity. The z-average and PDI were obtained at an angle of 90° using disposable polystyrene cells having 10 mm diameter cells at 25°C.
Drug content
Drug content was determined for all the batches of nanoparticles. Nanoparticles (50mg) were dissolved in 50 ml methanol and sonicated about 1 h. The solution was filtered using Whatman filter paper and resultant filtrate was diluted with methanol. The concentration of drug was determined by scanning the filtrate at UV spectrophotometer (Shimadzu 1700, Japan) at wavelength 263 nm and drug content was calculated.
In vitro drug release
In vitro drug release of itraconazole from nanoparticles of all batches was studied by using Franz diffusion cell apparatus Electrolab (Bombay, India). Dialysis membrane was soaked 24 h in PBS (pH 7.4) before it is used for diffusion study. Weighed quantity (50mg) of nanoparticles was placed in donor compartment and receptor compartment was filled with PBS (pH 7.4). Dialysis membrane was placed in between donor and receptor compartment as a diffusion membrane and tight with clamp. The temperature of receptor compartment media were maintain at 32 °C (± 0.5 °C) under 100 rpm by using Teflon coated stirring bar. 3mL aliquot was collected at predetermined time interval 1, 2, 3, 4, 5 and 6 h and sink condition was maintained. The collected aliquots were scanned at UV-visible spectrophotometer at wavelength 263nm and % cumulative drug release was calculated.
Infra-red spectroscopy
The formulation NF2 was subjected for preparation of the pellet before scanning at FT-IR spectrophotometer by using pellet press method. Nanoparticles of NF2 batch was mixed with potassium bromide (KBr) of IR grade in the ratio of 1:100 and compressed using motorized pellet press at 10 tones pressure. The pellet was then scanned using FT-IR spectrophotometer, in IR range of 4000-400 cm-1 and the IR spectrum was obtained.
Scanning electron microscopy (SEM)
The morphology of optimized (NF 2) and non-optimized (NF 3) batch was studied by using scanning electron microscopy (JSM 6390, Japan). Samples were prepared by placing the droplet of nanoparticulate dispersion of samples on to aluminium metal plate and dried under vacuum to form a dry film, which was then observed under the scanning electron microscope and then images were taken using 20kV electron beam at magnification X30, X500, X1000, X3000.
X-ray powder diffraction study
X-ray powder diffraction analysis of pure drug itraconazole and optimized batch (NF 2) was performed using x-ray powder diffractometer (Brucker AXS, D8 Advance, Germany). The samples were mounted on a sample holder. The samples were irradiated with monochromatized Cu Kα radiation and XRPD patterns were scanned in the range of 3–80° (2θ) at a chart speed of 5° per min. The voltage and current used were 30 kV and 30 mA, respectively.
Physicochemical properties of topical nanogel
Determination of pH: pH of all batches of topical nanogel were measured by using digital pH meter Electrolab (Bombay, India). Before actual measurement 1gm topical nanogel was transferred into 50ml beaker containing 20 mL purified water. The electrode of pH meter was dipped into topical nanogel and pH of topical nanogel was noted.
Homogeneity
Homogeneity study of topical nanogel was examined by using visual inspection of topical nanogel. All developed topical nanogels were filled into flint color vial containers and subjected for visual inspection to check the prepared topical nanogel was homogenous and presence of aggregates or not.
Spread ability
The spread ability of topical nanogel was measured by using two slides (5 cm2). The 0.5g topical nanogel of each batch were placed between two slides and left for 1 min. Diameter of spread circle of topical nanogel measured and compared with each other.
Viscosity
The viscosity of all formulated batches of topical nanogel was measured using Brookfield Rheometer (Brookfield, USA). Topical nanogel was filled into sample holder up to mark on sample holder. The spindle CCT-14 was attached to the Brookfield Rheometer. The spindle was dip into topical nanogel previously filled in sample holder. Then sample holder was attached to instrument and viscosity of topical nanogel was measured.
Drug content
Topical nanogel (0.5g) was weighed and dissolved into 50 mL of methanol. It was sonicated for 15 min to dissolve the itraconazole completely into the methanol. The solution was filtered through whatman filter paper and resultant filtrate was diluted with methanol. The aliquot subjected for scanning at wavelength 263 nm using UV spectrophotometer (Shimadzu 1700, Japan) and drug content was calculated.
In vitro drug release study
In vitro drug release of topical nanogel of all batches was studied by using Franz diffusion cell apparatus electrolab (Bombay, India). Dialysis membrane was soaked in PBS (pH 7.4) before its utilization for diffusion study. Weighed quantity (0.5g) of topical nanogel was placed in donor compartment and receptor compartment was filled with PBS pH 7.4. Dialysis membrane was placed in between donor and receptor compartment as a diffusion membrane and it kept tight using clamp. The temperature of receptor compartment media were maintain at 32 °C (± 0.5 °C) under 100 rpm by using Teflon coated stirring bar. 3ml aliquots was collected at every prefixed time interval 1, 2, 3, 4, 5 and 6 h and sink condition was maintain over period of complete diffusion study. The collected aliquots were scanned by using UV-visible spectrophotometer (Shimadzu 1700, Japan) at wavelength 263nm and % cumulative drug release of topical nanogel was calculated.
In vitro antifungal study
The antifungal activity was assessed by using cup plate method. The activity was observed against Candida Albican. Sabouraud dextrose agar (SDA) media was used for in vitro antifungal activity. Accurately, weighed 4.7 g of media was added to distilled water (100 mL) and heated to boiling point to dissolve the SDA media completely. Sabouraud dextrose agar was sterilized by autoclave at 121 °C for 15 min (15 psi). After autoclaving, it was cooled to 40- 50°C. Microbial suspension of test organism (Candida Albican) was prepared. The resultant microbial suspension was added to media and mixed well. The media was then transferred to Petri plate. The topical nanogel was applied to previously prepared cavities in solidified media under laminar air flow in aseptic area (Class 1000). The plate was then incubated for 18 h and the zone of inhibition was measured.
Stability study
Accelerated stability of optimized topical nanogel (NG2) was carried out according to ICH Q1A (R2) guidelines. The stability study was performed at 25 ±2°C and 60 ±5% RH in an environmental stability chamber over a period of three months to assess the stability of topical nanogel. The topical nanogel was transferred to amber-colored glass vials, which were plugged and kept in the stability chamber. The viscosity, drug content and in vitro drug release measured after three months.
Results
Solubility study
The solubility study of itraconazole was carried out in different medium like purified water, methanol, 0.1N HCl (pH 1.2), PBS (pH 6.8) and PBS (pH7.4) (Table 1). Based on solubility study results, it was found that the itraconazole was insoluble in water, 0.1N HCl (pH 1.2), PBS (pH 6.8) and PBS (pH7.4).
Physicochemical properties of itraconazole nanoparticles
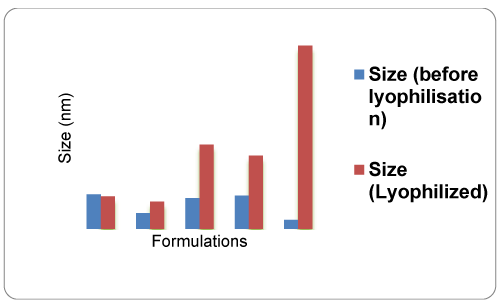
Particle size, PDI and Zeta Potential: The NF2 batch was formulated with pluronic F127 stabilizer. The nanosuspension NF2 batch gave the particles size 93.11 nm and 97.25 nm before and after freeze drying, respectively (Table 2). NF5 batch containing polyvinyl alcohol had desired particle size but after freeze drying, these nanoparticles was converted to micro particles 1.090 µm due to agglomeration of particles. Batch NF1, NF3 and NF4 had the desired particle size but there was significant increase in particle size after freeze drying (Figure 1). PDI of prepared nanoparticles revealed the homogeneity among the particles. PDI ranges from 0 to 1, closure the value to 0 higher the homogeneity and NF 2 formulation showed lowest PDI about 0.208 (Table 2). The zeta potential give the clear indication about the stability of formulation and all batches gave desired zeta potential. The lyophilized NF3 batch showed lowest zeta potential value about -11.6 (Figure 1; Table 2).
Drug content
Drug content is used to determine amount of drug present in formulations. The drug content of NF2 batch contains maximum drug as compared to the other batches. Drug content of NF2 batch was found to be 94.8 % (Table 2).
In vitro drug release study
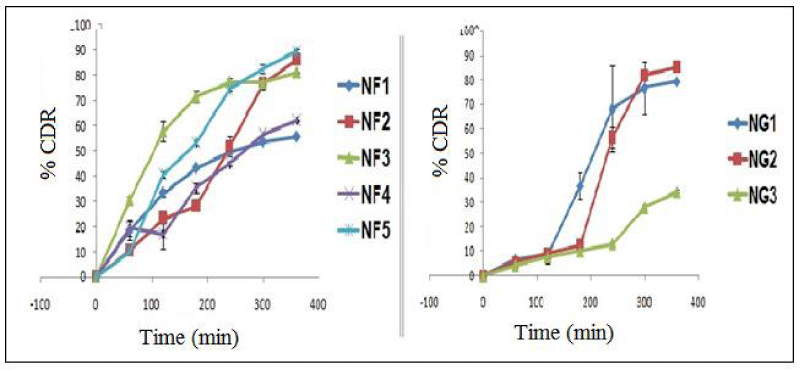
The % cumulative drug release was studied and % release of drug for all the batches of nanoparticles are shown in figure 2. The drug releases of all the batches were compared and significant difference was found. Cumulative drug release of formulation NF2 and NF5 was found to be 86.15% and 89.58%, respectively. Other batches of had no significant effect drug release profile. The drug release profile of NF2 batch was better than other batches.
Infra-red spectroscopy
The IR spectra of drug, physical mixture (drug and excipients) and optimized batch NH2 were obtained after scanning using IR spectrophotometer (Figure 3; Table 3). In this FTIR study, it is revealed that the drug was compatible with all excipients. The batch NF 2 has showed the intense peak at 2939 cm-1, which showed chemical bonding for C-H aliphatic (stretch). Other peaks observed at 1381cm-1, 1319cm-1, 1261cm-1 and 1197cm-1 for C-O and C-N chemical bonding.
X-ray powder diffraction study (XRPD)
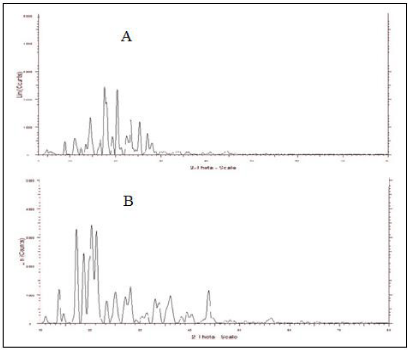
The X-ray powder diffraction study of pure drug and optimized batch (NF2) was performed using X- ray diffractometer. The 2ϴ values were recorded for both samples. In the diffractogram of drug (Figure 4), 2ϴ values presents are 4.76°, 8.79°, 10.95°, 12.32°, 13.45°, 14.42°, 16.54°, 17.84°, 17.50°, 19.25°, 20.32°, 21.15°, 22.40°, 23.40°, 25.30°, 27.03°, 27.98° and 28.76°. These recorded 2ϴ values of drug matches with reported values of drug; it means the drug is present in crystalline form. The 2ϴ values 4.76°, 8.79°, 12.32°, 14.42°, 16.54°, 17.84°, 17.50°, 19.25°, 22.40°, 23.40°, 25.30°, 27.98° and 28.76° of ITZ were not found in the diffract graph of optimized batch NF2. This showed that, itraconazole might be converted from one crystalline to other crystalline form.
Scanning electron microscopy (SEM)
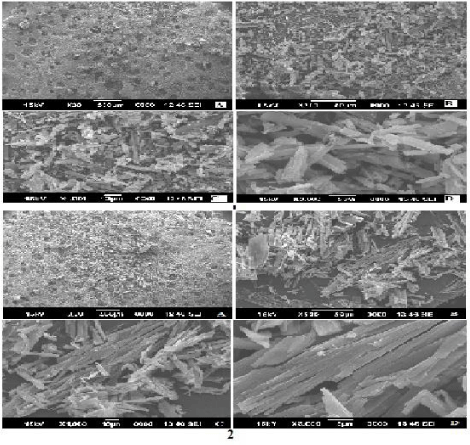
The surface topography of optimized (NF2) and non optimized (NF3) nanoparticles was studied. The X30, X500, X1000 and X3000 magnification images were taken for both NF2 and NF3 samples (Figure 5). The morphology of prepared nanoparticles of optimized batch and non optimized batch were found non-spherical and irregular shape. The few particles were cylindrical in shape with smooth surface.
Physicochemical properties (evaluation) of topical nanogel
Determination of pH: The change in pH of topical nanogel was observed when concentration of Carbopol 940 was changed. It was changed from pH 6.5 to pH 6.8 when concentration of gelling agent changed 1% and 2% of topical nanogel formulation NG1 and NG2, respectively. When concentration of Carbopol 940 further increased to 3% in topical nanogel formulation NG3 there was no change in pH was observed (Table 4). The pH of topical nanogel in between 5.5 to 7.0 avoids tendency of irritation due to pH when applied to skin.
Homogeneity
Homogeneity of topical nanogel formulations NG1, NG2 and NG3 were observed after filling of topical nanogel into flint colored glass containers. The homogeneity was observed of respective batches of topical nanogel to check, wither presence of aggregates or not. All topical nanogel formulations were homogeneous. The concentration of gelling agent does not affect homogeneity of topical nanogel.
Spread ability
Spread ability was used to check the spreading capacity of the topical nanogel formulations [28,29]. The spread ability of topical nanogel formations NG1, NG2 and NG3 was found to be 3cm, 2.2cm and 2cm, respectively (Table 4). It was observed that when concentration of gelling agent that is Carbopol 940 increased 1%, 2% and 3% in NF1, NG2 and NG3 formulations, respectively; the spreading capacity of topical nanogel was decreased. NG2 topical nanogel batch was selected as optimized batch, because it has good consistency and spread ability.
Viscosity
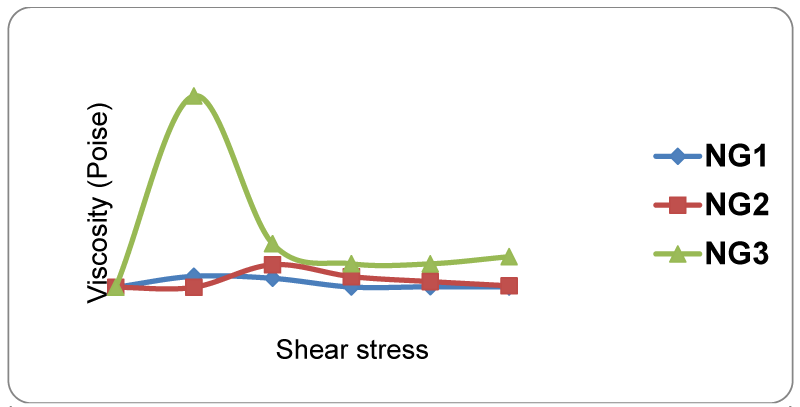
The viscosity of topical nanogel formulations NG1, NG2 and NG3 was found to 107.4 cp, 160.2 cp and 1434.5 cp, respectively (Table 4). It reveals that, as concentration of the gelling agent increases, the viscosity also increases (Figure 6). From all these batches, NG2 batch was selected as optimized batch because of its desired viscosity with consistency.
Drug content
Drug content was calculated for all the prepared batc hes of topical nanogel. NG2 topical nanogel formulation showed maximum drug content (77%) as compared to NG1 and NG3 formulations (Table 4), therefore, NG2 batch was selected as optimized formulation.
In vitro drug release study
Drug release of topical nanogel formulation was studied by using Franz diffusion cell apparatus. The collected aliquots were scanned using UV-Visible spectrophotometer and % cumulative drug release was calculated. % cumulative drug release of NG1, NG2 and NG3 was found to be 79.33 ± 0.20, 85.24 ± 0.91 and 34.37 ± 0.53, respectively at 6 h. NG2 batch has showed maximum drug release as compared to NG1 and NG3 formulations.
In vitro antifungal study
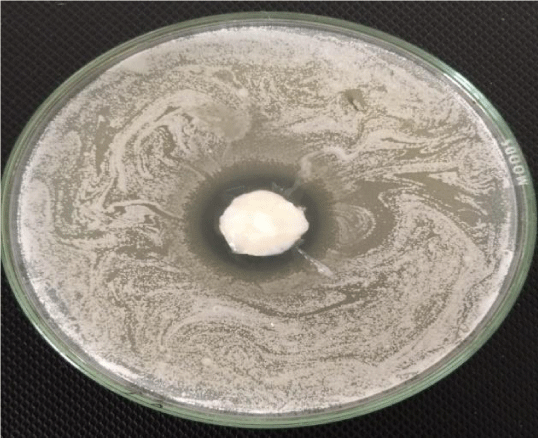
The in vitro antifungal activity of topical nanogel (NG2) was studied using cup plate method. The Sabouraud dextrose agar media was used for this antifungal study. After 18 h of incubation the zone of inhibition was observed. The zone of inhibition was found to 4 mm. It indicated that the topical nanogel containing itraconazole was penetrated into fungal cell and the inhibition of ergosterol synthesis in cell wall of fungal cell was occurred and cell death was possible (Figure 7).
Stability atudy
Stability study of optimized batch of topical nanogel (NG2) was carried out in stability chamber at 25 ±0.5°C at 60 ±5% RH over period of three months. The parameters evaluated were viscosity, drug content and in vitro drug release. The viscosity, drug content and cumulative drug release of NG2 was 671.9 cps, 69.5 % and 83.5 %, respectively. Based on these results, the prepared topical nanogel formulation was stable in short term stability study as per ICH guidelines.
Discussion
Itraconazole is had low solubility in water and it may lead to low bioavailability about 55%. This drug produces hepatic and gastrointestinal side effect after oral administration. In long term treatment of fungal infection, the adverse effect of itraconazole may increases to chronic. To overcome these problems, the itraconazole was formulated into topical nanogel. The drug nanocrystals were prepared by using solvent diffusion method using suitable stabilizers. These prepared nanocrystals of itraconazole were then incorporated to the gel. This topical nanogel was hypothesized to treat fungal infection.
In brief, Nanoparticle were prepared by solvent diffusion method. The drug was dissolved in dichloromethane and added to surfactant solution, then produces emulsion. Emulsion was then further diluted with water and passed through high pressure homogenizer to obtain nanoemulsion. The prepared nanoemulsion was stabilized by using different stabilizers. Different batches of nanoparticles was formulated NF1, NF2, NF3, NF4, NF5 by using Tween 80, Pluronic F127, sodium lauryl sulfate, span 80, polyvinyl alcohol (PVA) as a stabilizers. From these batches, NF2 gave stable emulsion. After pouring of water into emulsion and further homogenization to high pressure homogenizer, it causes diffusion of drug from solvent. This leads to formation of nanospheres. To recover these nanoparticless freeze drying was necessary. Cryotectant was added 3% w/v to avoid any drug degradation due to lyophilization. All batches of prepared nanoparticles were characterized through particle size, PDI, zeta potential, drug content, in vitro drug release study, FTIR and XRPD. The NF2 batch showed smallest particle size 167.2 nm as compared to other batches. PDI of NF2 batch was lowest about 0.208 which is closer to zero. Zeta potential gives the indication of stability. Zeta potential of NF2 (-17.8mV) indicates that the prepared nanoparticles were stable, free flowing with no aggregation. The NF2 batch had higher drug content and desired drug release. IR spectra revealed the nature and its presence had no interaction between excipients. The X-ray diffractogram showed that crystalline form was converted to other crystalline form. The SEM showed that, the shape of nanoparticles of NF2 batch was non spherical. Therefore, by considering desired results of the parameter like particle size, PDI, ZP, drug content, % cumulative drug release, IR spectra, X-ray powder diffractometry and SEM, NF2 formulation was optimized batch for further preparation of the topical nanogel.
Topical nanogel was prepared using Carbopol 940 as gelling agent. The drug nanocrystal were (1.12 g) was added to get 1% ITZ in topical nanogel (for 5g topical nanogel batch). The batches were developed by changing concentration of gelling agent. The concentration of carbopol 940 as 1%, 2%, 3% was used in formulation batches NG1, NG2, NG3 respectively. The obtained topical nanogel was then evaluated.
Topical nanogel was evaluated as for pH, homogeneity, spreadability, viscosity, drug content, % cumulative drug release study. The pH of all topical nanogel was good, because all prepared topical nanogel batches has pH in range of 5.5 to 7.0. This pH of nanogel was suitable for skin and do not casues irritation. The topical nanogel was also subjected to know the homogeneity. All topical nanogel batches were found to homogeneous. The spreading capacity of topical nanogels were studied, the NG1 topical nanogel showed desired spreading. Viscosity of NG1 and NG2 was optimum. The drug content of NF2 batch was in the official limit and showsed maximum release from topical nanogel. Base on the results of evaluation of topical nanogel, NG2 batch of topical nanogel was selected as optimized batch and it is further evaluated for in vitro antifungal activity.
Conclusion
Itraconazole nanoparticles were prepared by solvent diffusion method and incorporated into the topical gel successfully. The nanosuspension NF2 batch gave the particles size 93.11 nm and 97.25 nm before and after freeze drying, respectively. Based on particle size, zeta potential, PDI, drug content and in vitro drug release, nanoparticles formulation NF 2 was optimized batch. Nanoparticles of optimized batch were further used to prepare topical nanogel. The NG2 topical nanogel had desired physicochemical properties required for topical gel. The percent cumulative drug release of NG1, NG2 and NG3 was found to be 79.33 ± 0.20, 85.24 ± 0.91 and 34.37 ± 0.53, respectively at 6 h. In conclusion, prepared nanogel of itraconazole was stable and could be used with promising potential for fungal infection of skin.
- Khullar R, Kumar D, Seth N, Saini S (2012) Formulation and evaluation of mefenamic acid emulgel for topical delivery, Saudi Pharm J. 20: 63-67. https://goo.gl/r1Jkzp
- Müller R, Radtke M, Wissing (2002) Solid lipid NPs (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations, Adv Drug Delv Rev 54S: 131-S155. Link: https://goo.gl/QRMN7k
- Bhalekar M, Pokharkar V, Madgulkar A, Patil N (2009) Preparation and evaluation of miconazole nitrate-loaded solid lipid NPs for topical delivery, AAPS Pharm Sci Tech 10: 289-296. Link: https://goo.gl/WshNWS
- Murthy S, Shivakumar H (2010) Topical and transdermal drug delivery, Handbook of Non-Invasive. 1-36. Link: https://goo.gl/UTakBw
- Verma P, Pathak K (2012) Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation, Nanomed: Nanotechnol Biol Med 8: 489-496. Link: https://goo.gl/wH6b3t
- Barakat N (2010) Evaluation of glycofurol-based gel as a new vehicle for topical application of naproxen, AAPS Pharm Sci Tech 11: 1138-1146. Link: https://goo.gl/dxzqAt
- Barot B, Parejiya P, Patel H, Mehta D, Shelat P (2012) Microemulsion-based antifungal gel delivery to nail for the treatment of onychomycosis: formulation, optimization and efficacy studies, Drug Delv Transl Res 2: 463-476. Link: https://goo.gl/jAKnhN
- Rao J, Geckeler K (2011) Polymer NPs: preparation techniques and size-control parameters, Prog. Polym. Sci 36: 887-913. Link: https://goo.gl/HZYMPv
- Reis C, Neufeld R, Ribeiro A, Veiga F (2006) Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles, Nanomed: Nanotechnol Biol Med 2: 8-21. Link: https://goo.gl/AzCjgn
- Hu F, Jiang S, Du Y, Yuan H, Ye Y, et al. (2005) Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system, Colloids Surfaces B 45: 167-173. Link: https://goo.gl/3pgpag
- Hu F, Yuan H, Zhang H, Fang M (2002) Preparation of solid lipid nanoparticles with clobetasol propionate by a novel solvent diffusion method in aqueous system and physicochemical characterization, Int J Pharm 239: 121-128. Link: https://goo.gl/uddGNe
- Murakami H, Kobayashi M, Takeuchi H, Kawashima Y (1999) Preparation of poly (DL-lactide-co-glycolide) NPs by modified spontaneous emulsification solvent diffusion method, Int J Pharm 187: 143-152. Link: https://goo.gl/mBV43k
- Niwa Takeuchi H, Hino T, Kunou N, Kawashima Y (1993) Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D, L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method and the drug release behavior, J Controlled Release 25: 89-98. Link: https://goo.gl/6Df2Jy
- Trotta M, Debernardi F, Caputo O (2003) Preparation of solid lipid NPs by a solvent emulsification–diffusion technique, Int J Pharm 257: 153-160. Link: https://goo.gl/t9jg5S
- Trotta M, Gallarate M, Pattarino F, Morel S (2001) Emulsions containing partially water-miscible solvents for the preparation of drug nanosuspensions, J Controlled Release 76: 119-128. Link: https://goo.gl/NB3qaX
- Rose S, Miller R (1940) Studies with the Agar Cup-plate Method, American J Med Sci 199: 338-342. Link: https://goo.gl/Qn2z7f
- Dolenc A, Kristl J, Baumgartner S, Planinsek O (2009) Advantages of celecoxib nanosuspension formulation and transformation into tablets, Int J Pharm 376: 204-212. Link: https://goo.gl/MZvm77
- Elnaggar Y, Talaat S, Bahey-El-Din M, Abdallah O (2016) Novel lecithin-integrated liquid crystalline nanogels for enhanced cutaneous targeting of terconazole: development, in vitro and in vivo studies, Int J Nanomed 11: 5531-5547. Link: https://goo.gl/Vikziz
- Ige PP, Solanki ND (2015) Preparation and in vitro–in vivo evaluation of surface-modified poly (lactide-co-glycolide) NPs as controlled release carriers for flutamide delivery, J Microencapl 32: 231-239. Link: https://goo.gl/zwYDgK
- Jana S, Manna S, Nayak A, Sen K, Basu S (2014) Carbopol gel containing chitosan-egg albumin NPs for transdermal aceclofenac delivery, Colloids Surfaces B 114: 36-44. Link: https://goo.gl/etRn4o
- Joshi M, Patravale V (2008) Nanostructured lipid carrier based gel of Celecoxib, Int J Pharm 346: 124-132. Link: https://goo.gl/eJR7da
- Laxmi R, Karthikeyan R, Babu P, Babu R (2013) Formulation and evaluation of antipsoriatic gel using natural excipients, J Acute Dis 2: 115-121. Link: https://goo.gl/pD3S94
- Pople P, Singh K (2006) Development and evaluation of topical formulation containing solid lipid NPs of vitamin A, AAPS Pharmscitech 7: E63-E69. Link: https://goo.gl/bzmTjh
- Takeuchi H, Yamamoto H, Kawashima Y (2001) Mucoadhesive nanoparticulate systems for peptide drug delivery, Adv. Drug Dev Rev 47: 39-54. Link: https://goo.gl/HZ8HD7
- Bachhav Y, Patravale V (2009) Microemulsion based vaginal gel of fluconazole: formulation, in vitro and in vivo evaluation, Int J Pharm 365: 175-179. Link: https://goo.gl/dLmvqA
- De Beule K (1996) Itraconazole: pharmacology, clinical experience and future development, Int J Antimicrob Agents 6: 175-181. Link: https://goo.gl/hEJM7R
- Kocbek P, Baumgartner S, Kristl J (2006) Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs, Int J Pharm 312: 179-186. Link: https://goo.gl/UcAUnM
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
