International Journal of Pharmaceutical Sciences and Developmental Research
Effects of Duration of Treatment, HIV and HCV Co-Infection on Hematological and Hepatic Functions in Libyan Patients with Pulmonary Tuberculosis
Fadya A Menesi1, Mabroka A El-Majdob2, Saleh E Mghil3, Isam Denna4, Ghazala Othman2, Mustafa YG Younis5, Faraj El -Shari5, Abdulkader H El-Debani3, Fathi M Sherif6* and Awad G Abdellatif2
2Department of Pharmacology, Faculty of Public Health, University of Benghazi, Libya
3Department of Internal Medicine, Faculty of Public Health, University of Benghazi, Libya
4Department of Nutrition, Faculty of Public Health, University of Benghazi, Libya
5Department of Biochemistry, Faculty of Medicine, University of Benghazi, Libya
6Department of Pharmacology and Clinical Pharmacy, University of Tripoli, Tripoli, Libya
Cite this as
Menesi FA, El-Majdob MA, Mghil SE, Denna I, Othman G, et al. (2017) Effects of Duration of Treatment, HIV and HCV Co-Infection on Hematological and Hepatic Functions in Libyan Patients with Pulmonary Tuberculosis. Int J Pharm Sci Dev Res 3(1): 017-023. DOI: 10.17352/ijpsdr.000011Background: Pulmonary tuberculosis (TB) remains a major global health problem despite the availability of efficient treatment over the last decades. TB is the most common opportunistic infection among HIV patients and complicated the outcome of treatment globally.
Aims: The prevalence of HCV infection among TB patients has not fully been investigated and limited data on rates of HCV co-infection in TB patients exist. Therefore, this study was aimed to investigate the effects of duration of treatment with first line anti-TB drug, HIV, HCV and co-infection on haematological and hepatic functions in Libyan patients with pulmonary TB.
Methods: A total of 120 Libyan newly diagnosed pulmonary TB patients (74 males and 46 females) with age range of 26 to 41 years old were enrolled in this study. They were selected for a regular follow up on the basis of inclusion and exclusion criteria. Pulmonary TB was confirmed by chest X-ray and sputum smear in all the patients. Patients were divided into three groups; the first group of 75 patients with no HIV and HCV infections (positive control), the second group of 35 patients who had TB and HIV co-infection, before starting anti-microbial therapy, while the third group consists of 20 patients who had TB and HCV co-infection before starting anti-HCV treatment. All patients received a confirmation of the 1st line anti-TB drug (isoniazid, rifampin, ethambutol and pyrazinamide for five consecutive weeks) simultaneously.
Results: The results showed a significant decrease in white blood cells (WBCs) in all the groups of patients and significant changes in other haematological parameters, there were also significant increases in the hepatic enzyme activities, alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP), in all the groups which indicate hepatic toxicity.
Conclusion: Treatment with first line anti-TB drug simultaneously produced hepatotoxicity after two weeks which is more in HIV and HCV co-infection patients.
Abbreviations
WHO: World Health Organization; TB: Tuberculosis; HIV: Human Immune Deficiency Virus: HCV: Hepatitis C Virus; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; ALP: Alkaline Phosphatase; Hb: Haemoglobin; WBC: White Blood Cells; Plts: Blood Platelets; ESR: Erythrocyte Sedimentation Rate.
Introduction
Pulmonary tuberculosis (TB) is a serious health problem in Libya [1]. World Health Organization (WHO) estimated TB incidence in Libya for the period of 1990 to 2010 as 40 per 100000 [2]. This incidence rate is considered too high according to the recommendation of the National Institute for Health and Clinical Excellence (NICE) for vaccination and screening in England and Wales [3]. In the late 60s and early 70s, a large number of people from neighbouring countries come to Libya to seek for employment. This increased the TB cases in Libya as large number of TB patients entered the country. In 1973, based on the advice of the Central TB Committee, two legislative actions were implemented by the government. First was to screen all foreigners and local workers for TB before seeking employment or in the course of employment for those already employed. The second concerned anti-TB drugs where TB hospitals and centres became the only dispensing organizations for anti-TB drugs. A study from 1971 to 1976 revealed that a significant decrease in the prevalence of primary and acquired drug resistance [4]. The authors indicated that the decline in new and retreatment cases was mainly due to introduction of the two legislations in 1973.
Anti-TB drugs have been altered throughout several years according to their efficacy, safety and emergence of resistant strains of mycobacterium TB. For this reason, the 1st anti-TB drug “streptomycin” is no longer considered because of the toxicity and elevated incidence of drug resistance [5]. Therefore, according to WHO, the current 1st anti-TB drug regiment consists of isoniazid, rifampin, ethambutol and pyrazinamide. These drugs act by inhibiting cell membrane synthesis. Agreeing to WHO [6], the risk factors for adverse reactions for anti-TB drugs include, age (> 60 years), hepatic and/or renal diseases, human immune deficiency virus (HIV) or hepatitis C virus (HCV) co-infection as well as sodium or albumin deficiency. Adverse drug reactions of anti-TB drugs can be classified as gastrointestinal tract (GIT), neurologic, hepatic, immune mediated and some other adverse drug reactions. Isoniazid, pyrazinamide and rifampin have hepatotoxic potential and can lead to such reactions during anti-TB chemotherapy. Most of the hepatotoxic reactions of anti-TB drugs are dose related and usually occur in the initial few weeks of intensive therapy [7].
Till now, the exact mechanism of anti-TB drug hepatotoxicity is not completely understood but the toxic metabolites are suggested to play a crucial role, at least in case of isoniazid [8]. The degree of severity of hepatotoxicity induced by anti-TB drugs is identified according to the WHO toxicity classification standards [9,10] as grade-I: mild hepatotoxicity is defined as alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) elevation of < 3X the upper limit of normal activities (< 120 IU/L) and resolved spontaneously despite continued anti-TB therapy. Grade II: moderate hepatotoxicity occurs as both enzymes elevated 3-5X the upper limit of normal activities (121-200 IU/L). Grade III: very severe hepatotoxicity exists where enzymes elevation > 10X the upper limit of normal activities (201-400 IU/L) and grade IV: very severe hepatotoxicity > 10X the upper limit of normal of ALT and/or AST activities (> 400 IU/L) or more than 250 IU/L, if accompanied by symptoms such as nausea, vomiting, abdominal pain, and jaundice. Previous studies also revealed that hepatotoxicity induced by anti-TB drugs is increased four- to five-fold in patients with HIV and HCV co-infection, respectively [11,12].
Tuberculosis is considered to be the most common opportunistic infection among HIV patients and complicated the outcome of treatment [13]. In adults, it is estimated that about 15% of all new TB patients are attributed to the HIV infection [14]. HIV infection significantly increases risk of progression from latent to active TB [15,16]. The first priority for HIV-positive TB patients is to initiate TB treatment followed by anti-retroviral drugs and cotrimoxazole [9]. It is well known that HIV infection causes a slow decline in cluster of differentiation 4 (CD4+) cells in most patients and consequently, the prevalence leukopenia is increased with declined CD4+ cell counts [17]. For long period of time, CD4+ cell counts were considered to be the best predictor of the disease stage and risk of developing AIDs related complication. HCV has also emerged as an important global health problem. WHO estimated that 3% of the world population is infected with HCV and more than 170 million chronic carriers are at risk of developing liver cirrhosis and/or liver cancer. Globally, the prevalence of HCV infection among patients with TB has extensively been investigated, however, with a very limited data on rates of HCV co-infection among patients with TB exists [9,18]. To the best of our knowledge, no previous studies have been reported regarding the effects of duration of treatment and co-infections with HIV and HCV on patients with pulmonary TB. In Libya, the prevalence of HCV infection among TB patients has also not been investigated at all, and inadequate data on rates of HCV co-infection in TB patients exist. Thus, the aim of the present study was to explore the effects of duration of treatment with 1st line anti-TB drug, HIV and HCV co-infection on haematological and hepatic functions in Libyan patients suffering from pulmonary TB.
Materials and Methods
Protocol of the study
This study was planned as unicenter study and conducted at Alquefia Chest Hospital, Benghazi, Libya (2015). The protocol was approved by the Ethical Research Committee of University of Benghazi (2014). All the participants of the study gave informed consent to the doctor for using their data determined during the course of treatment at the hospital for this purpose. The protocol was designed as retrospective study.
Retrospective study
The study included pulmonary TB patients with negative HIV and HCV infection (positive control), pulmonary TB patients with positive HIV and pulmonary TB patients with positive HCV who were subjected to 1st line anti TB drug when admitted to the hospital and before start of the study. For all the patients, the efficacy and safety of anti-TB drugs were evaluated.
Patients
A total of 120 Libyan newly diagnosed pulmonary TB patients were enrolled in this study. They were selected for a regular follow up on the basis of inclusion and exclusion criteria. The number of male and female patients were 74 and 46, respectively. Patients who participated in this study were aged between 26 to 41 years old and had sputum smear positive AFB. All the patients were interviewed for their medication history, concomitant diseases before their participation in the study. Patient’s name, gender, age, file number, date of admission and discharge from the hospital were recorded.
Exclusion criteria
Non Libyan pulmonary TB patients, extra pulmonary TB patients, pulmonary TB patients returning after defaulting or relapsing from their first treatment course (previously treated by anti-TB drugs), MDR-TB, alcoholic, drug abusers, smokers, liver diseases except in the third group, HIV infection except in the second group, renal and cardiovascular diseases, patients receiving other potentially hepatotoxic drugs (methotrexate, phenytoin, valproate, acetaminophen, fluconazole), diabetes mellitus, thyroid and connective tissue diseases.
The patients were divided into three groups according to the presence or absence of co-infection (Table 1). First: pulmonary TB patients with negative HIV and HCV infection (positive control group) received 1st line anti-TB drug for five weeks. Second: pulmonary TB patients with positive HIV (HIV group) received 1st line anti-TB drug for five weeks (before administration of anti-retroviral drugs). Third: pulmonary TB patients with positive HCV (HCV group) received 1st line anti-TB drug for five weeks (newly diagnosed HCV infection did not received anti-hepatitis drugs). Four drugs isoniazid, rifampicin, ethambutol and pyrazinamide were used as 1st line anti-TB drug. All drugs were used simultaneously by all the patients.
Data collection
All data was collected from the files of the patients: patients name, age, gender, body weight (kg), nationality, date of admission and discharge, date of start anti-TB drug therapy, medical history and drug history. Patients were subjected to following investigations:
Baseline values: Chest x-ray PA view, sputum direct smear and culture for AFB, complete blood count (white blood cells and haemoglobin, Hb) and blood platelets (PLTs), erythrocyte sedimentation rate (ESR), liver function test (total bilirubin, ALT, AST and Alkaline phosphatase (ALP). All investigations were carried out at Biochemistry and Microbiology Laboratory of Alquefia Chest Hospital, AFB smear microscopy was performed using the Ziehl-Neelson staining method, culture was performed using solid, Lowenstein-Jensen, media and incubated for 45 days, Fasting blood glucose (FBG) checked by DIAGON Ltd D-cell 60 auto hematology analyzer, ESR checked manually. LFT, RFT, uric acid and serum electrolytes were determined by VITROUS chemistry system 350. Prior to the start of treatment, blood samples from all the patients were tested for HBs Ag, anti-HCV and HIV by ELISA serologic test. All the patients were subjected to regular follow up after two and five weeks. The same investigations were repeated for all the patients and results were recorded again.
Efficacy and safety evaluations
Efficacy outcome: patients were observed for improvement of the primary efficacy variables (WBC and ESR) reduction and rise of Hb at two and five weeks of treatment relative to the baseline. Sputum was also examined for follow up.
Safety outcome: Safety was assessed on the basis of adverse events reported during the study and it was measured by the effect of drugs on hepatic and renal functions.
First line anti-TB drug used for all the patients were:
Isoniazid tablet 300 mg O. D. (5 mg/kg/day),
Rifampicin tablet 600 mg O. D. (10 mg/kg/day),
Ethambutol tablet 1200 mg O. D. (15 - 20 mg/kg/day) and
Pyrazinamide tablet 1500 mg O. D. (25 mg/kg/day).
Other drugs used
Pyridoxine (vitamin B-6) as prophylaxis treatment of peripheral neuropathy for the side effect of isoniazid. Prophylaxis treatment of opportunistic infection for HIV infection patients, co-trimoxazole (sulfamethoxazole 400 mg with trimethoprim 80 mg) was used. Treatment of hyperuricemia secondary to pyrazinamide: allopurinol was used. Treatment of GIT reactions by omeprazole and metoclopromide.
All data were expressed as mean ± S.E.M. Data were analysed by analysis of variance test (one-way ANOVA). If this analysis indicated a significant difference among the means then multiple comparisons between the individual groups were tested by post-hoc test (LSD) or unpaired t-test. A value of *p < 0.05 is considered significant, **p < 0.01 is highly significant and ***p < 0.001 is very highly significant.
Results
Effects of duration of anti-TB therapy, HIV and HCV infections on haemoglobin concentration in pulmonary TB patients
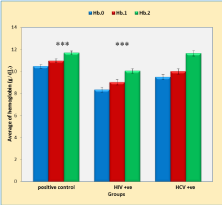
Figure 1 shows that five weeks duration of treatment causes a very highly significant increase (p < 0.001) in Hb levels (Hb.2) in positive control, TB with HIV and TB with HCV groups compared with the baseline Hb levels (Hb.0) in the same groups. On the other hand, HIV infection caused a significant decrease in the Hb levels (p < 0.001) compared with positive control group. However, HCV infection did not affect the levels of Hb at five weeks of anti-TB treatment (Hb.2, p = 0.98) compared with the positive control group.
Effects of duration of anti-TB therapy, HIV and HCV infection on white blood cells in pulmonary TB patients
Table 2 shows values of white blood cells (WBC) determined at the baseline, two and five weeks after the start of anti-TB treatment. The duration of treatment (after 2 weeks and 5 weeks) produced highly significant decrease in WBC counts (p < 0.001) compared with that of the baseline counts in all the groups.
However, HIV infection produced a highly significant decrease in WBC compared with positive control group (p < 0.001). In HCV infection patients although there was a change in WBC but this change was found to be insignificant (p = 0.846) in comparison with positive control group.
Effects of duration of anti TB treatment, HIV and HCV infection on blood platelets in pulmonary TB patients
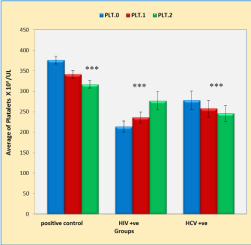
Figure 2 shows that duration of anti-TB treatment (5 weeks) produces very highly significant decrease in blood platelets (PLTs) counts in positive control and HCV groups compared to the baseline counts (p < 0.001 and p < 0.001, respectively). On the other hand, the HIV group showed no significant increase in the PLTs counts compared to the baseline counts. The overall number of PLTs was very highly decreased in both HIV and HCV infection patients as compared with the positive control group (p < 0.001 and p < 0.001, respectively).
Effects of duration of anti-TB treatment, HIV and HCV infection on erythrocyte sedimentation rate in pulmonary TB patients
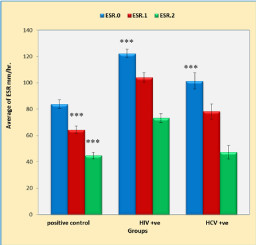
Figure 3 shows that duration of anti-TB treatment (2 and 5 weeks) significantly lowers ESR in positive control, HIV and HCV groups compared to that of baseline values (p < 0.001, p < 0.001 and p < 0.001, respectively). However, both HIV and HCV infection caused significant increase in ESR values at the start of the study compared with the positive control group (p < 0.0001, p < 0.019, respectively). On the other hand, 2 and 5 weeks of anti-TB treatment caused non-significant increase in HCV infection group compared with that of the positive control group (p = 0.859).
Effects of duration of anti-TB treatment, HIV and HCV infection on bilirubin levels in pulmonary TB patients
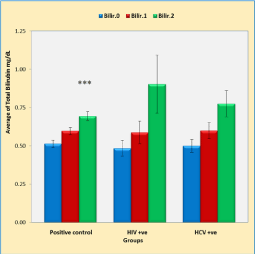
Figure 4 shows that duration of anti-TB treatment (5 weeks) leads to a significant increase in bilirubin levels (p < 0.001) in positive control TB patients with negative HIV or HCV infection compared to the baseline bilirubin. In addition, HIV and HCV caused a significant increase in bilirubin levels (p < 0.01, p < 0.01, respectively). This increase was not significant when compared with that of the positive control group (p = 0.191).
Effects of duration of anti-TB treatment, HIV and HCV infection on alanine aminotransferase activities in pulmonary TB patients
Table 3 shows duration of treatment (2 and 5 weeks) produced a significant increase in the activities of ALT in positive control, HIV and HCV co-infection groups compared with that of baseline ALT levels (p < 0.001 in every comparison) for each group. However, in HIV group, an increase in ALT was found to be very highly significant (p < 0.001) compared with the positive control group mainly after five weeks of the start of treatment. Also, in HCV infection group, there was a highly significant increase ALT compared with the positive control group (p < 0.01).
Effects of duration of anti TB treatment, HIV and HCV infection on aspartate aminotransferase activities in pulmonary TB patients
Table 4 shows duration of anti-TB treatment (2 and 5 weeks) caused highly significant increase in the activities of AST in the positive control, HIV and HCV (co-infected TB patients) groups compared with that of baseline (p < 0.001, p < 0.007 and p < 0.01, respectively) and after 5 weeks (p < 0.001, p < 0.001 and p < 0.001, respectively). On the other hand, both HIV and HCV (co-infected TB patients) groups showed a highly significant increase in AST levels compared with that of positive control group (p < 0.001 and p < 0.000, respectively), this increase was more obvious as anti-TB treatment duration increased to 5 weeks.
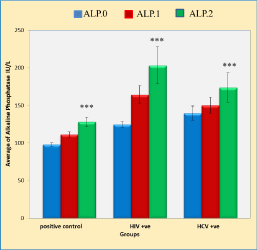
Effects of duration of anti TB treatment, HIV and HCV infection on alkaline phosphatase activities in pulmonary TB patients
Figure 5 shows five weeks period of anti-TB treatment significantly increased ALP activities in the positive control patients, HIV and HCV groups compared with that of the baseline ALP activities (p < 0.001, p < 0.001 and p < 0.001, respectively). However, in both HIV and HCV TB co-infected groups caused more significant increase in the activities of ALP compared with that of the positive control group (p < 0.001 and p < 0.01, respectively).
Discussion
Pulmonary TB remains a major global health problem for the last decades despite the availability of effective pharmacotherapy. WHO declared TB a global public health emergency in 1993 [19]. The disease affects mainly third world countries. According to WHO, eight thousand million people are infected by this disease. Pulmonary TB is responsible for 8.9 million new cases each year, and for two million deaths around the world [20]. Pulmonary TB is considered to be high in Libya despite the enforced legislations to control it, the legislations was first introduced in 1973. These legislations caused decline in primary and acquired resistance [4]. Drug resistance is a major problem in treatment of TB and drug combination is used to minimize this problem. TB is also considered to be the most common opportunistic infection among HIV patients, and complicated the outcome of treatment [13]. There is a lack of data concerning the effect of HIV and HCV co-infection in Libyan patients with TB. Therefore, this study was conducted to explore the effects of HIV and HCV co-infection on hematological and hepatic functions in Libyan patients suffering from pulmonary TB.
The present study showed that duration of anti-TB treatment produces very high significant increase in the levels of Hb in control patients, HIV and HCV groups after five weeks of treatment. This increase may be due to the eradication of the infection and due to the improvement of the general health of the patient. A study by Saathoff and others [21] among TB patients indicated that anemia is strongly associated with HIV co-infection. Other studies by Taha et al., and Kibret et al. [22,23], are in line with the present data and showed that patients having higher Hb levels were less likely to develop TB than those with low Hb levels.
The present results also indicated a significant decrease in the counts of WBC with duration of anti-TB therapy in all the groups. This decrease was most significant in HIV groups. The results indicated eradication of TB infection due to anti-TB therapy, however, HIV co-infected patients had a very low WBC counts compared with the other groups due to immune deficiency in HIV group. These findings are in line with the previous reported findings [24]. The present data also revealed that number of blood platelets was significantly declined in HIV and HCV co-infected groups in comparison with the control group. Rifampin induces thrompocytopenia which is not common but potentially life threatening complication [25]. It has been known that rifampin-induced thrombocytopenia is caused by the presence of anti-rifampin antibodies [26]. These antibodies fix a complement on the blood platelets in the presence of rifampin resulting in platelet destruction [27]. Other studies by Rieg et al., and Torre et al. [28,29], showed that thrombocytopenia is a common finding among HIV infected patients due to bone marrow suppression. On the other hand, chronic infection with HCV may produce a significant autoimmune reaction to blood platelets, leading to thrombocytopenia [30]. Other mechanisms of thrombocytopenia include a decrease of thrombopietin levels or reduced bone marrow production of platelets [31].
The present findings indicated a highly significant decrease in ESR in positive control and HCV groups after two weeks of start of anti-TB therapy. However, HIV and HCV co-infection caused significant increase in the levels of ESR compared with the control group. This increase in ESR may be due to inflammation resulted from infections. Another study elucidated a correlation between elevated ESR in HIV-infected patients and the clinical and immunologic condition of HIV infection and suggested the use of ESR in monitoring HIV/AIDS disease [32]. Other possible explanation for the elevated ESR in HCV infected patients is the host response to HCV infection [33]. In the present study, treatment with 1st line anti-TB drug caused a significant increase in the levels of total bilirubin in all the patients, and there was no difference in total bilirubin of HIV and HVC groups when compared with control group. This increase may be due to rifampin which may inhibit the major bile salt exporter [34]. Asymptomatic elevated bilirubin may also result from dose dependent competition with bilirubin for clearance at the sinusoidal membrane or from impeded secretion at the canalicular level [35].
In this study, the pattern of alteration of liver enzymes was evaluated. Thus, the normal maximum values in the laboratory are 45 IU/L for ALT and 40 IU/L for AST which were same cut-offs for male and female. The activities of ALT and AST after treatment with anti-TB drugs were significantly elevated above the upper normal limit but remained below the three times the upper normal limit (< 120 IU/L) indicating grade I hepatotoxicity according to WHO classification [6]. These increases in hepatic enzyme activities were transient and resolved with continued use of anti-TB drugs. These findings are in good agreement with previous study [9]. The time between initiation of anti-TB therapy and elevation of liver transaminase enzyme activities of most of our cases occurred from two to five weeks from start of anti-TB drugs. Different previous studies reported comparable findings [36]. The exact mechanism of anti-TB drug induced hepatotoxicity is unknown but toxic metabolites are suggested to play a major role at least in case of isoniazid [8]. Ungo and his colleagues [11] reported that co-infection with HIV and HCV increased the risk of grade I hepatotoxicity which is in line with the present findings. Additionally, the present data indicated that treatment with anti-TB drugs significantly increase ALP activities in all the groups, however, this increase in HIV and HCV co-infected patients was more significant compared with that of the control group. This finding is in agreement with the findings by Toppet et al. [37], who reported an increase in the serum ALP levels in pulmonary TB patients on anti-TB therapy. Elevations of ALP and/or bilirubin with little or no increase in ALT activities indicated cholestasis while increase of ALT and AST activities indicated a sign of hepatic necrosis. Thus, it is concluded that treatment with 1st line anti-TB drug produced hepatotoxicity after two weeks from start of the treatment and it is more observed in HIV and HCV co-infection patients. However, clinical signs of jaundice were negative.
- Gheghesh KS, Rahouma A, Tawil K, Zorgani A, Franka E (2013) Antimicobal resistance in Libya: 1970-2011. Libyan J Med 8: 1-8. Link: https://goo.gl/SbiKJM
- World Health Organization (2011) Global tuberculosis control. WHO ? HTM ? TB ?. Geneva, Switzerland. Link: https://goo.gl/L6HzPj
- World Health Organization (2010) Tuberculosis Fact sheet N°104. Retrieved 26 July 2011.
- Khalil A, Sathianathan S (1978) Impact of anti-tuberculosis legislation in Libya on the prevalence of primary and acquired resistance to the three main drugs at a major tuberculosis centre. Tubercle 59: 1-12. Link: https://goo.gl/VOgomh
- Centers for Disease Control and Prevention (2003) Treatment of tuberculosis. MMWR. 53: 1-77. Link: https://goo.gl/b8KCD8
- World Health Organization (1979) Collaborating Center for International Drug Monitoring. Adverse drug reaction terminology.
- Yew WW, Leung CC (2006) Anti-tuberculosis drugs and hepatotoxicity. Respiratol 11: 699-707. Link: https://goo.gl/uSyfC6
- Tostmann A, Boeree MJ, Aarnoutse RE, De Lange WC, van der Ven AJ, et al. (2008) Anti-tuberculosis drug induced hepatotoxicity. J Gastroen Heaptol 23: 192-202. Link: https://goo.gl/uUlCbF
- World Health Organization (1999) Global surveillance and control of hepatitis C: Report of a WHO consultation organized in collaboration with the Viral Hepatitis Prevention Board, Antwerp, Belgium. J Viral Hepat 6: 35-47. Link: https://goo.gl/FS9JYI
- Lomtadze N, Kupreishvili L, Salakaia A, Vashakidze S, Sharvadze L, et al. (2013) Hepatitis C virus co-infection increases the risk of anti-tuberculosis drug-induced hepatotoxicity among patients with pulmonary tuberculosis. PLoS ONE 8(12): e83892. Link: https://goo.gl/EaFY9o
- Ungo JR, Jones D, Ashkin D, Hollender ES, Bernstein D, et al. (1998) Anti-tuberculosis drug-induced hepatotoxicity. The role of hepatitis C virus and the human immunodeficiency virus, Am J Respir Crit Care Med 157: 1871-1876. Link: https://goo.gl/XXcKQi
- Hassen AA, Belachew T, Yami A, Ayen WY (2013) Anti-tuberculosis drug induced hepatotoxicity among TB/HIV co-infected patients at Jimma University Hospital, Ethiopia: nested case-control study. PLoS ONE 8: e64622. Link: https://goo.gl/8tYIE5
- World health Organization (2010) Treatment of tuberculosis: guidelines-4th ed, Geneva, Switzerland. Link: https://goo.gl/JYsOtM
- Pozniak A, Coyne K, Miller RF, Lipman MCI, Freedman AR, et al. (2011) British HIV Association guidelines for the treatment of TB/HIV co infection. British HIV Association, HIV Medicine 12: 517-524. Link: https://goo.gl/3Cz4ht
- Perlman DC, El-Sadr WM, Nelson ET, Matts JP, Telzak EE, et al. (1997) Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus-related immunosuppression. The Terry Beirn community programs for clinical research on AIDS (CPCRA). The AIDS clinical trials group (ACTG). Clin Infect Dis 25: 242-246. Link: https://goo.gl/fvKwbF
- Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8: e1002464. Link: https://goo.gl/2J5EPE
- Yinzhong Shen, Jiangrong Wang, Zhenyan Wang, et al. (2015) Across-sectional study of leukopenia and thrombocytopenia among Chinese adults with newly diagnosed HIV/AIDS. Bio Sci Trends 9: 91-96. Link: https://goo.gl/PWJRP3
- Shephorad CW, Finelli L, Alter MJ (2005) Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 5: 558-567. Link: https://goo.gl/4a96Hx
- Singla R, Sharma SK, Mohan A, Makharia G, Sreenivas V, et al. (2010) Evaluation of risk factors for anti-tuberculosis treatment induced hepatotoxicity. Indian J Med Res 132: 81-86. Link: https://goo.gl/H9jRf3
- Elkhabbazi H, Benkirane R. Khadmaoui A, Sefiani H, Quyou A, et al. (2015) Evaluation of adverse effects of anti-tuberculosis in El-Idrissi Hospital, Kenitra, Morocco. IOSR J Pharmacy 5: 06-11. Link: https://goo.gl/tuW7wD
- Saathoff E, Villamor E, Mugusi F, Bosch RJ, Urassa W, et al. (2011) Anemia in adults with tuberculosis is associated with HIV and anthropometric status in Dar el Salaam, Tanzania. Int J Tuberc Lung Dis 15: 925-932. Link: https://goo.gl/DCY72b
- Taha M, Derbew A., Tessema F, Assegid S, Duchateau L, et al. (2001) Risk factors of active tuberculosis in people living with HIV/AIDS in southwest Ethiopia: a case control study. Ethiop J Health Sci 21: 131-139. Link: https://goo.gl/VnuWtJ
- Kibret KT, Yalew AW, Belaineh BG, Asres MM (2013) Determinant factors associated with occurrence of tuberculosis among adult people living with HIV 1: 122-130. Link: https://goo.gl/c8lrQo
- Wrotkowska M, Stalke P, Smiatacz T, Zaucha JM (2014) Hematological complications in chronic hepatitis C virus infection. Blood. 124: 2169. Link: https://goo.gl/SfLMhO
- Garg R, Gupta V, Mehra S, Singh R, Prasad R (2007) Rifampicin induced thrombocytopenia. Indian J Tuberc 54: 94-96. Link: https://goo.gl/WuTlmy
- Mehata YS, Jijina FF, Badakere SS, Pathare AV, Mohanty D (1996) Rifampin-induced immune thrombocytopenia. Tuberc Lung Dis. 77: 558-562. Link: https://goo.gl/2alOyq
- Hadfied JW (1980) Rifampicin-induced thrombocytopenia. Postgrad Med J 56: 59-60. Link: https://goo.gl/XzBx5e
- Rieg G, Yeaman M, Lail AE, Donfield SM, Gomperts ED, et al. (2007) Platelet count is associated with plasma HIV type 1 RNA and disease progression. AIDS Res Human Retroviruses 23: 1257-1261. Link: https://goo.gl/OmRPCs
- Torre, Donato; Pugliese, Agostino (2008) Platelets and HIV-1 infection: old and new aspects. Current HIV Res 6: 411- 418. Link: https://goo.gl/DW34M7
- Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, et al. (1996) Thrombocytopenia associated with hepatitis C viral infection. J hepatol 24: 129-251. Link: https://goo.gl/fZvUNI
- Tana MM, Zhao X, Bradshaw A, Moon M, Page S, et al. (2015) Factors associated with the platelet count in patients with chronic hepatitis C. Thrombosis Res 135: 823-828. Link: https://goo.gl/g6VQB6
- Ndakotsu MA, Salawu L, Durosinmi MA (2008) Relation between erythrocyte sedimentation rate, clinical and immune status in HIV-infected patients. Niger J Med 17: 420-422. Link: https://goo.gl/AlHM8b
- Najafizadeh M, Farhadi N, Sarkari B (2007) Th1 cytokine profiles in hepatitis C virus infected patients and their contribution to inflammatory responses. Shiraz E-Med J 8: 22-27. Link: https://goo.gl/JoLvD4
- Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, et al. (2002) The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterol 123: 1649-1658. Link: https://goo.gl/vQPABG
- Chitturi S, Farrell G (2002) Drug-induced liver disease. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff's diseases of the liver, 9th ed. Philadelphia: Lippincott, Williams & Wilkins: 1059-1128. Link: https://goo.gl/O36IE7
- Pukenyte E, Lescure FX, Rey D, Rabaud C, Hoen B, et al. (2007) Incidence of and risk factors for severe liver toxicity in HIV-infected patients on anti tuberculosis treatment. Int J Tuberc Lung Dis 11: 78-84. Link: https://goo.gl/6v8Z9y
- Toppet M, Vainsel M, Cantraine F, Franckson M (1985) Course of serum alkaline phosphatase during treatment with isoniazid and rifampicin. Arch Fr Pediatr. 42(2): 79-80. Link: https://goo.gl/xLReob
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
