International Journal of Pharmaceutical Sciences and Developmental Research
Development and Evaluation of Rutin-HPβCD Inclusion Complex Based Mouth Dissolving Tablets
Hitendra S Mahajan*and Krishna G Bhalkar
Cite this as
Mahajan HS, Bhalkar KG (2017) Development and Evaluation of Rutin -HPβCD Inclusion Complex Based Mouth Dissolving Tablets. Int J Pharm Sci Dev Res 3(1): 001-006. DOI: 10.17352/ijpsdr.000009The objective of the present study was to explore inclusion complex of Rutin to improve the aqueous solubility and dissolution rate along with rapid mouth dissolving tablets for oral drug delivery. Rutin is a BCS class II drug having low aqueous solubility and therefore low oral bioavailability. In the present study, inclusion complex of rutin with hydroxypropyl-b-cyclodextrin were prepared by kneading method. Inclusion complex were characterized by 1H NMR, X-ray diffractometry (XRD), differential scanning calorimetry (DSC) studies, and Fourier transform infrared spectroscopy and evaluated for phase solubility study, saturation solubility and in vitro dissolution studies of plain drug (Rutin) and HPβCD inclusion complex. DSC and XRD study demonstrated that there was a significant decrease in crystallinity of pure drug present in inclusion complex (Rutin- HPβCD), which resulted in an increased dissolution rate of Rutin and 1H NMR studies strongly, confirmed that the inclusion complex has formed. The formulation so prepared were characterized for its disintegration, wetting properties, dissolution studies etc. Inclusion complexation results in improvement in solubility and dissolution rate. The inclusion complexation would be suitable method for dissolution and bioavailability enhancement of Rutin. Inclusion complex prepared by kneading method is promising approach for enhancing dissolution rate which increases oral bioavailability of poorly water soluble drugs.
Introduction
Rutin is a plant pigment (Flavonoid). Rutin(2-(3,4-dihydroxyphenyl)-4,5-dihydroxy-3-[3,4,5-trihydroxy-6-[(3,4,5-6-[(3,4,5 trihydroxy-6 methyl-oxan-2-yl)oxymethyl] oxan-2-yl]oxy-chromen-7-one). Also known as querecetin-3-Rutinosoid or sophori. Rutin has significant scavenging properties on oxidizing species such as OH radical, superoxide radical, and peroxyl radical. Therefore, it shows several pharmacological activities including anti-allergic, anti-inflammatory, and vasoactive, antitumor, antibacterial, antiviral, and anti-protozoal properties. Rutin is BCS Class II drug, however the use of Rutin is relatively limited due to its low water solubility and poor bioavailability. So for this class solubility enhancement is the promising way to increase the bioavailability of the drug [1,2]. There are various techniques to improve the solubility of drug such as particle size reduction, modification of crystal habit, drug dispersion in carrier, complexation with cyclodextrins and use of surfactants [3]. Among all techniques; inclusion complexation with cyclodextrins is the most efficient technique. Inclusion complexation with cyclodextrin has been extensively used to increase the solubility [4,5] dissolution and consequently the bioavailability of many practically insoluble or poorly water soluble drugs such as, artemether, celecoxib, meloxicam, Rutin [6–9].
Cyclodextrins (CDs) are cyclic (α-1, 4)-linked oligosaccharides of D-glucopyranose containing a relatively hydrophobic central cavity and a hydrophilic outer surface. CDs are able to form inclusion complexes with poorly water-soluble drugs. These inclusion complexes have been shown to improve stability, solubility, dissolution rate, and bioavailability. This improvement in hydrophilicity may be attributed either to the formation of inclusion complexes or to the highly homogeneous assembly between CDs and drugs in the solid state. In most cases, this association increases the solubility of poorly soluble drugs. The drug-CD binary systems are also useful in dosage form development for increasing the solubility, dissolution, and absorption rates of poorly soluble drugs in tablet or capsule form [10] The chemically modified amorphous hydroxypropyl-β-cyclodextrin (HPβCD) has higher water solubility and greater solubilizing and complexing properties than β-cyclodextrin, and is obtained by partial etherification of the crystalline parent cyclodextrins with a hydroxylalkyl group [10].HPβCD has very low toxicity by the parenteral route and no adverse effects have been observed in humans. Complexation of the drug with carrier material gives more surface area to drug for solubility enhancement [11-13].
Experimental
Materials
Rutin was obtained from Vishal-CHEM Mumbai, India. Hydroxy propyl- β-cyclodextrin was obtained as gift sample from Gangwal Chemicals PVT LTD. Malad West, Mumbai - India.Methanol and other chemicals used were of analytical grade.
Phase solubility studies: Phase solubility studies were performed according to the method reported by Higuchi and Connors [14]. Excess of Rutin inclusion complex (equivalent to 100mg) was added to 10 ml of distilled water containing various concentrations of HPβCD (0.02-0.1 mM), taken in series of test tubes covered with black paper and suspension were shaken for 48 hours on orbital shaker. The suspensions were equilibrated and filtered using Whatman filter paper (No. 40). The filter sample were suitably diluted and assayed for Rutin content by UV analysis against blank prepared in same concentration of HPβCD. The experiments were performed in triplicate. The phase solubility diagram was constructed by plotting the dissolved Rutin concentration against the respective concentration of HPβCD. The binding constant Ka was calculated from phase solubility diagram using its slope and intercept value [14].
Preparation of inclusion complex: Inclusion complex of Rutin with hydroxypopyl-β-cyclodextrin were prepared by kneading method. Drug and polymer was taken in molar ratio (1:1).The mixture of Rutin and hydroxypopyl-β-cyclodextrin was triturated in a mortar with a small volume of water – ethanol (1:2 v/v) solution. The thick slurry that formed was kneaded for 45 min and then dried at 45°C. The dried mass was pulverised and sieved through sieve no. 80. Store in cool place and in air tight container.
Characterization studies of inclusion complex
Fourier transform infrared spectroscopy (FTIR): FTIR spectra were obtained using FTIR spectrometer (8400S, Shimadzu, Japan).FTIR of Rutin, hydroxypopyl-β-cyclodextrin (HPβCD), physical mixture and inclusion complex was carried out using the KBr disk method (2 mg sample in 200 mg KBr). The scanning range was 400 to 4000 cm -1.and the resolution was 1 cm -1.
Differential scanning calorimetry (DSC): The DSC measurements of pure drug and inclusion complex were performed on a differential scanning calorimeter (Mettler Toledo) with a thermal analyzer. The thermal behavior of Rutin, HPβCD and physical mixture were determined using differential scanning calorimeter at heating rate of 5° C /min. The measurements were performed at a heating range of 40°C to 400° C under nitrogen atmosphere [15].
X-ray diffraction studies (XRD): The powder X-ray diffraction patterns of Rutin, physical mixture, Rutin -HPβCD inclusion complex were evaluated by using Philips diffractometer (PW3710) and Cu-Kα line as a source of radiation which was operated at the voltage 40 kV and the current 30 mA. All samples were measured in the 2θ angle range between 20° and 60° with a scanning rate of 3° /min and a step size of 0.02°.
1H NMR studies [16]: NMR spectroscopy assures the existence of complexes in solution and also predicts its geometry by determining the chemical shift changes of drug protons due to its insertion into the hydrophobic cavity of cyclodextrins. 1H-NMR may also be used to determine the direction of penetration of guest molecules into the cyclodextrin cavity. The H-3 and H-5 atoms of cyclodextrin, which are directed towards the interior of the cyclodextrin will show a significant upfield shift if inclusion does indeed occur and the H-1, H-2 and H-4 atoms, located on the exterior of the cavity will show only marginal upfield shifts. The spectrum of the guest molecule may also be changed upon inclusion complex formation [17,18].
Saturation solubility study
In order to analyze the improvement in solubility by various complexation methods, saturation solubility studies were performed as per Li et al. in distilled water, and pH 6.8 in triplicate. Excess of pure drug (Rutin) and inclusion complexes were added to 5 ml of distilled water in glass vials which were subsequently tightly closed and shaken for 24 h in a mechanical shaker at room temperature to achieve the equilibrium. In preliminary studies, it was found that equilibrium solubility was achieved in 24 h and therefore, samples were shaken for 24 h. Appropriate aliquots were then withdrawn, filtered, diluted, and were analyzed spectrophotometrically at 257nm [19].
In vitro dissolution studies
The dissolution rate of rutin and optimized inclusion complex of rutin was determined in 900 ml Phosphate buffer solution pH 6.8 using USP type II dissolution test apparatus. Paddle speed and bath temperature were set at 100 rpm and 37.0±0.5 0C, respectively. Five milliliters sample was withdrawn at specific intervals replaced with a fresh dissolution medium. These samples were filtered using a filter paper of pore size 0.45µm. The concentration of samples was analyzed in an ultraviolet spectrophotometer (UV-1700, Shimadzu, Japan) at 257 nm.
Formulation and development
Rutin inclusion complex (100 mg), superdisintegrant in different ratios and excipients were blended using mortar and pestle. The drug inclusion and the superdisintegrant were sieved through mesh # 60 before blending. The mixture was evaluated for angle of repose, bulk density and compressibility. The mixture was mixed with magnesium stearate, talc as lubricant and aerosil as dispersing agent they are previously sieved through mesh # 60. The granules were then compressed into tablet on a ten station rotary punch tablet machine (Rimek Mini Press) using 8 mm punch. The hardness was adjusted to 2-4 kg/cm2.Composition of tablets formulations is given in Table 1.
Evaluation of mouth dissolving tablets
MDTs were evaluated against hardness, friability, wetting time, assay of rutin, disintegration test and in vitro dissolution test, etc.
Disintegration test (IP 2010): Introduce one tablet into each tube and add a disc to each tube. Suspend the assembly in the beaker containing the specified liquid and operate the apparatus for the specified time. Remove the assembly from the liquid. The tablets pass the test if all of them have disintegrated.
In vitro dissolution studies of MDTs: In vitro dissolution studies will carry out by USP II (paddle type) dissolution test apparatus at 50 rpm. The dissolution medium consisted of 900ml of 6.8 pH Phosphate buffer. This is maintained at 37±0.50C. Aliquots of 5 ml were withdrawn and equivalent amount of fresh dissolution medium is added. Aliquots withdrawn were filtered and analyzed at 257 nm spectrophotometrically. All the release studies were conducted in triplicate and the mean values were plotted versus time with standard deviation less than three indicating reproducibility of result [20].
Result and Discussion
Inclusion complex obtained by kneading method was free flowing powder. Percent yield of inclusion complex 1:1 drug and polymer ratio was found 94.66±0.84 %.
Phase solubility study
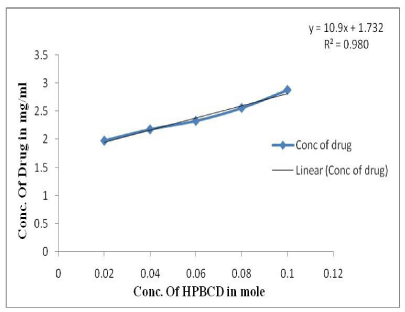
Phase solubility diagram for the complex formation between Rutin and HPβCD in water was AL type. The HPβCD solubility diagram shows a typical curve (Figure 1) whose initial rising portion is followed by a plateau region; the apparent stability constant (Kc) was calculated from the straight-line position of solubility diagram, assuming that 1:1 M complex was initially formed. The slope was less than 1 for HPβCD studied. The coefficient of regression value was 0.980. The stability constant (Kc) of Rutin-HPβCD inclusion complex was found to be 635.93M-1.
Characterization studies of inclusion complex
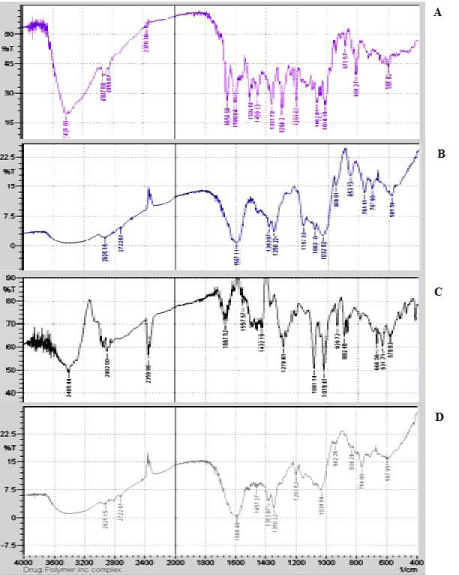
Fourier transform infrared spectroscopy (FTIR): IR spectra recorded for rutin, hydroxypopyl-β-cyclodextrin, physical mixture and inclusion complex are shown in Figure 2. Pure Rutin spectra showed sharp characteristic peaks at 3421 cm-1 assigned to –COH group. The peak at 2856 cm-1 can be assigned to acid COOH very broad. The characteristic peak at 2937 cm-1 can be assigned to alkene C-H Stretch usually weak. The characteristics peak at 1654 cm-1 revealed presence of alkene C=C Stretch groups, while peaks at 1249.91 cm-1 can be assigned to alkene C-C Stretch group respectively. The characteristics peak at 879.57 cm-1 revealed the presence meta-disubstAr ring =C-H bend three peaks. All the above characteristic peaks of drug appears in the spectra of physical mixture at the same wave number indicating no modification or interaction between drug and the polymer while some diminution and disappearance of some peaks in inclusion complex may be due to encapsulation of drug into the polymer. As shown in Figure 18, two peaks of –alcohol, COH or amine, CNR2. Alcohol is strong, broad amine is usually weak / medium, broad 2 peaks = primary amine, 1 peak = secondary amine of Rutin and physical mixture was observed at 3421and 3405.44 cm-1. The diminution of these peaks in inclusion complex indicates that there may be interaction of hydroxyl group with the secondary amine.
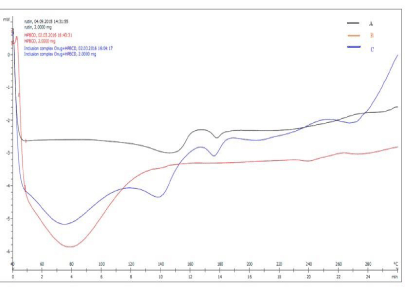
Differential scanning calorimetry: The DSC thermograms of Rutin, HPβCD and inclusion complex are shown in Figure 3. DSC analysis of crystalline Rutin showed a single sharp fusion endotherm at 148.86°C. It is revealed from DSC thermogram of pure drug and inclusion complex containing hydroxypopyl-β-cyclodextrin. There was decrease in sharpness and intensity of characteristic endothermic peak of drug which could be attributed to the conversion of most of the crystalline form of the drug to the amorphous. DSC thermogram of Rutin showed sharp endothermic peak at 148.86 oC, while in inclusion complex containing hydroxypopyl-β-cyclodextrinshowed only a little endothermic peak at 139.64oC indicating conversion of crystalline drug into amorphous form, due to kneading method. This observed no interaction between rutin and hydroxypopyl-β-cyclodextrin. The characteristic endothermic peak corresponding to melting peak of Rutin was broadened and shifted towards lower temperature (176.35 °C), with reduced intensity in the inclusion complex. This might be due to higher concentration and molecular dispersion of the drug in the polymer, resulting in complete miscibility of drug.
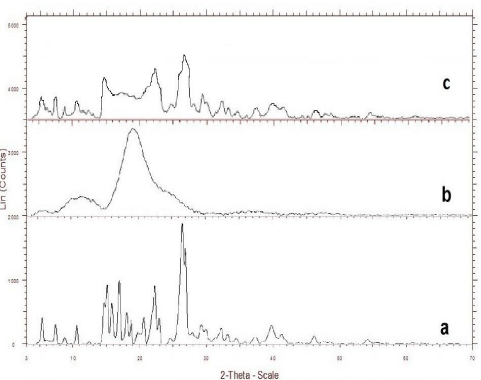
X-ray diffraction studies: The diffraction pattern of pure Rutin showed that the drug was highly crystalline nature, as demonstrated by numerous distinct peaks at 2θ of 28.84ο, 26.353ο respectively. However, the intensity of the peaks in inclusion complex prepared by kneading method was reduced when compared to that of the drug and Intensity of peak sharpness was greater in physical mixtures of RUTIN-HPβCD than in kneaded products. The results indicate that the drug in kneading method was amorphous as compared to the pure drug; hence the dissolution of the drug was improved (Figure 4).
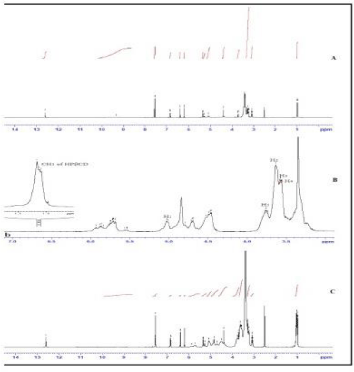
1H NMR studies: In order to explore the possible inclusion mode of the HPβCD/RUTIN complex, we compared the 1H NMR spectra of HPβCD in the absence and presence of Rutin (Figure 5). The 1H resonances of HPβCD were assigned according to the reported method.After inclusion complexation with RUTIN, the H-3 proton of HPβCD shifted 0.005 ppm and the H-5 proton of HPβCD shifted 0.007 ppm. Both H-3 and H-5 protons are located in the interior of the Cyclodextrin cavity, with H-3 protons near the wide side of cavity and H-5 protons near the narrow side. These results may indicate that RUTIN should be included in the HPβCD cavity from the wide side. Figure 5 shows the 1H NMR spectra of HPβCD, RUTIN and HPβCD – RUTIN complex in DMSO. In the 1H NMR spectrum of the HPβCD- RUTIN inclusion complex, the presence of hydrogen atom signals belonging to both HPβCD and RUTIN molecules strongly confirmed that the inclusion complex has formed. Thus. Almost all the peaks were same as in the 1H NMR spectra of RUTIN except a few peaks arise of HPβCD. In the structure of HPβCD it is a cyclodextrin which contain cyclic aliphatic structure all peaks appeared in downfield region. Pyranose ring contain -CH2-OH proton appeared as a singlet at 1.037 ppm. As per the 1H NMR spectra of drug polymer inclusion complex there is no chemical interaction between the drug and polymer; Hence RUTIN is fully intact inside the structure of HPβCD.
Saturation solubility study
Solubility data for Rutin and inclusion complex (IC) (Optimized for solubility enhancement in 1:1 ratio) Increment in solubility of Rutin from inclusion complex might be due to highly porous and amorphous nature and improved wettability provided by hydrophilic nature of HPβCD [21].
Dissolution study of inclusion complex: Dissolution efficiencies after 30 and 60 min are shown in Table 2. This table shows DE. 60% of the inclusion complex compared to rutin powder. About 75% of the inclusion complex of rutin dissolved in 30 min and complete dissolution occurred in 60 min. However only 44% of untreated rutin dissolved in 60 min and achieved complete dissolution up to the end of 120 min testing period. The increase in dissolution rate of the drug in the presence of HPβCD may be attributed to both an improvement in drug wettability and the formation of a readily soluble complex. Furthermore, the Rutin-HPβCD complex shows a markedly higher dissolution rate than that of the physical mixture. This is a consequence of increasing the drug-carrier contact surface. This result agrees with the previously reported data regarding the high affinity of HPβCD for drugs.
Evaluation of Mouth Dissolving Tablets: Tablet properties of all batches like hardness, friability, disintegration time, wetting time are given in Table 3. In Formulation Batches F1 & F4 it was found that the disintegration time of these tablets was increased. On comparing Batch F3 it was found that the disintegration time of tablet can be lowered by increasing the concentration of the Crospovidone. Among these the rapid disintegration as well as rapid wetting was observed in the F3 formulation, hardness of the tablet is 2.0±0.2 kg/cm2. Friability of the F3 batch is 0.65±0.05 it was found that the friability of the tablet was not more than 1%, the batch was disintegrate faster than other batches. Batch F3 showed ideal disintegration time .The physical parameters of F3 was found to be satisfactory and complies with official specification. Hence, F3 was considered as optimized formula for preparation of mouth dissolving tablets containing Rutin. Assay of MDT containing rutin as active constituent The % drug content of rutin F3 formulation was found to be 97.33±1.52% W/W which is within the limit. (95 to 110% as per USP)
In vitro dissolution profile of MDT: The dissolution profile of batch F3 in phosphate buffer pH 6.8 showed that 80% of drug was dissolved within 6 minute (80.45 ± 0.95). Thus it is concluded that the batch F3 will be well suitable as mouth dissolving tablets.
Conclusion
Inclusion complex of RUTIN have been successfully prepared with hydroxypropyl-b-cyclodextrin as water soluble carrier, using kneading method, which can be scaled-up industrially. DSC and XRD studies confirmed amorphism of drug (RUTIN) after inclusion complexation with HPβCD. There is no interaction between the drug and carrier was confirmed with the help of FTIR. Inclusion complex showed significantly higher drug dissolution in comparison with pure drug. Inclusion complex prepared by kneading method is promising approach for enhancing dissolution rate which increases oral bioavailability of poorly water soluble drugs.
Authors are thankful to Gangwal Chemicals PVT LTD. Malad West, Mumbai – India, for providing gift sample of Hydroxy propyl- b-cyclodextrin. The authors are also grateful to Principal (R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur) for providing necessary facilities and infrastructure for carrying out this work.
- Mauludin R, Müller RH, Keck CM (2009) Development of an oral rutinnanocrystal formulation. Int J Pharm 370: 202-209. Link: https://goo.gl/SzFnPQ
- Yang J, Guo J, Yuan J (2008) In vitro antioxidant properties of rutin. Food Sci and Tech 41: 1060-1066. Link: https://goo.gl/Ma41Sa
- Reddy BBK, Karunakar A (2011) Biopharmaceutics classification system: a regulatory approach. Dissol Technol 18: 31–37. Link: https://goo.gl/rM64Hz
- Antoniadou-Vyza E, Buckton G, Michaleas SG, Loukas YL, Efentakis M (1997) The formation of inclusion complex ofmethocarbamol with hydroxypropyl-b-cyclodextrin: the effect of chemical stability, solubility and dissolution rate. Int J Pharm 158: 233–239. Link: https://goo.gl/9OSmio
- Mahajan HS, Shah SK, Surana SJ (2011) Nasal in situ gel containing hydroxypropyl-b-cyclodextrin of artemether: development and in vitro evaluation. J Incl Phenom Macrocycl Chem 70: 49–58. Link: https://goo.gl/HpcSrB
- Yang B, Lin J, Chen Y, Lin Y (2009) Artemether/hydroxypropylb- cyclodextrin host–guest system: characterization, phase solubility and inclusion mode. J Bioorg Med Chem 17: 6311–6317. Link: https://goo.gl/kxIOfQ
- Rawat S, Jain SK (2004) Solubility enhancement of celecoxib using b-cyclodextrin inclusion complexes. Eur J Pharm Biopharm 54: 263–267. Link: https://goo.gl/WXMrVk
- Nandi S, Debnath S, Manjunath SY, Mallareddy V, Babre NP, et al. (2011) Improvement of dissolution characteristics of meloxicam by complexation with cyclodextrins. Int J Pharm Sci Nano 3:1263–1270.
- Boudad H, Legrand P, Lebas G, Cheron M, Duchene D, et al. (2001) Combined hydroxypropyl-beta-cyclodextrin and poly (alkylcyanoacrylate) nanoparticles intended for oral administration of saquinavir. Int J Pharm 218: 113–124. Link: https://goo.gl/BXYXB7
- Williams III RO, Mahaguna V, Sriwongjanya M (1998) Characterization of an inclusion complex of cholesterol and hydroxypropyl-β-cyclodextrin. Eur J Pharm Biopharm 46: 355-360. Link: https://goo.gl/SrmzOV
- Pinnamaneni S, Das N, Das S (2002) Formulation approaches for orallyadministered poorly soluble drugs. Pharmazie 57: 291–300. Link: https://goo.gl/6UMMbo
- Nalluri BN, Chowdary KPR, Murthy KVR, Hayman AR, Becket G (2003) Physicochemical characterization and dissolution properties of nimesulidecyclodextrin binary systems. Pharm- SciTech 4: 6. Link: https://goo.gl/HCcRxq
- Amit K, Rajesh V, Suresh P, Anil B (2011) Innovation of cyclodextrin in novel drug delivery system. J Nat Consci 2: 293–305.
- Higuchi TK, Connors A (1965) Phase-solubility techniques. Adv Anal Chem Instrum 4: 117–122. Link: https://goo.gl/rPYWvY
- Gaisford S, Saunders M (2012) Essentials of pharmaceutical preformulation. John Wiley & Sons 45-59. Link: https://goo.gl/vi4eD0
- Singh R, Bharti N, Madan J, Hiremath SN (2010) Characterization of cyclodextrin inclusion complexes—a review. J Pharm Sci Techno 2: 171-183.
- Sambasevam KP, Mohamad S, Sarih NM, Ismail NA (2013) Synthesis and characterization of the inclusion complex of β-cyclodextrin and azomethine. Int J molecular sci 14: 3671-3682. Link: https://goo.gl/Z6aUTT
- Fronza G, Mele A, Redenti E, Ventura P (1996) 1H NMR and molecular modeling study on the inclusion complex β-cyclodextrin-indomethacin. J Org Chem 61: 909-914. Link: https://goo.gl/1PcfQr
- Hirlekar R, Kadam V (2009) Preformulation study of the inclusion complex irbesartan-β-cyclodextrin. Pharm Sci Tech 10: 276-281. Link: https://goo.gl/9MPha9
- Patel M, Tekade A, Gattani S, Surana S (2008) Solubility enhancement of lovastatin by modified locust bean gum using solid dispersion techniques. Pharm Sci Tech 9:1262-1269. Link: https://goo.gl/C3rSoK
- Zingone G, Rubessa F (2005) Preformulation study of the inclusion complex warfarin-β-cyclodextrin. Int J Pharm 291: 3-10 . Link: https://goo.gl/IbHBA8
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
