International Journal of Pharmaceutical Sciences and Developmental Research
Diabesity Increases Inflammation and Oxidative Stress
Dayre A1, Pouvreau C1, Butkowski EG2, de Jong B2 and Jelinek HF2,3*
2School of Community Health, Charles Sturt University, Albury, Australia
3Australian School of Advanced Medicine, Macquarie University, Sydney, Australia
Cite this as
Dayre A, Pouvreau C, Butkowski EG, de Jong B, Jelinek HF (2016) Diabesity Increases Inflammation and Oxidative Stress. Int J Pharm Sci Dev Res 2(1): 012-018. DOI: 10.17352/ijpsdr.000006Background: Inflammation and oxidative stress are two pathophysiological mechanisms that link obesity, type 2 diabetes mellitus (T2DM) and diabesity. However how levels of inflammatory and oxidative stress markers differ between patients with obesity, T2DM and diabesity has not been completely elucidated.
Objectives: This study aimed to investigate the interactions between emerging biomarkers of oxidative stress and inflammation and the differences in biomarker levels between T2DM, obesity and diabesity in a clinical setting.
Methods: A total of 270 patients attending a diabetes health screening clinic (57 T2DM; 37 obese; 44 diabesity, and 132 age, gender, and weight-matched controls) participated in the study. All patients were selected on clinical grounds. Differences in the level of biomarkers of oxidative stress (erythrocyte GSH/GSSG, 8-hydroxy-2-deoxy-guanosine (8-OHdG) and urinary 8-iso-prostaglandin F2α), inflammation (CRP, IL-6, IL-10 and IL1β), and coagulation (C5a, D-dimer) were determined.
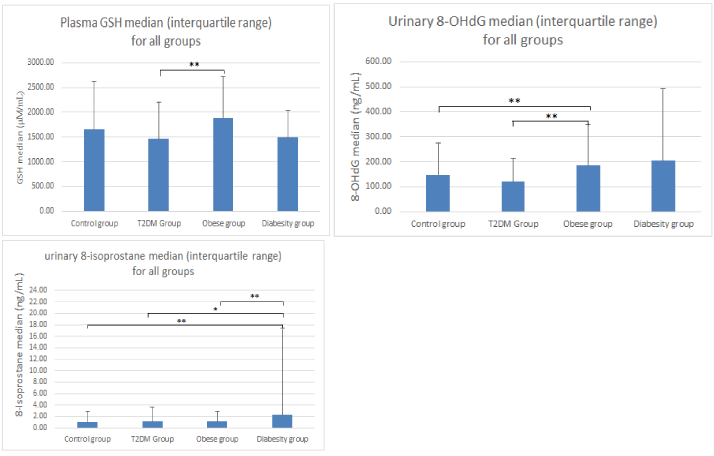
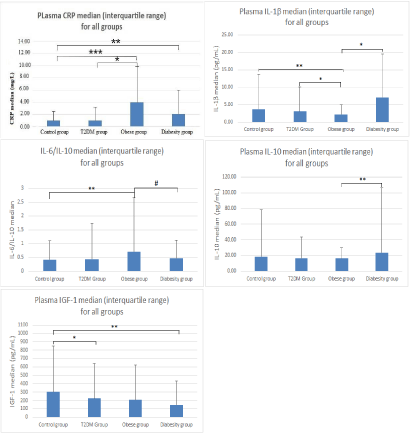
Results: Both inflammatory markers and oxidative stress differed significantly between the three clinical groups. GSH revealed a significant difference between the T2DM (1456.6 µM/mL ± 737.5, p<0.03) and obese groups (1890.7 µM/mL ± 823.3). 8-OHdG increased significantly in the obese group (185.5ng/mL ± 162.4, p<0.03) compared to the control group (146.8ng/mL ± 127.5). Similarly 8-OHdG was significantly higher in the obesity group (185.5ng/mL ± 162.4) compared to the T2DM group (119.2ng/mL ± 92.9, p<0.03). A significant increase was also found for 8-iso-PGF2α in the diabesity group (2.3 ± 15.0 ng/mL) compared to the control group (1.0 ± 1.9 ng/mL, p<0.03) and the T2DM group (1.1±2.5ng/mL, p<0.05). 8-iso-PGF2α in the diabesity group (2.3±15.0 ng/mL) was significantly higher than the obese group (1.1ng/mL ±1.8, p<0.03). A significant decrease occurred in IGF-1 levels for diabesity (144.6 ± 285.7 ng/mL) compared to the control (302.8 ± 547.2 ng/mL, p <0.03) and the T2DM groups (225.1ng/mL ± 417, p<0.05). A statistically significant increase in the inflammatory marker ratio IL-6/IL-10 between the control group (0.41 ± 0.7) and the obesity group (0.71 ± 1.94, p<0.03) was also observed.
Conclusion: The results obtained indicate that 8-iso-PGF2α, 8-OHdG as markers of oxidative stress and IGF-1, IL-6/IL-10 are associated with diabesity and could be used diagnostically for risk assessment.
Abbreviations
Eda: Edaravone; 3-methyl-1-phenyl-2-pyrazolin-5-one; AAPH: 2,2’-azobis[2-methylpropionamidine] dihydrochloride; EDD: Embryo Development Day; MDA: Malondialdehyde; SOD: Superoxide Dismutase; CAMs: Chorioallantoic Membranes; IPP: Intel Integrated Performance Primitives; TBA: Thiobarbituric Acid
Introduction
Obesity and diabetes mellitus type 2 (T2DM) as well as other chronic diseases have been shown to be associated with inflammation and oxidative stress processes [1-3]. Whether the level of these inflammatory and oxidative stress markers differs in diabesity and how they interact has not been investigated. Overweight and/or obesity is now estimated to be over 30% of the global population, with approximately 12% being diagnosed with type 2 diabetes mellitus (T2DM) [4,5]. Obesity and diabetes are linked, with the strong association between these diseases resulting in the creation of the term obesity-dependent diabetes or “diabesity [6,7]. Diabesity is associated with cardiovascular disease (CVD), dyslipidaemia, hyperuricaemia, respiratory disease, osteoarthritis and depression [8]. Two possible pathophysiological mechanisms involved in obesity and T2DM may be associated with diabesity. These two mechanisms are oxidative stress and inflammatory processes. Chronic inflammation as a result of several pro-inflammatory markers such as cytokines and chemokines have been shown to precede weight gain and obesity [9]. Similar observations are seen with a number of oxidative stress markers in obesity [10,11]. The link between inflammatory and oxidative stress markers observed in obesity is also a contributory factor in the development of T2DM and CVD [8,12].
The inflammatory process in obesity includes the activation of macrophages to produce pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-6 (IL-6) [13-15]. These pro-inflammatory cytokines lead to increased levels of C-reactive protein (CRP) with a demonstrable decrease in the anti-inflammatory interleukin-10 (IL-10). IL-1β, IL-6, CRP and IL-10 are linked to abnormal fat and glucose metabolism, which provides further evidence of a link between obesity and diabetes by increasing insulin resistance [8,16].
During the hyperglycaemic state, cytokine production is also accompanied by increased levels of monocyte chemoattractant protein-1 (MCP-1) and decreased levels of insulin-like growth factor-1 (IGF-1) (17-21). MCP-1 has chemotactic effects, and attracts immune cells such as leukocytes and macrophages to the inflamed sites. It has been shown that an increase in plasma MCP-1 level may cause a macrophage infiltration into adipose tissue and induce adipocyte de-differentiation, which contribute to obesity, hyperinsulinaemia and T2DM via insulin resistance [17,19,20]. IGF-1 functions in association with insulin-like growth factor binding protein-1 (IGFBP-1) [21]. Both insulin and IGF-1 play a role in glucose homeostasis, and reduce blood glucose levels (BGL). In diabetes low levels of IGF-1 predominate, leading to a decrease in glucose homeostasis [18]. This decrease may be due to a suppression of IGFBP-1 and therefore leads to a weight gain [21]. A slightly different pathology however is observed in obesity where, IGF-1 levels are stable [22].
Studies have shown that markers of coagulation such as D-dimer and C5a are increased in diabetes and obesity [23-26]. D-dimer is a product of fibrin degradation and its level has been related to lower levels of reduced glutathione (GSH) [23,24,27]. An increase in oxidative stress first leads to a depletion in GSH followed by a rebound increase [28]. The decrease in GSH observed closer to the onset of T2DM is due to the activity of plasminogen activator inhibitor-1 (PAI-1) and thrombin-activated fibrinolysis inhibitor (TAFI), followed by an increase in erythrocyte GSH levels associated with erythrocyte oxidative stress [23,24,27]. However, during T2DM disease progression, the physiological response to GSH synthesis/regeneration is overwhelmed by continued oxidative stress due to free radical activity, which leads eventually to a decrease in GSH concentration and an increase in D-dimer levels [23,27].
The complement and coagulation protein, C5a, is a protein fragment released from cleavage of complement component C5. In cases of obesity and T2DM there is an increase in phospholipid antibody (aPL) and increasing complement proteins such as C5a. These stimulate transmembrane glycoprotein tissue factor (TF) synthesis [29] initiating the coagulation cascade [30]. Adipose tissue inflammation has been related to an up regulation of TF synthesis resulting in an increase in coagulopathy [29,30].
Inflammation induces the development of diabesity, however inflammation not only precedes the development of diabesity but persistent high levels of plasma FFAs, IL-6, CRP, IL-1β and low level of IL-10 continue to be a factor in disease progression through several mechanisms including oxidative stress [13,31].
Oxidative stress is defined as free radical-mediated damage caused by an excess of reactive oxygen species (ROS) [32]. Several oxidative stress markers such as 8–hydroxy-2-deoxy guanosine (8-OHdG), 8-iso-prostaglandin F2α (8-iso-PGF2α), reduced glutathione (GSH) and its oxidized form glutathione disulfide (GSSG) have been extensively studied [32,33]. Oxidative stress then leads to a less reduced GSH with a concomitant increase in GSSG [34]. This decrease in GSH is accompanied by an increase in 8-iso-PGF2α and has been shown to have associations with MetS, which includes obesity and diabetes as factors [35].
Chronic disease progression is often associated by an interaction between inflammation and oxidative stress. IL-6 is related to enhanced superoxide radicals and oxidative stress that decreases the effective processing of excess FFA’s and causes mitochondrial uncoupling and an increased production of ROS. Increased ROS then leads to further oxidative stress effects and exacerbating the inflammatory process [36].
Diabesity has been suggested to be a combination of T2DM and obesity with the link being inflammation and oxidative stress [13]. Increased levels of 8-iso-PGF2α accompanied by an increase in urinary 8-OHdG levels correlating to body mass index (BMI) have been reported in diabesity [11,37]. Several inflammatory markers have also been suggested [19]. The role of inflammatory markers and oxidative stress and how these interact in diabesity has not been extensively investigated when compared to T2DM and obesity and is therefore explored in this study.
Methods
The study protocol was reviewed and approved by the Ethics in Human Research Committee of Charles Sturt University (CSU). A total of 270 participants (female: male, 163:107) were selected within a rural diabetes screening clinic at CSU (DiabHealth) for analysis of blood and urine samples. All measurements carried out by clinical staff were in accordance with the relevant legislation, professional standards of practice and policy directives. General biochemistry, including lipid studies, HbA1c, CRP and D-dimer were conducted by the local pathology provider a National Accredited Testing Authority (NATA) laboratory. Enzyme linked immunosorbent assays (ELISA) and GSH were carried out using commercially available kits.
Participants were excluded by age (< 40 years), CVD or kidney disease. As a part of the screening process body mass index (BMI), gender, lipid profile including triglycerides (TG), total cholesterol (TC), high density lipoproteins (HDL)-cholesterol, low density lipoproteins (LDL)-cholesterol and TC/HDL ratio were determined for all participants.
Diabetic participants were selected on the basis of having been diagnosed with an oral glucose tolerance test (OGTT) and/or being prescribed antihyperglycaemic medication. Control participants were included in the study if they had no background of diabetes, cardiovascular, renal or respiratory disease. Participants were comparable for age, gender, smoking and alcohol consumption, diet and physical activity and divided into 4 groups: a control group (FBG <5.6mmol/L and BMI <30.0), a diabetic group (FBG ≥7mmol/L and BMI <30.0), an obese group (FBG <5.6mmol/L and BMI ≥30.0) and an obese diabetic group (FBG ≥7mmol/L and BMI ≥30.0). Fasting blood glucose level (FBG) for the two groups were defined according to the criteria of the American Diabetes Association 2004 [38].
Sample collection
Venous blood was collected into EDTA, serum-separating (SST) and sodium-citrate-(SC) tubes. Blood tubes were immediately centrifuged in a Universal 32R (Hettich Zentrifugen, Germany) for 15 min at 800 g. Plasma and serum for inflammatory, coagulation and oxidative stress biomarkers were stored at -80°C until analysis was performed. For measurement of GSH and GSSG, erythrocyte lysates were prepared by washing cells in normal saline followed by the addition of 4 × volume of ice-cold 5% metaphosphoric acid. Post centrifugation lysate was stored at -80°C until analysis.
Midstream urine specimens were collected for the determination of 8-isoprostaglandin F2α and 8-OHdG. Additional urine aliquots for creatinine and plasma and serum from the blood EDTA, SST and SC-tubes were referred to the local accredited Dorovitch Pathology Laboratory for lipids, glucose, HbA1c, CRP and D-Dimer levels.
Anthropometric variables
Weight was measured using standardized beam weight scales without footwear and with only light clothes. Height was measured with the subjects barefoot and standing with the feet together. Waist circumference was measured in centimeters. BMI, defined as weight in kilogram per height in meters squared and is independent of gender and age was calculated for each client. Overweight clients were classified as a BMI between 25.0-29.9 kg/m2 and obese when BMI was equal or greater than 30.0 kg/m2 [39]. Blood pressure (BP) was recorded, after patients were rested in a supine position for 5 minutes, using a stethoscope and BP cuff measured at the brachial artery.
A screening fasting blood glucose levels (FBG) was determined on all patients using the Accu-Chek® (Roche Australia Pty Ltd).
Measurement of oxidative stress and DNA damage
Urinary 8-isoprostaglandin F2α (8-iso-PGF2α) was determined using OxiSelectTM 8-iso-Prostaglandin F2α ELISA Kits (Cell BioLabs, INC).The assay incorporates a competitive binding ELISA strategy, allowing the 8-iso-PGF2α contained in samples and standards to compete with 8-iso-PGF2α - HRP conjugate for binding to an anti-8-iso-PGF2α antibody fixed to a goat anti-rabbit antibody pre-coated microplate.
Erythrocyte reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined using the CaymanTM Total Glutathione (GSSG/GSH) Assay Kit. Total glutathione was measured by incorporating glutathione reductase to reduce reducing GSSG to GSH in the presence of Nicotinamide adenine dinucleotide phosphate (NADPH). GSSG was measured by subtraction after the addition of 2 vinyl pyridine.
Urinary 8-OHdG was determined using OxiSelectTM Oxidative DNA Damage ELISA Kit 8-OHdG Quantitation (Cell BioLabs, INC). 8-OHdG contained in samples and standards competes with an HRP conjugate secondary antibody for binding to an 8-OHdG/BSA conjugate coated microplate.
Inflammation biomarkers
Plasma IL-6, IL-1β, IL-10, MCP-1 and IGF-1 were determined using Human IL-6 ELISA Kit provided by Elisakit.com, Melbourne, Australia.
Coagulation and fibrinolysis
Plasma C5a was determined using Human C5a Platinum ELISA (eBioscience, Affymetrix, North America).
All ELISA measurements conducted on the inflammatory, oxidative stress and coagulation biomarkers were carried out with a Thermo Scientific Multiskan FC (Fisher, China). Data analysis for the ELISA analysis utilised a 4-parameter logistic curve fit.
Statistical analysis
Descriptive data was expressed as mean ± standard deviation (x ± SD). Statistical analysis was performed with SPSS (Version 20, IBM Co). To determine if there were significant differences in biomarker levels between the control, the diabetic, the obese and the obese diabetic group, an analysis of variance (ANOVA) was used. To correct for age and gender, an analysis of covariance (ANCOVA) was used. A p-value 0.05 was considered as significant.
Results
Anthropometric, clinical and biochemistry data for the 270 patients who participated in the study and for whom blood and urine samples were available are shown in Table 1 Comorbidities and medication history were noted and documented.
BMI – Body mass index, SBP – systolic blood pressure, DBP – diastolic blood pressure, TC – Total cholesterol, HDL – high density lipoprotein, LDL – low density lipoprotein,
Significant differences were found for anthropometric data including age, waist circumference and BMI, which increased from control to T2DM, obese and diabesity status. Waist circumference for females was lower than for men in all groups, with significant differences for both genders between all groups other than the obese and diabesity group in the male cohort. There were significant differences in systolic blood pressure (SBP) between the control, T2DM and diabesity groups. General biochemistry profiles including screening fasting glucose and HbA1c were elevated in T2DM and diabesity but were low in obese with some significant differences between groups. Lipid profiles also showed significant differences between the groups (Table 1).
Coagulation and fibrinolysis investigated with C5a and D-dimer showed no significant differences between groups in our study. However C5a results trended upwards in the diabetic group compared to control and decreased again in the obese and diabesity groups.
Figure 1 indicates a significant difference for GSH between the T2DM (1456.6 µM/mL ± 737.5, p<0.03) and obese groups (1890.7 µM/mL ± 823.3, p<0.003). However GSSG levels did not show significant differences between any of the other groups. 8-OHdG increased significantly in the obese group (185.5ng/mL ± 162.4, p<0.03) compared to the control group (146.8ng/mL ± 127.5) with a further nonsignificant increase in the diabesity group (204.6ng/mL ± 286.8). The obesity group (185.5ng/mL ± 162.4) was significantly increased to the T2DM group (119.2ng/mL ± 92.9, p<0.03). The T2DM group results (119.2ng/mL ± 92.9) were less than the control group but were not significant (Figure 1). A significant increase was also found in 8-iso-PGF2α in the diabesity group (2.3 ± 15.0 ng/mL) compared to the control group (1.0 ± 1.9 ng/mL, p<0.03) and the T2DM group (1.1±2.5ng/mL, p<0.05). The diabesity group (2.3 ± 15.0 ng/mL) was significantly increased to the obese group (1.1ng/mL ± 1.8, p<0.03). A slight but not significant increase in the T2DM and obese groups was also found for 8-iso-PGF2α (Figure 1).
Results for CRP levels for all groups compared to the control (1.0mg/L ± 1.50) were significantly higher for the obese group (4.0mg/L ± 5.80, p<0.001) and the diabesity group (2.0mg/L ± 4.0, p<0.05). There was also a significant increase between the obese group (4.0mg/L ± 5.80, p<0.05) and the T2DM group (1.0mg/L ± 2.20) (Figure 2). CRP levels decreased between obese group and diabesity, however results were not significant. IL-6 results showed no significant difference between any groups, however the T2DM group did demonstrate an increase above the other groups including the control group. IL-1β levels were significant between the control (3.70pg/mL ± 10.10) and obese groups (2.20pg/mL ± 2.70, p<0.03), the T2DM (3.10pg/mL ± 7.0) and obesity groups (2.20pg/mL ± 2.70, p<0.05) and the obesity and diabesity group (7.10pg/mL ± 12.50, p <0.05). IL-10 decreased slightly but not significantly from the control group to the T2DM group and obesity group, but did increase significantly between the obese group (16.20pg/mL ± 13.20) and diabesity group (23.60pg/mL ± 82.30, p<0.03). No significant results were obtained for MCP-1 between any groups. IGF-1 revealed a significant decrease in the control (302.8pg/mL ± 547.2) and T2DM (225.1pg/mL ± 417.0, p<0.05) and the diabesity group (144.6pg/mL ± 285.7, p<0.03) (Figure 2). There was a trending down of IGF-1 in all groups observed.
To further our investigations into inflammatory marker interactions we also performed comparisons with inflammatory marker ratios: IL-6/IL-10, IL-1β/IL-10, (MCP-1/IGF-1)*IL-6 and (MCP-1/IGF-1)*IL-1β ratios. The IL-6/IL-10 ratio was significantly higher in the obesity group (0.71 ± 1.94) versus the control group (0.17 ± 0.35, p<0.03), the level of significance between the obesity group and the diabesity group (0.47 ± 0.66) was p<0.06. IL-1β/IL-10 and (MCP-1/IGF-1)*IL-1β results were not significant. However the (MCP-1/IGF-1)*IL-6 results showed an increasing but not significant trend.
Discussion
Diabesity is related to several comorbidities and chronic disease states, which may all have a pathophysiological basis in oxidative stress and inflammation. Previous studies have related obesity dependent diabetes to an increase in inflammation, and have shown that biomarkers of inflammation interact with each other. In the current study we have shown that the ratio of IL-6, MCP-1 and IGF-1 was the lowest in the control group and increased with obesity, diabetes and diabesity. During the inflammatory process as part of chronic disease pathophysiology an increase of proinflammatory biomarkers (IL-6, IL-1β) coupled with a decrease in anti-inflammatory biomarkers (IL-10) has been demonstrated [13-16]. Other studies have shown that IL-6 and IL-1β also increased MCP-1 levels by inducing MCP-1 mRNA expression [40-43]. These two proinflammatory biomarkers also inhibit IGFBP-2 and IGFBP-4 secretion resulting in a decrease in IGF-1 levels (44-47). A negative feedback loop between IGF-1 and MCP-1 then also decreases MCP-1 [48]. Our study demonstrated that the increase in the IL-6/IL-10 ratio between control and obesity was significant but dropped back down in the diabesity group. This drop still remained higher than the control and the T2DM group and suggests a cyclic interaction or negative feedback loop between these inflammatory markers and other biomarkers involved in disease progression. This is further confirmed with CRP being significantly increased in the obese and diabesity groups compared to control. In our study a significant decrease was observed in IGF-1 for the diabesity compared to the control group. The significant changes in the inflammatory markers in this clinical study were found despite patients being on several medications such as statins, antidiabetic and antihypertensive medications suggesting their utility in clinical practice as diabesity risk markers.
A similar association was seen for oxidative stress markers. We observed a significant increase in 8-iso-PGF2α in the diabesity group and between the obesity and T2DM groups. These results are indicative of lipid peroxidation increasing due to oxidative stress occurring in the diabesity state similar to T2DM and obesity and demonstrates the potential for 8-iso-PGF2α as a marker of oxidative stress in the development of the diabesity state. A significant increase in 8-OHdG levels in the T2DM and obese groups compared to controls was also observed. Oxidative stress is apparent in diabesity and should therefore be subject to further analysis to confirm our findings.
A novel finding was that the IL-6/IL-10 ratio was significantly different between the control group and the obesity group with the difference between the obesity and diabesity group were approaching significance (p<0.06), indicating that further research is required with increased cohort numbers to assess the role of IL-6/IL-10 in diabesity. The (MCP-1/IGF-1)*IL-6 while not significant did trend upwards throughout the four groups, whereas the (MCP-1/IGF-1)*IL-1β ratio appeared to trend down with the obese group and upwards with the diabesity group. We have previously observed significant differences in IL-6/IL-10, as a measure of the pro/anti-inflammatory response in endurance training and high intensity interval training when compared to a control group [49]. Our own work on mild cognitive decline also suggests that the interaction between IL-6 and IL-10 measured as a ratio is part of the pathophysiological process [50]. Analysis of inflammatory ratios may therefore provide additional information on the pathophysiology of diabesity.
- Rodriguez-Hernandez H, Simental-Mendia LE, Rodriguez-Ramirez G, Reyes-Romero MA (2013) Obesity and Inflammation: Epidemiology, Risk Factors, and Markers of Inflammation. Int J Endocrinol 2013: 678159 .
- Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, et al. (2015) Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer Disease. Med Inflamm 2015: 17 .
- Al-Aubaidy HA, Jelinek HF (2011) Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol 164: 899-904 .
- Basdevant A, Bouillot JL, Clement K, Oppert JM, Tounian P (2011) Traité médecine et chirurgie de l'obésité. Lavoisier, France: Médecine Sciences Publications 09/2011. 800 .
- Wilborn C, Beckham J, Campbell B, Harvey T, Galbreath M, et al. (2005) Obesity: prevalence, theories, medical consequences, management, and research directions. J Int Soc Sports Nutr 2: 4-31 .
- Farag YMK, Gaballa MR (2011) Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant 2011: 28–35 .
- Zimmet P (2003) Diabesity in Australia: an affair of the heart. Heart Lung Circ 12: 95-98 .
- Golay A, Ybarra J (2005) Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab 19: 649-663 .
- Engström G, Hedblad B, Stavenow L, Lind P, Janzon L, et al. (2003) Inflammation-Sensitive Plasma Proteins Are Associated With Future Weight Gain. Diabetes 52: 2097-2101 .
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752-1761 .
- Al-Aubaidy HA, Jelinek HF (2011) Oxidative DNA damage and obesity in type 2 diabetes mellitus. Eur J Endocrinol 164: 899–904 .
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286: 327-334 .
- Schmidt Maria I, Duncan Bruce B (2003) Diabesity: An Inflammatory Metabolic Condition. Clin Chem Lab Med 41: 1120-1130 .
- Bergmann K, Sypniewska G (2013) Diabetes as a complication of adipose tissue dysfunction. Is there a role for potential new biomarkers? Clin Chem Lab Med 51: 177-185 .
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N (2014) Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 105: 141-150 .
- Blüher M, Fasshauer M, Tönjes A, Kratzsch J, Schön M, et al. (2005) Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol 113: 534-537 .
- Chadt A, Scherneck S, Joost HG, Al-Hasani H (2014) Molecular links between Obesity and Diabetes: “Diabesity”: NCBI; 2014 .
- Teppala S, Shankar A (2010) Association between Serum IGF-1 and Diabetes among U.S. Adults. Diabetes Care 33: 2257-2259 .
- Al Hannan F, Culligan KG (2015) Human resistin and the RELM of Inflammation in diabesity. Diabetol Metab Syndr 7: 54 .
- Deshmane SL, Kremlev S, Amini S, Sawaya BE (2009) Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J Interferon Cytokine Res 29: 313-326 .
- Lewitt MS, Dent MS, Hall K, Huang P (2014) The Insulin-Like Growth Factor System in Obesity, Insulin Resistance and Type 2 Diabetes Mellitus. J Clin Med Res 3: 1561-1574 .
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, et al. (1997) Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord 21: 355-359 .
- Nwose EU, Jelinek HF, Richards RS, Tinley P, Kerr PG (2009) Atherothrombosis and oxidative stress: the connection and correlation in diabetes. Redox Rep 14: 55-60 .
- Nwose EU, Richards RS, Jelinek HF, Kerr PG (2007) D-dimer identifies stages in the progression of diabetes mellitus from family history of diabetes to cardiovascular complications. Pathology 32: 252-257 .
- Krupinski J, Turu MM, Font AM, Ahmed N, Sullivan M, et al. (2007) Increased tissue factor, MMP-8, and D-dimer expression in diabetic patients with unstable advanced carotid atherosclerosis. Vasc Health Risk Manag 3: 405-412 .
- Maschirow L, Khalaf K, Al-Aubaidy HA, Jelinek HF (2015) Inflammation, coagulation, endothelial dysfunction and oxidative stress in prediabetes — Biomarkers as a possible tool for early disease detection for rural screening. Clin Biochem 48: 581-585 .
- Nwose EU, Jelinek HF, Richards RS, Kerr PG (2006) Erythrocyte oxidative stress in clinical management of diabetes and its cardiovascular complications. Br J Biomed Sci 64: 35-43 .
- Jelinek HF, Al-Aubaidy H, Maschirow L, Meidinger S, Jamil D, et al. (2014) Glutathione:Glutathione sulfide redox imbalance in early impaired fasting glucose. Int J Cardiol Angiol 2: 223-229 .
- Chu AJ (2011) Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam 2011: 30 .
- Samad F, Ruf W (2013) Inflammation, obesity, and thrombosis. Blood 122: 3415-3422 .
- Harder-Lauridsen NM, Krogh-Madsen R, Holst JJ, Plomgaard P, Leick L, et al. (2014) Effect of IL-6 on the insulin sensitivity in patients with type 2 diabetes. Am J Physiol Endocrinol Metab 306: E769-E78 .
- Calabrese V, Cornelius C, Leso V, Trovato-Salinaro A, Ventimiglia B, et al. (2012) Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. BBA Mol Bas 1822: 729-736 .
- D'Archivio M, Annuzzi G, Varì R, Filesi C, Giacco R, et al. (2012) Predominant role of obesity/insulin resistance in oxidative stress development. Eur J Clin Invest 42: 70-78.
- Raza H, John A, Howarth F (2012) Alterations in glutathione redox metabolism, oxidative stress, and mitochondrial function in the left ventricle of elderly Zucker diabetic fatty rat heart. Int J Mol Sci 13: 16241-16254 .
- Mure K, Yoshimura N, Hashimoto M, Muraki S, Oka H, et al. (2015) Urinary 8-iso-prostaglandin F2alpha as a marker of metabolic risks in the general Japanese population: The ROAD study. Obesity 23: 1517-1524 .
- Codoñer-Franch P, Valls-Bellés V, Arilla-Codoñer A, Alonso-Iglesias E (2011) Oxidant mechanisms in childhood obesity: the link between inflammation and oxidative stress. Transl Res 158: 369-384 .
- Al-Aubaidy HA, Jelinek HF (2015) 8-Hydroxy-2-deoxy-guanosine identifies oxidative DNA damage in a rural pre diabetes cohort. Redox Rep 15: 155-160 .
- American Diabetes Association (2004) Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 27: 5-10 .
- WHO (2003) Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. World Health Organization 9789241209168 .
- Biswas P, Delfanti F, Bernasconi S, Mengozzi M, Cota M, et al. (1998) Interleukin-6 Induces Monocyte Chemotactic Protein-1 in Peripheral Blood Mononuclear Cells and in the U937 Cell Line. Blood 91: 258-265 .
- Arendt B, Velazquez-Dones A, Tschumper R, Howell K, Ansell S, et al. (2002) Interleukin 6 induces monocyte chemoattractant protein-1 expression in myeloma cells. Leukemia 16: 2142-2147 .
- Klouche M, Bhakdi S, Hemmes M, Rose-John S (1999) Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J Immunol 163: 4583–4589 .
- Parry GCN, Martin T, Felts KA, Cobb RR (1998) IL-1b–induced monocyte chemoattractant protein-1 gene expression in endothelial cells is blocked by proteasome inhibitors. Arterioscler Thromb Vasc Biol 18: 934-940 .
- Street ME, Miraki-Moud F, Sanderson IR, Savage MO, Giovanelli G, et al. (2003) Interleukin-1β (IL-1β)and IL-6 modulate insuline-like growth factor-binding protein (IGFBP) secretion in colon cancer epithelial (Caco-2) cells. J Endocrinol 179: 405-415 .
- Al-Shanti N, Stewart CE (2012) Inhibitory effects of IL-6 on IGF-1 activity in skeletal myoblasts could be mediated by the activation of SOCS-3. J Cell Biochem 113: 923-933 .
- Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, et al. (2003) Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab 88: 2019-2025 .
- Rosen CJ (2000) Growth hormone and aging. Endocrine 12: 197-201 .
- Chiu K, Yeung SC, So KF, Chuen-Chung Chang R (2010) Modulation of morphological changes of microglia and neuroprotection by monocyte chemoattractant protein-1 in experimental glaucoma. Cell Mol Immunol 7: 61-68 .
- Kaspar F, Jelinek HF, Perkins S, Al-Aubaidy HA, deJong B, et al. (2016) Acute-Phase Inflammatory Response to Single-Bout HIIT and Endurance Training: A Comparative Study. Med Inflamm 2016: 6 .
- Fabrègue F, Butkowski E, Voigt A, Mouquot G, de Jong B, et al. (2016) Association of Inflammation and Possible Mild Cognitive Decline Measured by the Stroop Cognitive Function Test. J Alzheimers Dis Parkinsonism 6 .
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
