International Journal of Oral and Craniofacial Science
Prevalence of Temporomandibular Joint Disorders among Yemeni University students: A prospective, cross-sectional study
Jabr Saleh Al-sanabani1*, Essam Ahmed Al-Moraissi2 and Abdulrazaq Ahmed Almaweri1
2Assistant Professor, Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, Thamar University, Yemen
Cite this as
Al-sanabani JS, Al-Moraissi EA, Almaweri AA (2017) Prevalence of Temporomandibular Joint Disorders among Yemeni University students: A prospective, cross-sectional study. Int J Oral Craniofac Sci 3(2): 053-059. DOI: 10.17352/2455-4634.000032Purpose: The aim of this study was to estimate prevalence of temporomandibular Joint disorders (TMDs), among dental university student in Yemen.
Material and methods: A prospective cross-sectional study was conducted in the Department of Oral Medicine and Oral Diagnosis, Faculty of Dentistry, Thamar University, Yemen. Study sample consisted of 207 university students (114 males and 93 females). Predictor’s variables were age, gender and marital status. The outcomes variables were the signs and symptoms of TMDs using the Helkimo clinical dysfunction (Di) and anamnestic (Ai) indices.
Results: The results revealed that prevalence of TMDs among Yemeni dental students was 41.07 % (mild: 14.01%, sever: 27.05%). In the clinical examination, 49.76 % (mild: 20.77%, moderate; 12.08% and sever; 16.91%) showed some degree of dysfunction. Females showed a significant higher prevalence of TMDs than males. There was significant association between the dysfunction of TMD and gender (P > 0.005).
Conclusions: The result of this study showed that prevalence of TMDs among Yemeni dental students was higher than some studies have reported in the literature. Also, the results revealed that majority of studied sample were not aware of the presence of their TMD
Introduction
Temporomandibular joint (TMJ) function has been the subject of considerable study for over a century, and despite voluminous literature, the multifactorial etiology of temporomandibular dysfunction is even today a cryptic issue [1].
Temporomandibular disorders (TMD) have defined as a collecting term comprising a variety of clinical signs and symptoms confined to the temporomandibular joint (TMJ) and/ or the related structures (masticatory musculature, bone and facial structures). Signs and symptoms of the TMD include a symptom as facial pain, headache, earache, and joint pain both on rest position and during jaw movement; and signs as limited jaw movement, jaw deviations, joint noises (clicking and popping), jaw locking, and dislocation. In addition, traumatic occlusion and wear of dentition due to parafunctional habits (clenching and bruxism, anxiety, stress) had been experienced in by patients with TMD [2,3].
Several research and clinical diagnostic criteria were introduced have been used to diagnose the TMDs including: Helkimo index (HI) - in 11.7% research studies, criteria (RDC/TMD) for TMDs; 23.5%, craniomandibular index (CMI) 58.8%, anamnesis questionnaires 35.2% [4], and Fonseca’s anamnesis index (FAI) [5]. It has been report that prevalence of TMDs ranged from 20% to 50 % [6], but those who seek treatments accounts about 2-7% [7], the most affected age group varied between 20 years to 40 years with female predilection. A higher ratio of women has been seeking treatment (ranging from 3:1 to 9:1) [8-10].
It has been well established, by means of epidemiological studies in which signs and symptoms of TMDs are common in adults of all ages [11]. Reports have shown that signs and symptoms of temporomandibular disorder (TMD) increase with age; however, other studies have shown a decrease in symptoms with increasing age [12]. Over a 20-year period, investigations on TMD have revealed predominately mild signs and symptoms already present in childhood. An increase in symptoms occurs until young adulthood, after which they level out [4,13]. The concept of TMD may be attributable to specific genes that are inheritable. There are evidences to suggest that anxiety, stress, and other emotional disturbances may exacerbate TMDs, especially in patients who clinically experience chronic pain [3,14].
Nevertheless, the cause of the signs and symptoms of TMDs is not clearly understood and various opinions on their etiology have been offered. It is evident from the numerous epidemiologic studies on the occurrence of temporomandibular disorders that signs of temporomandibular disorders appear in about 60% - 70% of the general population and yet only about one in four people with signs are actually aware of or report any symptoms [15]. The frequency of severe disorders that are accompanied by headache and facial pain characterized by urgent need of treatment is 1% - 2% in children, about 5% in adolescents and 5% - 12% in adults [16]. A non-patient population has been reported prevalence rates vary broadly (from 26% to 50%) [17].
The Helkimo index was one of the first to be referred to in the literature as having the reliability to identify signs and symptoms of TMD. Because it has the following advantages including: 1) Its allow to collect a large number of information in a short period of time; 2) it is a low-cost application; 3) Easy for perception-based evaluation ;4) does not influence the appraiser in obtaining answers; 5) a simple self-administered questionnaire would offer the advantage of faster application and, thus, low cost. This makes epidemiological surveys and treatment follow-up by using this index is more feasible. An additional advantage is that a self-applied questionnaire would provide a severity index with less influence from the examiner and less variability in the measures [5,11,17].
Helkimo reported that prevalence of TMD varied from 12% and 57% for anamnestic symptoms and between 28% and 88% for clinical signs [18]. In Asian population, 43% of Taiwanese university students had a prevalence of one or more signs of TMD [19]. About 19% had anamnestic symptoms and that over 36% showed clinical sign in university students have reported by Jagger [20].
A higher prevalence of TMD has been reported among male university students in Riyadh, Saudi Arabia (46.8%) [21]. another study in north Saudi university students showed that prevalence of TMD was higher (94.7%) [22]. Whilst, a low rate of TMD (25.4%) among students was estimated in Arab students at Gulf Medical University Ajman, UAE [23]. Additionally, the prevalence of TMD in Indian university students was (45.16%) and showed women slightly higher than men (36.58% and 31.48% respectively) [2,14]. A higher prevalence of TMD among the university students in Brazil was 57.7% and showed the women higher prevalence than men (68.7% and 48.2%) [24].
Others epidemiological studies estimated the prevalence of TMDs in various communities including: Southern Portugal (25.2 %) [16]. Caucasian population (23.78% in male and 25.32 in female) [25], Syrian (28%) [26].
A higher prevalence rate of TMDs has been documented among university Students in Sudan and Jordan (77.8% and 68.6 % respectively) [15,27].
Up to date, there is no study estimated prevalence rate of the TMDs among Yemeni population. To the best of author’s knowledge, this is the first epidemiological study identifying the rate of signs and symptoms of TMDs among Yemeni university students. Therefore, authors of this study aimed to estimate prevalence rate of TMDs among Yemeni university students, using Helkimo anamnestic index and dysfunction index.
Material and Method
Study design
A cross-sectional, prospective study was conducted in the Department of Oral Medicine and Oral Diagnosis, Faculty of Dentistry, Thamar University, Dhamar, Yemen. An approval for this study was obtained from the scientific ethic committee.
Study sample
The study sample comprised 207 dental students studying at the following institutions: from the Faculty of Dentistry, Thamar University and Faculty of Dentistry, Saba University.
Inclusion criteria
1. Dental students of the Thamar and Saba University
2. Both male and female were including
3. Age ranged from 18 to 26 years with mean age 22.4 age
4. Students signed a consent form agreeing to participate in the study.
Exclusion criteria
1. Students with pervious history of TMDs like: fibromyalgia, trigeminal neuralgia, burning mouth syndrome, atypical facial pain, migraine, atypical odontalgia, cervical, neuropathic pain and those with a history of previous TMD treatment were excluded from the study.
2. Those students who have not signed the consent form.
3. Students with history of systemic, musculoskeletal or neurological disorders.
4. Student with history of orthodontic treatment.
Predictors and outcomes variable
Interview
The subjective symptoms were obtained by asking the students the following questions with adequate explanation as needed which history of emotional stress, maxillofacial surgery, orthodontic treatment and history of trauma by dental work. Then information about related factors were obtained and recorded, which included headache more than twice a week or more, previous trauma to head and neck and oral parafunctions, the subjects was asked if s/he frequently did one or more of the following oral habits (grinding, clenching, nail-, object-, lip-cheek biting, chewing gum, chewing on one side and sleeping on their face.
Age, gender and marital status are the predictor›s variables. The outcomes variables were the signs and symptoms of TMDs, assessing through questionnaire using a Helkimo index and dysfunction of TMJ assessed through clinical examination using a dysfunction index [28].
To assess symptoms of the TMDs using anamnestic Helkimo index (Ai), it was subdividing into three parts namely: First, (Ai0) involves a complete absence of symptoms. Second: (AiI), involves a mild symptom including, one or more of the following symptoms were reported in anamnesis: joint sound, feeling of fatigue and feeling of stiffness of the jaws on awaking. Third, (AiII), involves severe symptoms of dysfunction, one or more of the following symptoms were reported in anamnesis as difficulty in opening the mouth widely, locking, subluxation, pain on movement of the mandible, facial and jaw pain, pain and tiredness on chewing (Table 1).
Clinical Examination
All clinical examinations and assessments for articular and masticatory components of the TMJ was performed by the first author (J S A). A clinical examination was conducted to assess severity of clinical signs TMDs, based on the clinical dysfunction index by Helkimo (Di). The dysfunction index was subdividing into 4 categories (Di0 = free symptoms, DiI = mild dysfunction, DiII = moderate dysfunction and DiIII = sever dysfunction). Each category was obtaining an index value according to clinical examination for the following variables 1) maximal mouth opening; 2) TMJ function as clicking, crepitation, deviation, locking, luxation; 3) Masticatory muscle pain or tenderness; 4) TMJ pain during palpation and mandibular movement (Table 2).
Statistical analysis
Statistical analysis was performed using SPSS v.22.0 (IBM, USA). To Descriptive statistics were performing to all variables in the study. After that, Chi-squared Test of Independence was applied in order to evaluate associations between the occurrence of TMD and gender, age, and severity of the TMDs through Helkimo index.
In order to satisfy the requirements of applicability of Chi-squared Test for Independence, the variable TMD was grouped into absence and presence (included mild, moderate, and severe TMD).
Results
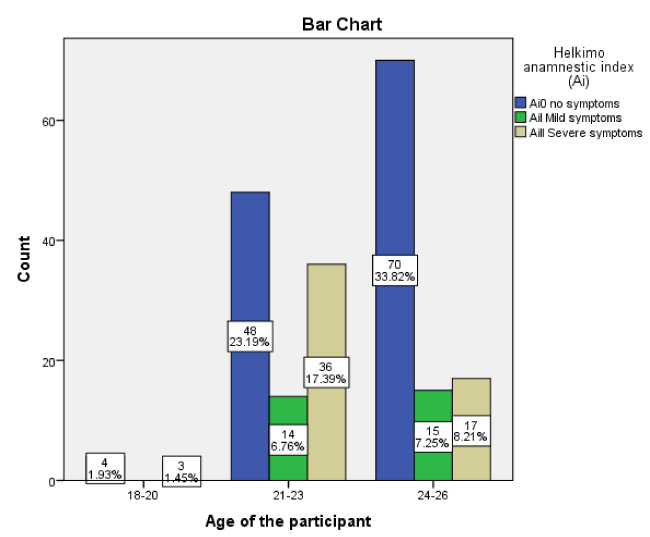
A total of 207 dental students (114, 55.07% was male and 93, 44.93% was female) were enrolled in this a prospective, cross sectional study. All participants were categorized into three groups: 1) 18 to 20 years (include seven participants, 3.38 %); 2) 21 to 23 years (include 98 participants, 47.34 %); 3) 24 to 26 years (include 102 participants, 49.28%).
Prevalence of the symptoms of TMD based on anamnestic index (Ai)
The prevalence of TMD was observed in 41.07% (n = 85) and 58.93 % (n = 122) was free of TMDs, 14.01 % (n = 29) had a mild TMDs and 27.05 % (n = 56) a sever TMDs. (Figure 1).
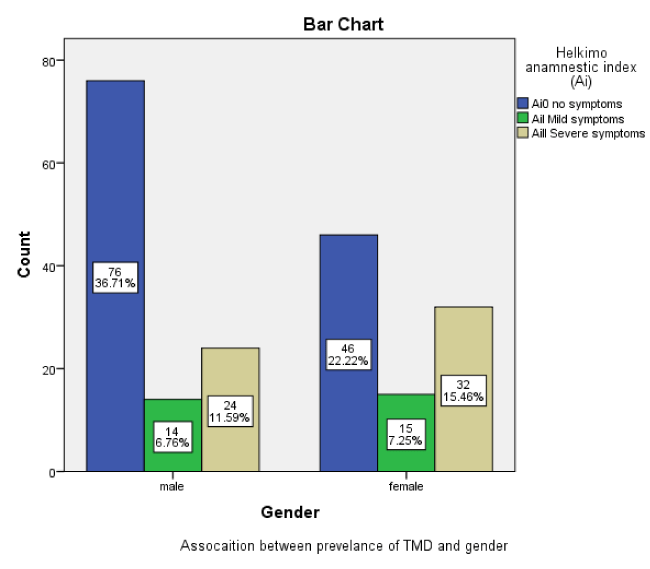
Of 41.07 % (n = 85) whose had TMDs, 18.35% (n = 38) and 22.70 % (n = 47) were male and female respectively. There was a significant association between TMD prevalence and gender (P < 0.005). (Figure 2).
Severity of the TMDs based on clinical dysfunction index (Di)
In respect of clinical dysfunction of TMD, 49.76 % (n = 103), had one or more signs of TMD dysfunction (TMJ pain and clinking, deviation and limited mouth opening, masticatory muscle tenderness). Of 49.76 %, whose had one or more TMDs, 20.77 (n =43) had, mild TMD dysfunction (11.11 % were females and 9.66% were males), 12.08 (n= 25) had a moderate TMD dysfunction (7.73% were females and 4.35 were males) and 16.91 % (n = 35) had a severe TMD dysfunction (10.63% were females and 6.28 % were males). A 50.24% (n= 104) were a free of symptoms. Females (29.45%, n = 61) were had a greater severity of TMD than males (20.29%, n = 42). 7.25%. (Figure 3).
There was significant association between the TMD dysfunction (Di) of TMD and gender (P > 0.005).
Discussion
To the best of author’s knowledge, there has been no study estimated the prevalence and severity of the TMDs among Yemenis population. The objective of present study was to identify prevalence of TMDs among Yemeni university students, using Helkimo anamnestic index and dysfunction index. The main key findings of this study were that, prevalence of TMD was 41.06 % (n = 85). There was a significant association between TMD prevalence and gender (P < 0.005). This is in consistent with others studies [1,15,27,29,30], and inconsistent with others reports (slightly lower than our results) [10,16,24,31,32], In contrast, some studies reported a higher rate of signs and symptoms of TMDs in compared to our finding [22,32-34].
Regarding prevalence of TMDs in male and female population, there was slightly higher prevalence of TMDs in student female (22.70, n = 47), than male gender (18.35%, n = 38). This agrees of majority of previous studies [10,24,35-39], and in disagreement with others reports [14,26,33,40].
The discrepancy between our results and others previous studies may be due to different racial, cultural and economic environments.
Based on anamnestic Helkimo index, a majority of studied sample were suffered of sever TMDs (27.1, n = 56), this is a higher and inconsistent with previous studies [14,41].
The prevalence of clinical dysfunction in our study was 49.876 % (Di0: 50.24 %, DiI: 20.8%, DiII; 12.8 % and DiIII; 16.91%). This is similar to others studies [36,42-46]. There was significant association between the dysfunction of TMD and gender (P > 0.005). This in accordance with other previous studies [43].
Limitations of the present study were: 1) Helkimo index has been used to assess prevalence and severity of TMDs, it does not serve for diagnosis and classify of TMD. The result given through using of this index are limited to the identification of the severity of signs and symptoms of TMD.
Strengths of this study were: 1) both subjective (Ai, anamnestic index) and objective (Di, dysfunction index) have been investigated; 2) study sample was collected from two separated governorates in Yemen. Thamar University is a governorate located at middle of country, while Saba University located in Sanaa (capital of country). Thus, study sample consisted of students coming from all regions in Yemen. Therefore, this is meant that results of this study can be generalized and fulfilled the principal of internal validity of good study.
The results of this study confirm that prevalence of TMD are more frequent among female that male. This is accordance with many studies [14,36,41-48]. The best explanation for that is hormonal factors are believed to account for at least some of the gender difference in prevalence rates. 45 Some studies have assessed the role of hormonal fluctuations in the frequency or intensity of musculoskeletal pains, such as TMD, where episodes tend to be longer than for headache. Dao et al. showed on variability of myofascial pain of TMD over three menstrual cycles in 12 female subjects. Consumers of oral contraceptives tended to show less variable pain intensity levels, and fewer pain-free days than women suffering hormonal fluctuations related to their naturally occurring menstrual cycles; nevertheless, the differences were not statistically significant and a predominant temporal pattern could not be discerned in this small sample [49]. In the normal menstrual cycle, estrogen levels are at their lowest during menses. Estrogen secretion rises gradually during the early part of the follicular phase and then exponentially in the days before ovulation. Ovulation happens about 10–12 h after the LH peak, around Day 14 in the ‘typical’ menstrual cycle. There is a precipitous decrease in estrogen in the days following ovulation and then a gradual increase during the early to mid-luteal phase. Estrogen then drops again during the late luteal phase just prior to menses [50].
As far as, this study assessed prevalence of TMD among of Yemeni population, disc positions were not investigated to identify causes of TMJ clicking. This is because unavailability of magnetic resonance image. Additional disadvantages of this study the external validity because presence of khat chewing habits among Yemeni population will increase in the chance of development of TMD due to increased total number of chewing hours per day. Recently study showed that there was significant difference in prevalence of TMD between khat chewer students and non khat chewer students [51].
The etiology of TMDs has been associated to several factors, namely traumatic injury, immune-mediated systemic disease, neoplastic, emotional stress, occlusal discrepancies, malocclusion or loss of teeth, postural changes, disease of the masticatory musculature and adjacent structures, extrinsic and intrinsic changes of TMJ structure, bruxism , tooth clenching habits, or a combination of such factors [52,53]. Prosthodontic rehabilitation, orthodontic treatment, orthognathic surgery, and mandibular fractures have been associated with TMJ changes and worsening of existing TMD [54]. Loading, altered jaw position, and mechanical stress in response to the aforementioned treatments induce morphological changes in the TMJ, due to its inherent adaptive capacity [14].
Concerning a TMD assessment tool, the research diagnostic criteria for temporomandibular disorders (RDC/TMDs) is universally accepted tool, which have since been used in multiple clinical and epidemiological investigations [55]. Recently [56], a proposed a new refinement and modification version of the RDC/TMDs, known as the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD). They claim that the DC/TMD includes a valid and reliable screening questionnaire, as well as diagnostic algorithms for the most common pain-related TMDs. Despite their advantages, the RDC/ TMD and DC/TMD are quite cumbersome assessment tools in that they require the individual to be present in order to render a TMD diagnosis, and they are difficult to use on large samples. The so called Fonseca’s anamnestic index (FAI) is a self-administered questionnaire for the non-patient population. It has been proposed as a low-cost, easily applied alternative TMD assessment tool and can be used by both general practitioners and epidemiologists [57], in the present study, Helkimo anamnestic index and dysfunction index was applied to assess prevalence of TMD. This is similar to most previous studies.
Emotional and psychological stress is also an important factor predisposing to the TMD development. The studied student population is particularly susceptible to the impact of this factor [58]. Psychological stressors may involve large number of duties, the pressure of getting a well education, an uncertain future, low income, living far away from house, and working in a foreign environment. Additionally, students also face social, emotional, physical, and family problems [59]. This is study did not assess the psychological factors in developing TMD Because the Helkimo index specified on certain points. Base on present literature this percentage could reach 72% [60], or even 90% [61], in student sample. It has been concluded that being under stress increases the activity of the masticatory muscles, which subsequently results in TMD [61].
Conclusion
In conclusion, the results of the present study revealed that among dental university students was 41.07 % of studied sample showed symptoms (Ai) of TMD. Also, this study has shown that 49.76 % of studied sample revealed some degree of dysfunction (Di).
Further investigations with calculation of sample size and using diagnostic research criteria to assess prevalence and severity of TMD among Yemeni population are needed before a final conclusion can be drawn.
- Modi P, Shaikh SS, Munde A (2012) A Cross Sectional Study of Prevalence of Temporomandibular Disorders in University Students. Inter J Scien and Res Pub 2:1-3. Link: https://goo.gl/5MZbp1
- Sakrania H, Ghandhib D, Kamalc AT (2015) Prevalence of signs and symptoms of temporomandibular disorders in different malocclusion groups. POJ 7: 2-7 Link: https://goo.gl/XgJB2v
- Milanez Oliveira FB, Rocha Paula AB (2016) Association between Temporomandibular Dysfunction and Depression in People Living with HIV/AIDS. International Archives of Medicine 9: 393 Link: https://goo.gl/xt879F
- Sena MF, Mesquita KS, Santos FR, Silva FW, Serrano KV (2013) Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr 31: 538-545. Link: https://goo.gl/1Wdbde
- Campos JADB, Gonçalves DAG, Camparis CM, Speciali JG (2009) Reliability of a questionnaire for diagnosing the severity of temporomandibular disorder. Rev Bras Fisioter 13: 38-43. Link: https://goo.gl/Xru6ih
- Graber TM, Rakosi T, Petrovic AG (2009) Functional Analysis—Examination of Temporomandibular Joint and Condylar Movement. In: Dentofacial Orthopedics with Functional Appliances, 2nd Edition, Mosby, St. Louis, 135-140. Link: https://goo.gl/GTYNkJ
- Köhler AA, Hugoson A, Magnusson T (2013) Clinical signs indicative of temporomandibular disorders in adults: time trends and associated Factors. Swedish Dental Journal 37: 1-11. Link: https://goo.gl/4CP4s2
- Guimarães TB, Ferreira-Cabrini MB, Quaglio CL, Guimarães AS, Smith RL, Antunes SV, et al. (2015) Temporomandibular disorder: prevalence among hemophiliac patients. Int. J. Odontostomat. 9: 295- 300. Link: https://goo.gl/7Y8sH9
- Bagis B, Ayaz EA, Turgut S, Durkan R, Özcan M (2012) Gender difference in prevalence of signs and symptoms of temporomandibular joint disorders: a retrospective study on 243 consecutive patients. Int. J Med Sci 9: 539-544. Link: https://goo.gl/WpKG21
- Manfredini D, Chiappe G, Bosco M (2006) Research diagnostic criteria for temporomandibular disorders (RDC/TMD) axis I diagnoses in an Italian patient population. J Oral Rehabil 33: 551-558. Link: https://goo.gl/5R5NWh
- Kalanzi D, Osman YI, Shaikh A (2005) Prevalence of Sign and Symptoms of the Temporomandibular Join Dysfunctions in Subjects with Different Occlusion Using Helkimo Index. Thesis for Degree of Masterscience in Restorative Dentistry, University of Western Cape, Cape Town Link: https://goo.gl/tj7avB
- Hiltunen K, Schmidt-Kaunisaho K, Nevalainen J, Närhi T, Ainamo A (1995) Prevalence of Signs of Temporomandibular Disorders among Elderly Inhabitants of Helsinki, Finland. Acta OdontolScand 53: 20-23. Link: https://goo.gl/Ug2QZc
- Magnusson T, Egermarki I, Carlsson GE (2005) A Prospective Investigation over Two Decades on Signs and Symptoms of Temporomandibular Disorders and Associated Variables. A Final Summary. Acta Odontol Scand 63: 99-109 Link: https://goo.gl/SxF7MT
- Majumder K, Sharma S, JK DR, Siwach V, Arya V, et al. (2015) Prevalence and Sex Distribution of Temporomandibular Disorder and Their Association with Anxiety and Depression in Indian Medical University Students. International Journal of Clinical Medicine 6: 570-578. Link: https://goo.gl/wabLV3
- Ryalat S, Baqain Z, Amin W, Sawair F, Samara O, et al. (2009) Prevalence of Temporomandibular Joint Disorders among Students of the University of Jordan. J Clin Med Res 1:158-164. Link: https://goo.gl/i8jqcj
- Minghelli B, Cardoso I, Porfírio M, Gonçalves R, Cascalheiro S, et al. (2014) Prevalence of Temporomandibular disorder in children and adolescents from public schools in Southern Portugal. North Am J Med Sci 6:126-132. Link: https://goo.gl/Zxt8NT
- Sena MF, Mesquita KS, Santos FR, Silva FW, Serrano KV (2013) Prevalence of temporomandibular dysfunction in children and adolescents. Rev Paul Pediatr 31: 538-545. Link: https://goo.gl/VqPHSW
- Vojdani M, Bahrani F, Ghadiri P (2012) the study of relationship between reported temporomandibular symptoms and clinical dysfunction index among university students in Shiraz. Dent Res J (Isfahan) 9: 221–225. Link: https://goo.gl/j5nUgF
- Lee JY, Kim YK, Kim SG, Yun PY (2013) Evaluation of Korean teenagers with temporomandibular joint disorders. J Korean Assoc Oral Maxillofac Surg 39: 231-237. Link: https://goo.gl/rWe2hX
- Karatas O, Peker K, Balık A, Uysal O, Tuncer EB (2013) Identifying potential predictors of pain–related disability in Turkish patients with chronic temporomandibular disorder pain. J Headache Pain 14: 17. Link: https://goo.gl/mAh87S
- Habib SR, Al Rifaiy MQ, Awan KH, Alsaif A, Alshalan A, et al. (2014) Prevalence and severity of temporomandibular disorders among university students in Riyadh. Saudi Dent J 27: 125–130. Link: https://goo.gl/s671yQ
- Zwiri AM, Al-Omiri MK (2016) Prevalence of temporomandibular joint disorder among North Saudi University students. Cranio. 34: 176-181. Link: https://goo.gl/M6KF3p
- Elyasi M, Majeed S, Elyassi M, Aziz A, Rashid K, et al. (2016) Prevalence of temporomandibular joint disorder among Gulf Medical University students. Gulf Medical Journal 5: S123–S130. Link: https://goo.gl/Dn6wVb
- Cabral RP, Moiolli-Rodrigues ME, Motta FLK, Souza Motta FCK, da Silva JR, et al. (2016) Temporomandibular Disorder in University Students of the Parque das Rosas Campus, Universidade Estácio de Sá That Practice Sports. Health 8: 18-23. Link: https://goo.gl/YeE89t
- Tecco S, Crincoli V, Di Bisceglie B, Saccucci M, Macrĺ M, et al. (2011) Signs and symptoms of temporomandibular joint disorders in Caucasian children and adolescents. Cranio 29: 71–79. Link: https://goo.gl/tx2kA1
- Issa N, Baherly N, Mayhoube M (2015) Prevalence of Symptoms of Temporomandibular Joint Disorder in Lattakia-Syria, 1: 23–28. Link: https://goo.gl/xEwvtb
- Ahmed LI, Abuaffan AH (2016) Prevalence of Temporomandibular Joint Disorders among Sudanese University Students. J Oral Hyg Health 4: 200. Link: https://goo.gl/v5Mwdm
- Toscano P, Defabianis P (2009) Clinical evaluation of temporomandibular disorders in children and adolescents: a review of the literature. Eur J Paediatr Dent 10: 188-192. Link: https://goo.gl/UcPZrc
- Modi P, Shaikh SS, Munde A (2012) A Cross Sectional Study of Prevalence of Temporomandibular Disorders in University Students. International Journal of Scientific and Reserach Publications 2: 9–11. Link: https://goo.gl/zyqZvB
- Gopal SK, SRS, Vardhan BGH (2014) Prevalence of Temporomandibular Joint Disorders in Symptomatic and Asymptomatic Patients : A Cross-Sectional Study 1: 14–20. Link: https://goo.gl/Kex7mE
- Hama AM, Mahmood DK (2016) Development Research Evaluation of Temporomandibular Joint Disorders in partially edentulous patients. International Journal of Development Research 6: 8531-8533.
- Murrieta J, Alvarado E, Valdez M, Orozco L, Meza J, et al. (2016) Prevalence of temporomandibular joint disorders in a Mexican elderly group. Journal Oral of Research 5: 13–18. Link: https://goo.gl/jNMQQK
- Oliveira A, Dias E, Contato R, Berzin F (2006) Prevalence Study of Signs and Symptoms of Temporomandi- bular Disorder in Brazilian College Students. Brazilian Oral Research 20: 3-7. Link: https://goo.gl/x8g9pm
- Tanboga I, Durhan MA, Durmus B, Marks LA (2014) Temporomandibular Disorders in Young People with an Intellectual Disability: Prevalence of Signs and Symptoms. Eur J Paediatr Dent 15: 349-354. Link: https://goo.gl/s5Zrn5
- Vedolin GM, Lobato VV, Conti PC, Lauris JR (2009) the Impact of Stress and Anxiety on the Pressure Pain Threshold of Myofascial Pain Patients. J Oral Rehabil 36: 313-321. Link: https://goo.gl/VF5jgq
- Pedroni CR, Oliveira AS, Guaratini MI (2003) Prevalence Study of Signs and Symptoms of Temporomandi- bular Disorders in University Students. Journal of Oral Rehabilitation 30: 283-289. Link: https://goo.gl/DMhHFP
- Feteih RM (2006) Signs and Symptoms of Temporomandibular Disorders and Oral Parafunctions in Urban Saudi Arabian Adolescents: A Research Report. Head Face Med 2: 25. Link: https://goo.gl/NhkkMu
- Pollard H, Fernandez M (2004) Spinal Musculoskeletal Injuries Associated with Swimming: A Discussion of Technique. Australian Chiropractors and Osteopaths 12: 72-80. Link: https://goo.gl/JyyqZY
- Yuill E, Howitt SD (2009) Temporomandibular Joint: Conservative Care of TMJ Dysfunction in a Competitive Swimmer. J Can Chiropr Assoc 53: 165-172. Link: https://goo.gl/JenRE4
- Bonjardim LR, Lopes-Filho RJ, Amado G, Albuquerque RL, Goncalves SR (2009) Association be- tween Symptoms of Temporomandibular Disorders and Gender, Morphological Occlusion, and Psychological Factors in a Group of University Students. Indian J Dent Res 20: 190-194. Link: https://goo.gl/axw99K
- Iturriaga V, Navarro P, Cantín M, Fuentes R (2012) Prevalence of vertical condilar asymmetry of the temporomandibular joint in patients with signs and symptoms of temporomandibular disorders. Int J Morphol 30: 315-321. Link: https://goo.gl/Mmr53W
- kassa M, bakry A, Salem WS (2015) The Incidence of Temporomandibular Joint Disorders among Dental Students in Aljouf University, KSA. International Invention Journal of Medicine and Medical Sciences 2: 5-11. Link: https://goo.gl/W5rZuy
- Vojdani M, Bahrani F, Ghadiri P (2012) the study of relationship between reported temporomandibular symptoms and clinical dysfunction index among university students in Shiraz. Dent Res J (Isfahan) 9: 221–225. Link: https://goo.gl/eiUkwL
- DeRossi S, Stoopler E, Sollecito T (2004) Temporomandibular Disorders and Migraine Headache: Comorbid Conditions? The Internet Journal of Dental Science. 2: 1. Link: https://goo.gl/6UEi8m
- Kobs G, Bernhardt O, Kocher T, Meyer G (2005) Oral parafunctions and positive clinical examination findings. Stomatologija 7: 81- 83 Link: https://goo.gl/nTEqn2
- Nassif NJ, Al¬Salleeh F, Al¬Admawi M (2003) the prevalence and treatment needs of symptoms and signs of temporomandibular disorders among young adult males. J Oral Rehabil 30: 944–950 Link: https://goo.gl/o1nEvg
- LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF (2003) Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain 106: 253–261. Link: https://goo.gl/cMQoZz
- Koidis PT, Zarif A, Grigoriadou E, Garefs P (1993) Effect of age and sex on craniomandibular disorders. The Journal of Prosthetic Dentistry 69: 93–101. Link: https://goo.gl/549vyS
- Dao TT, Knight K, Ton-That V (1998) Modulation of myofascial pain by the reproductive hormones. J Prosthet Dent 79: 663–70. Link: https://goo.gl/NZeAWc
- Speroff L, Glass RH, Kase NG (1994) Regulation of the menstrual cycle. In: Clinical gynecologic endocrinology and infertility, 5th ed., Baltimore, MD: Williams & Wilkins 1994: 183–220. Link: https://goo.gl/14FgqN
- Al Moaleem MM, Okshah AS, Al-Shahrani AA, Alshadidi AA, Shaabi FI, et al. (2017) Prevalence and Severity of Temporomandibular Disorders among Undergraduate Medical Students in Association with Khat Chewing. J Contemp Dent Pract 18: 23-28. Link: https://goo.gl/HcERcB
- de Santis TO, Motta LJ, Gonzalez DAB, Ferrari RAM, Fernandes KPS, et al. (2014) Accuracy study of the main screening tools for temporomandibular disorder in children and adolescents. J Bodyw Mov Ther 18: 87–91. Link: https://goo.gl/QKH5sn
- Manfredini D, Lobbezoo F (2010) Relationship between bruxism and temporomandibular disorders: a systematic review of literature from 1998 to 2008. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109: e26–e50. Link: https://goo.gl/KwUs4V
- Al-Moraissi EA, Wolford LM, Perez D, Laskin DM, Ellis E (2017) Does Orthognathic Surgery Cause or Cure Temporomandibular Disorders? A Systematic Review and Meta-Analysis. Link: https://goo.gl/6uZorF
- Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord 6: 301–355. Link: https://goo.gl/DXt9Tc
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, et al. (2014) Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the international RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache 28: 6–27. Link: https://goo.gl/wLhHjN
- Da Fonseca DM, Bonfante G, Valle AL, de Freitas SFT (1994) Diagno´ sticopelaanamnese da disfunc¸ a˜ ocraniomandibular. Rev. Gauch de Odontol 4: 23–32.
- Yap AUJ, Dworkin SF, Chua EK, List T, Tan KBC, et al. (2003) Prevalence of temporomandibular disorder subtypes, psychologic distress, and psychosocial dysfunction in Asian patients. Journal of Orofacial Pain 17: 21–28. Link: https://goo.gl/zdwGfB
- Manfredini D, Lobbezoo F (2009) Role of psychosocial factors in the etiology of bruxism. J Orofac Pain 23: 153–166. Link: https://goo.gl/xPuaCU
- Eswi AS, Radi S, Youssri H (2013) Stress/ stressors as perceived by baccalaureate Saudi nursing students. Middle East Journal of Scientifc Research 14: 193–202. Link: https://goo.gl/ZpQtp1
- Shaikh BT, Kahloon A, Kazmi M, Khalid H, Nawaz K, et al. (2004) Students, stress and coping strategies: a case of Pakistani Medical School. Educ Health (Abingdon) 17: 346–353. Link: https://goo.gl/QMjW4L
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
