International Journal of Oral and Craniofacial Science
Autogenous Grafts for Orbital Floor Reconstruction: A review
Harish Saluja1*, shivani sachdeva2, Semmit shah3, Anuj Dadhich4, Parul Tandon5 and Vinayak More5
2Reader, Department of periodontics, India
3Professor & Head Department of oral & maxillofacial surgery, India
4Reader, Department of oral & maxillofacial surgery, India
5Senior lecturer Department of oral & maxillofacial surgery, India
Cite this as
Saluja H, Sachdeva S, Shah S, Dadhich A, Tandon P, et al. (2017) Autogenous Grafts for Orbital Floor Reconstruction: A review. Int J Oral Craniofac Sci 3(2): 046-052. DOI: 10.17352/2455-4634.000031Orbital fractures are relatively common midfacial injuries encountered in urban areas. Patients usually are seen with periorbitaloedema and restricted eye movements with or without changes in vision. A wide range of autogenous materials can be used in the reconstruction of orbital defects including bone grafts, cartilages and fascia each having its own strengths and weaknesses. The purpose of this paper is to provide a systematic literature review on various autogenous materials used for orbital floor reconstruction.
Introduction
Orbital floor reconstruction is usually carried out in patients with defects caused by facial trauma or tumor ablation. Orbital floor fractures are relatively common midfacial injuries encountered in urban areas [1], and were first described by Smith and Regan in 1957 [2]. It can occur as a part of zygomatico-maxillary complex fracture (57.4%) or as an isolated orbital floor fracture-the term coined as ‘blow out fracture’ up to 21.4% [3]. These orbital defects when big in size may hamper the function of the eye, mostly associated with double vision. The goal of surgical repair in orbital floor fractures is two-fold: to reposition herniated orbital fat and tissue back in the orbit; and to reconstruct the traumatic defect [4]. The reconstruction should release herniation of orbital contents, should avoid enophthalmia, diplopia [5], and should prove as a barrier against infection from the antrum [6].
Repair of the orbital floor defect is mandatory if the defect measures at least 50% of the size of the orbital floor bone. Two points are to be kept in mind in case of orbital floor defects, that is identification of cases which needs exploration and reconstruction and the other is selection & placement of appropriate materials for reconstruction. The ideal implant must be nonreactive, provide good structural support, be easily positioned, readily available, biocompatible, noncarcinogenic, and easy to place in position and free of any potential for disease transmission [1,2,4,7]. Reconstruction of the floor will avoid entrapment of fat as well as herniation of orbital fat. Even along with restoration of function; final form, shape and volume restoration of bony orbit will provide acceptable esthetics [8].
Lot of allogenic materials like hydroxyapetite, nylon, marlexmesh, porous polythene are in the market which are widely used for reconstruction of orbital floor, but still autogenous bone consider to be the best reconstructive material. Multiple autogenous bone grafts can be used for orbital floor reconstruction ranging from anterior ileac crest, clavicular graft, calvarial, antral wall, coronoid process, and lateral plate of mandibular ramus, body, lingual cortex and parasymphysis of mandible. Despite, autogenous grafts require a second surgical procedure to harvest, which may increase the morbidity , postoperative discomfort and sometimes loss of function related to donar site5,autologous bone grafts remain the ‘‘gold standard’’ for orbital floor reconstruction [9,10]. Benefits arise from inherent characteristics that promote their use in repairing defects of the orbital floor. They provide rigidity and molding capacity, vascularity, biocompatibility, and minimal immune reactivity [11], Autogenous bone has been the important material of choice for the last 30-40 years for the reconstruction of orbital blow out fracture. Some investigators have advocated the use of rib grafts and tibial grafts [12,13].
The choice of autogenous graft depends on many factors including surgical access, the size of the defect to be repaired, and donar site morbidity and depending on quality and quantity of available bone. While opting for a graft we should also keep in mind the basic principles of to harvest bone from areas you are familiar and contour of graft should fit the defect.
Review
Autogenous bone grafts are considered the reference standard for facial and orbital reconstruction, mainly because of their high biocompatibility and low rate of infection, graft exposure or displacement. The basic objective of reconstruction of orbital defect is to restore orbital volume, function and aesthetics [14,15]. Several study showed that the autogenous bone graft has been the method of choice in the reconstruction of orbital wall defects [16,17]. Although bone grafts have osteogeneic, osteoconductive and osteoinductive capacity, they are susceptible toresorption. Nevertheless, it is believed to be balanced by new bone growth, enabling a good final outcome.
According to the origin of bone, it may be divided into chondral & mesenchymal forms. In the growth of any long bone, chondral bone, is occasioned by cartilaginous growth of the epiphysis, which is gradually replaced by new bone from the diaphysis. Membranous bone is formed by the replacement of a membrane of preexisting condensed mesenchyme. It has been observed that membranous grafts are vascularised earlier than endochndral grafts [18] and a greater graft volume survival with membranousbone as compared to endochondral bone has been observed in the craniofacial region (14-10). Zins & Whitaker demonstrated in a rabbit model that membranous bone grafts revascularised earlier than endochondral bone grafts. Thus membranous bone grafts maintain volume and lead toresorption [19,20].
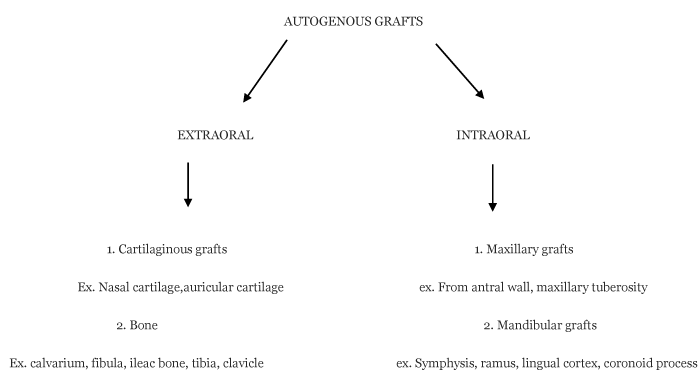
Seeing the availability of autogenous grafts we have broadly classified autogenus grafts for orbital floor reconstruction as-Here we will discuss various autogenous grafts individually which can be used for orbital floor reconstruction.
Anterior ileac crest
In 1908, Mauclaire first described the use of the iliac bone graft for calvarial reconstruction [21]. The ilium can be harvested as cortical, cortico-cancellous, or cancellous bone graft and it provides abundant cancellous bone. Autogenous bone graft from the anterior ileac crest is a favorable reconstruction material because of large quantity of available bone. Sculptured segments can be used for onlay, interposition or construction in craniomaxillofacial surgery. Large amount of coticocancellous bone can be made available from this region which can be used in large orbital floor defects. Iliac corticocancellous grafts are easily incorporated into rigid fixation plates and provide immediate mechanical strength. Cortical bone grafts are primarily useful for fixation-cancellous bone grafts are primarily advantageous for osteoinduction [22].
Iliac bone is of endochondral origin and may exhibit increased resorption rates compared with bone grafts of membranous origin such as calvarium [23]. Kontio [24], advocated harvesting grafts from the iliac bone, which is considered the preferred site owing to the easy moldability , abundance of bone , ability to harvest simultaneously while dissecting down the orbital floor, and low risk of complications Laurie et al. [25], in a combined retrospective and prospective study on iliac donor-site morbidity in 60patients found moderate postoperative pain ranging from 2 weeks to 2 months, with an average of 6 weeks in all patients and 10% had pain after 2 years. No patients had gait disturbances after 1 year.
Iliac grafts have the associated risk of peritonitis, pain, difficulty ambulating, and sensation loss [26]. Severalstudies concluded that with autogenous bone transplants taken from the anterior ileac region resulted in good esthetics and functional restoration. But on the other hand it was associated with fast unpredictable resorbtion because of more amount of cancellous bone. It has been observed that ileac crest undergo 75% resorption with only a thin shell of cortical bone and scant bone remaining. Also it is associated with neurovascular injuries, chronic donar site pain and gait disturbance, Paresthesia from injury to lateral femoral cutaneous nerves and rare occurrence of acetabular fractures. Also harvesting graft from this region requires more surgical skills.
Coronoid process
The coronoid process, coronoid meaning ‘crow’, has been described as one of the bony processes of the ramus of the mandible (Field et al. 1947). Williams et al. (1995), described the coronoid process as a flat triangular process. Triangular coronoid processes have been illustrated by Hamilton (1976), Romanes (1986) Snell (1986), and Basmaijan et al. (1989). Schafer et al. (1890), described the coronoid process as beak shaped [27]. Knowledge of the morphological shapes of thecoronoid process is useful for the maxillofacial surgeon. The coronoid process makes an excellent donor graft site for reconstruction of orbital floor deformities, (Mintz et al. 1998) [28].
Coronoid process is a membranous type of bone which can be removed intra-orally without any functional deficiency and facial disfigurement for reconstruction of orbital floor deformities [29].Coronoid process has been used in orbital floor reconstruction and paranasal augmentation [30]. Coronoid process offers other advantages of no facial scarring, no devitalization of the dentition, ease of access, good medullary bone source , adequate quantity for large orbital floor defects of size upto 27 mm [31]. On the effects of the resection of the coronoid process and detachment of the temporal muscle on mandibular movement and masticatory function need to be considered, Muto and Kanazawa [32], and Choungand Kim [30], noted that there was no problem with mandibular movement, occlusion or the temporo-mandibular joint after resections of the coronoid process and the anterior part of the ascending ramus.
Mandibular body & Ramus
Different areas of mandible have been used successfully in the reconstruction of osseous defects in the oral and maxillofacial region [30,32, 33-37]. According to Laskin and Edwards [36,38], lateral plate of mandibular ramus is very much suitable for orbital floor reconstruction, because of the contour of the donar site approximating the contour of orbital floor and rim. Ina study by Güngörmüs et al. [39], on 16 dry skulls, the dimensions of the bone grafts obtained from the different parts of mandible were evaluated, and it was determined that the average dimensions of the graft material obtained from the anterior part of the ascending ramus were 37.60 mm× 33.17 mm × 22.48 mm × 9.15 mm, and12.23 mm at the thickest part and 35.10 mm & 19.13mm from the mandibular body region.
Li and Schwartz [35], noted that a mean thickness of 3.6 – 4.0 mm of medullary bone was found between the inner surface of the buccal cortex and the mandibular canal in the region of the first and second molars. Mandibular body and ramus are the area which provides a large quantity of autogenous bone for grafting purpose.
Graft from the lingual plate of the mandible [40], has also been used for orbital floor reconstruction. However, it does not seem to be accessible site to harvest the graft in comparision to other sites of mandible.
Mandibular symphyses
Different parts of mandible can be used for orbital floor defect, however symphysis region is more accessible in comparison with other parts of mandible, also there are very less chances of neurovascular injuries. This has been previously shown by Bagatin [41], who reconstructed 6 orbital floor defects with mandibular symphyseal bone grafts. Grafts measuring 2.5 cm by 4cm can be harvested from the mandibular symphysis region. Additionally, the contour of the bone graft conforms perfectly to the orbital floor Graft of this dimension would find applicable in the repair of the majority of orbital floor defects. When bone grafts from the mandible [38,40], have been used for the repair of orbital floor defects, there have been no instances of infection. There should be no objection, therefore to harvest a bone graft from contaminated oral cavity and placing it in the orbital floor.
Montazemetal [42], reported harvesting of the graft, from the mandibular symphysis as difficult procedure, because of concavity of anterior mandible. Intraoral graft from mandibular symphysis region is well known for ridge augmentation and other reconstruction procedures, but its use for orbital floor defects is less popular. But this should be preferred for orbital floor defects because of contour, dimension, local site and slow rate of resorption. The cortical plate of mandible is thickest at the lower border and is maximal as one approaches the midline. Sufficient bone can be harvested at the lower border. The bone from this region can be harvested easily. The inferior border of symphyses region is to be left intact and osteotomy cut can be made above inferior border for harvesting graft, so that chin contour should be left unaffected. The osteotomy lines in the mandibular symphysis region can be made with 5 mm safety margins [39], caudal to the expected position of the mandibular dentition.
The most likely complications in harvesting the anterior part of the ascending ramus, mandibular symphysis and mandibular body are potential injuries to the inferior alveolar neurovascular bundle, tooth roots and mental foramen. We believe that these problems can be minimized if the surgeon has a clear understanding of mandibular anatomy.
In a comparative study by Garg et al. [43], in 2014, 20 patients with orbital floor fractures out of which 10 iliac crest grafts and 10 mandibular bone grafts were placed. In iliac crest group, diplopia got corrected in 6 out of 7 patient (85%), enophthalmos in 4 out of 5 patients (80%) and restricted ocular movement showed 100% correction and enophtalmos got corrected in 5out of 6 patients (83%).No statistically significant difference were found between the 2 groups. On the other hand more time required for the harvest of iliac graft and mandible graft was 30.2+-3.52 min and 16.8+-1.75min respectively.
There is no difference in the ability of mandible and anterior iliac crest bone grafts to correct post traumatic deformities. But he time and ease of harvest of the graft from mandible was comparatively less & easy. Secondly postop morbidity was low and quality and contour of the bone graft was very adaptable for the reconstruction of orbital floor.
Maxillary antral wall
In 1966, Kaye [44], introduced the concept of using bone from the anterior wall of the maxillary antrum. There are distinct advantages to using maxillary antral bone. The bone can be readily harvested because it lies in continuity with the orbital floor defect. This procedure obviates the need for a 2-team approach which is often required for iliac or rib bone graft and therefore decrease operating time. Furthermore, the need for 3-poit fixation and exploration of the maxillary buttress neccesiates an intraoral exposure, therefore there is no additional morbidity when this donor approach is used.
There are no external incisions or scar with the intraoral approach of harvesting the graft. The additional exposure via a gingivobuccal incision, can aid in visualisation and reduction of the prolapsed orbital content and allow the evacuation of blood and debris from the antrum. There are certain limitations to the use of this reconstructive technique however. The quantity of maxillary bone is limited and lacks the bulk that can be provided by other autogenous bone donor sources. Its usefulness may also be limited in cases of severely comminuted fractures or defects larger than 2.5cm and in the unusual cases of hypolastic maxillary antrum [45]. Because of its insufficient thickness, it is no he method of choice for correcting late enophthalmos. The uninvolved contralateral side however may still provide an excellent additional source of bone.
The antral wall has smooth contours like the orbital floor, however, harvesting this site can lead to dysesthesia of the infraorbital nerve, and the graft is essentially only cortical bone [46]. In a study by Lee et al. [45], 41 patients underwent repair of an orbital fractures with maxillary antral bone grafts. The size of the defects ranged from 0.5-2cm.There was no door site complication with respect to cosmetic deformity, infection or rhinosinusitis. In 2caaes persistent enophthalmos resolved after secondary reconstruction with cranial bone grafts. The quality and contour of the bone graft is very adaptable for the reconstruction of orbital floor. Same as with mandibular grafts, no instance of infection is attributable in harvesting maxillary antral graft from contaminated oral cavity. The maxillofacial surgeons should therefore consider using this readily available source of bone when reconstructing orbital floor. According to Hammack et al, this may be because of use of preoperative and postoperative antibiotics as well as the vascularity in the maxillofacial region [47,48].
Calvarial grafts
Marchac [49], and Tessier [50], introduced the use of both full thickness and split thickness calvarial bone grafts for orbital fracture reconstruction. Calvarial bone can be used in different ways: full thickness (bicortical), split thickness (unicortical), bone dusts, bone chips or shavings [51]. Tessier described the parietal bone as having the most appropriate shape for facial application50.Pensler and McCarthy demonstrated the consistent adequate thickness of calvarium as 7.45+-1.03.mm [52].
Calvarium is an ideal donor site for autogenous bone for reconstruction of orbit, nasal region and maxilla and for a limited extent over mandible since it is more resistant to resorption than endochondral bone. Calvarial bone grafts have been favored because of decreased infection and a slower rate of resorption. Calvarial graft represents a notable exception to the usual rule of late remodelling resorption seen in other corticocancellous bone grafts. This is due to diploic vascular system of this bone.
The numerous haversian and volkmann canal network of calvarial bone together with its thinness permit an early revascularization resulting in enhanced survival of osteocompetent cells and osteoblasts. So these grafts show little dimensional change during healing. This graft has been found to retain their original thickness even after 1 year and which had been completely replaced by new bone.
It offers several advantages. The scar is well hidden in the hair-bearing cal, and the skull offers a large harvest site for grafts of varying geometric proportions. When compared with the ilium or rib, cranial bone is one of the most dimensionally stable graft materials. The cranial bone graft can be shaped in such a way that the concave prominence can be placed into the orbital wall defect.
Eran Zunz et al. [53], found that calvarial bone was easier to graft, manipulate, mold and fit to the defect in the orbital floor. Kim et al. [54], presented a series of 82 orbital fractures reconstructed with calvarial bone grafts. No complications, such as graft extrusion, infection, significant eyelid malpositioning, ectropion, or a noticeable scar developed. They contemplated that the orbital bones are shaped to absorb the effect of the trauma and to fracture such that they protect the globe, this might be another argument to support the use of autologous bone grafts and not alloplastic materials and thus preserve the protecting mechanism, even if another post -reconstruction trauma occurs.
Series of 25 reconstructions that compared calvarial and iliac bone grafts, by Siddique and Mathog [55], failed to demonstrate a significant difference between the two types of bone grafts and stated that both were good for orbital reconstruction in their series [1]. They stated that calvarial bone undergoes less resorption than endochondral (iliac crest) bone and might possibly cause less residual enophthalmus. No complications were noted at the calvarial and mandibular graft harvest site. Zheng et al. [56], evaluated the effects of autogenous calvarial bone grafts on treatment of patients with defect of orbital floor from facial trauma.34 patients with orbital floor fracture were reconstructed by calvarial bone grafts. These grafts produces less donor site morbidity compared with other sites, non-visible scar as the incision is placed within the hair-bearing skin and the conjunctiva.
In a retrospective study of Iiankovan et al. [51], 222 patients were treated by using calvarial bone graft for orbital floor reconstruction out of which 13 developed complications of dural tears. All patients with dural tears were children in whom full thickness bone grafts were harvested. Kline and Wolfe reported his experience of 1000 patients with split-calvarial bone grafting. No patients developed neurologic damage [57].
A big disadvantage, however, is limited amount of available bone &the limited malleability of the calvarial bone, which makes restoration of correct anatomic situation of the orbit difficult&bone may easily fracture. Precise thicken of the calvarial graft is difficult to achieve when treating enophthalmos. Postop evaluation of the donor sites has revealed diminished strength upto50% in the area of calvarial bone graft harvest. Also harvesting graft from this region requires good surgical skills to prevent violation of dura and intracerebral haematoma. General risks for donor site include infection, hematoma and injury to healthy tissue, bony defect, additional scar, loss of hair growth, and possible need for drains [57], dural tears, subarachnoid hemorrhage, intracranial hematomas, and neurologic deficits [58]. Furthermore, harvesting autologous bone increases operative time. Counterpart to bone’s intrinsic strength is its difficulty in contouring to desired shape and size. This obstacle is harvest site dependent [59]. Certain donor sites including mandibular symphysis and calvarium are better molded [60].
Grafts from other parts of the body other than facial bones can also be used for orbital floor reconstruction, however the procedure will be too invasive for the patient.
Cartilage grafts
The predominant sources for cartilaginous grafting are auricular concha and nasal septum [61,62]. Characteristics of cartilage include a low anaerobic metabolism and relative vascularity. This combination allows cartilage grafts to survive with a minimal requirement for oxygen perfusion, thereby improving graft viability and reducing resorption rates compared with bone grafts [63]. Autologous cartilage grafts have a favorable application in orbital floor reconstruction owing to ease of access, malleability, and reliable support without evidence of resorption [64].
Auricular cartilages can be very well used for orbital floor bow out defects because of suitable size and easy harvesting. Conchal cartilage graft was used to span small orbital floor defects up to 2x2mm26.The use of auricular cartilage has wide application for small orbital floor defects. The conchal grafts are easy to harvest. It provides an optimal support function for the globe with minimum donor site morbidity. A graft of adequate size ensures adequate stability as well it provides adequate support to the orbital contents and literature suggests that this graft has very minimal donar site morbidity. Because the harvested graft is cartilage so it requires minimal remodelling. This graft is suitable for small size defects. Simplicity and speed of grafting is another point which favour its use as a graft material for floor reconstructions. Because it is near the field of repair, harvest can be performed without change in patient position.
Compared with nasal septum, auricular cartilage is anatomically better suited. This is secondary to its natural curve. It allows an improved inset in the inferior orbit. Although nasal septal grafts have a completely hidden scar, donor-site scars for auricular cartilage can be hidden with the posterior approach [65]. It advantages over other autogenous grafts include having a shape similar to the orbital floor, ease of harvest, malleability and limited morbidity at the donor site [3].
Conclusion
In determining the use of a particular reconstruction material, often the surgeons experience & comfort lays a major role. Although autogenous grafts require a second surgical procedure which increases donor site morbidity, however they have been appropriate material for repairing orbital defects.
- Shere JL, Boole JR, Holtel MR, Amoroso PJ. (2004) An analysis of 3599 midfacial and 1141 orbitalblowout fractures among 4426 United States Army Soldiers 1980-2000. Otolaryngol Head Neck Surg 130:164-170. Link: https://goo.gl/ThnvAX
- Kwon JH, Kim JG, Moon JH, Cho JH (2008) Clinical analysis of surgical approaches for orbital floor fractures. Arch Facial Plast Surg 10: 21-24 Link: https://goo.gl/1uA3Sg
- Seberer M, Sullivan WG, Smith DJ Jr, Phillips LG, Robson MC (1989) an analysis of 1423 Facial fractures in 788 patients at an urban trauma center. JTrauma 29: 388-390. Link: https://goo.gl/AZtC6W
- Castellani A, Negrini S, Zanetti U (2002) Treatment of orbital floor blowout fractures with conchal auricular cartilage graft: A report on 14 cases. J Oral Maxillofac Surg 60: 1413-1417. Link: https://goo.gl/UKBfR9
- Kontio R, Suuronen R, Konttinen YT, Halli D, Kainen C, et al. (2004) Orbital floor reconstruction with poly – L/D – lactide implants: Clinical, Radiological and immunohistochemical study in sheep. Int J Oral Maxillo fac surg 33: 361-368. Link: https://goo.gl/c5Z8rx
- Baumann, Burggasser G, Gouss N, Ewers R (2002) Orbital floor reconstruction with an Alloplastic resorbable polydioxanone sheet. Int J Oral MaxillofacSurg 31: 367-373. Link: https://goo.gl/r71jxC
- Kellman RM. Chapter 26: Maxillofacial Trauma in Cummings CW, Flint PW, Harker LA, HaugheyBH, Richardson MA, Robbins KT, Schuller DE, Thomas, JR, eds. Cummings Otolaryngology Head and Neck Surgery, 4th ed. Vol 4. Philadelphia (PA): Elsevier-Mosby, 2005. p. 602-36. Link: https://goo.gl/ycrE8d
- Rowe and William’s Maxillofacial injuries, second edition; volume 1; page number 553 Link: https://goo.gl/1DpUjN
- Vejayan Krishnan, Johnson JV (1997) Orbital floor Reconstruction with autogenous Mandibular symphyseal bone grafts. J Oral Maxillofac Surg 55: 327-330. Link: https://goo.gl/SSzaX6
- Schlickewei W, Schlickewei C (2007) the use of bone substitutes in thetreatment of bone defectsVthe clinical view and history. MacromolSymp 253: 10-23. Link: https://goo.gl/KwMyFV
- Chowdhury K, Krause GE (1998) Selection of materials for orbital floorreconstruction. Arch Otolaryngol Head Neck Surg 124:1398-1401. Link: https://goo.gl/a89QSJ
- Ronoevic R, Malinger B (1981) Experience with various procedures in the treatment of orbital floor fractures. J Maxillofac Surg 9: 81-84. Link: https://goo.gl/nSi6Bu
- Constantian MB (1982) Use of auricular cartilage in orbital floor reconstruction. Plast Reconstr Surg 69: 951-954. Link: https://goo.gl/Rd4KrE
- Rowe NL, Williams JL (1994) Fractures of zygomatic complex andorbit. Rowe and William’s Maxillofacial injuries 1:475-590. Churchill Livingstone.
- Courtney DJ, Thomas S (2000) Isolated orbital blow out fractures: Survey and review. Br J of Oral and Maxillofac Surg 38: 496-502. Link: https://goo.gl/Yn2PAu
- Ellis E 3rd, Tan Y (2003) Assessment of internal orbital reconstruction for pureblowout fractures: cranial bone grafts versus titanium mesh. J Oral Maxillofac Surg 61:442-453. Link: https://goo.gl/UTnZy5
- Siddique SA, Mathog RH (2002) A comparison of parietal and iliac crest bonegrafts for orbital reconstruction. J Oral Maxillofacial Surg 60:44-50. Link: https://goo.gl/yrKLWo
- Kusiakj f, Zins JE, WhitakerI A (1985) the early revascularization of membranous bone. Plast Reconstr Surg 76: 510-516. Link: https://goo.gl/d9Zq3d
- ZinsJ E, WhitakerI A (1983) Membranous versus enochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg 72: 778-785. Link: https://goo.gl/K2ScZ7
- Zins JE, Whitaker LA (1983) Membranous versus endochondral bones: implication for craniofacial reconstruction. Plast Reconstr Surg 72: 778-783. Link: https://goo.gl/DFb4R4
- Mauclaire H. (1908) Brechecraniennerestaureepar la prosthese metallique. Comments on article by Rouvillouis. Bull MemSocChir Paris 34: 232
- Butsele Van BLI, Mommaerts MY, Neyt LF, Clercq DE CA, Abeloos JV, et al. (1994) The use of skull bone in maxillofacial reconstruction and its potential use in orthopedic surgery. Acta Orthopaedic Belgica 60: 1. Link: https://goo.gl/ybZfCs
- Zins JE, Whitaker LA (1983) Membranous versus endochondral bone: implications for craniofacial reconstruction. Plast Reconstr Surg 72: 778-785. Link: https://goo.gl/rWsVLv
- Al-Sukhun J, Lindqvist C (2006) Christian Lindqvist “a comparative study of 2 implants used to repair inferior orbital wallbony defects : autogenous bone graft versus bioresorbable poly-l/dl-lactide plate” J Oral Maxillofac Surg 64:1038-1048. Link: https://goo.gl/rjpUvw
- Laurie SW, Kaban LB, Mulliken JB, Murray JE (1984) Donor-site morbidity after harvesting rib and iliac bone. Plast Reconstr Surg 73: 933-938. Link: https://goo.gl/rD6Ww8
- Talesh KT, Babaee S, Vahdati SA, Tabeshfar Sh (2009) Effectiveness of a nasoseptal cartilaginous graft for repairing traumatic fractures of the inferior orbitalwall. Br J Oral MaxillofacSurg 47:10-13 Link: https://goo.gl/9RhMU8
- Isaac B, Holla SJ (2001) Variations In The Shape Of The Coronoid Process In The AdultHuman Mandible. J Anat Soc India 50: 137-139. Link: https://goo.gl/8eHFYf
- Mintz SM, Ettinger A, Schmake T, Gleason MJ (1998) Contralateral coronoid process bone grafts for orbitalfloor reconstruction : an anatomic and clinical study. J Oral Maxillofac Surg 56: 1140-1145. Link: https://goo.gl/6MYfuJ
- Pradhan S, Bara DP, Patra S, Nayak S, Mohapatra C (2014) Anatomical Study of Various Shapes of Mandibular Coronoid Process in Relation to Gender & Age. Journal of Dental and Medical Sciences 13: 9-14. Link: https://goo.gl/LWaudf
- Choung PH, Kim SG (2001) the coronoid process forparanasal augmentation in the correction ofmidfacial concavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91: 28-33. Link: https://goo.gl/2bWbBS
- Mintz MS, Ettinger A, Schmakel T, Gleason MJ (1998) Contralateral coronoid process bone graft for orbital floor reconstruction:An anatomic and clinical study. J Oral Maxillofac Surg 56:1140-1145. Link: https://goo.gl/ao4WLg
- Muto T, Kanazawa M (1997) Mandibular reconstruction using the anterior part of ascending ramus: report of two cases. J Oral Maxillofac Surg 55: 1152-1156. Link: https://goo.gl/dULGM8
- Sindet-Pedersen S, Enemark H (1988) Mandibularbone grafts for reconstruction of alveolar clefts. J Oral Maxillofac Surg 46: 533- 537. Link: https://goo.gl/kVsCLM
- Jensen J, Sindet-Pedersen S (1991) Autogenous mandibular bone grafts and osseointegrated implants for reconstruction of the severely atrophied maxilla: a preliminary report. J Oral Maxillofac Surg 49: 1277 – 1287. Link: https://goo.gl/B3b6Tb
- Li KK, Schwartz HC (1996) Mandibular body bone infacial plastic and reconstructive surgery. Laryngoscope 106: 504-506. Link: https://goo.gl/GDuCDK
- Laskin JL, Edwards DM (1977) immediate reconstruction of an orbital complex fracturewith autogenous mandibular bone. J Oral Surg 35: 749-751. Link: https://goo.gl/HV7pZF
- So LL, Lui WW (1996) Alternative donor site foralveolar bone grafting in adults with cleft lipand palate. Angle Orthod 66: 9-15. Link: https://goo.gl/W1jECZ
- Kaye BL (1966) Orbital floor repair with antral wall bone grafts. Plast Reconstr Surg 37: 62-65. Link: https://goo.gl/rZJUD6
- Güngörmüş M, Yilmaz AB, Ertaş U, Akgül HM, Yavuz MS, Harorli A, et al. (2002) Evaluation of the Mandible as anAlternative Autogenous Bone Source for Oral and Maxillofacial Reconstruction. J Int Med Res 30: 260-264. Link: https://goo.gl/r5SbLL
- Roocevic R, Malinger B (1981) Experience with various procedures in the treatment of orbital floor fractures. J Maxillofac Surg 9: 81-84. Link: https://goo.gl/wxfnWt
- Hagatin M (1987) Reconstruction of orbital defects with autogenous bone from mandibular symphysis. J Cranio maxillofac Surg 15: 103-105. Link: https://goo.gl/LQxKXS
- Montazem A, Valauri DV, St-Hilaire H, Buchbinder D (2000) the mandibular symphysis as a donor site in maxillofacial bone grafting: a quantitative anatomic study. J Oral MaxillofacSurg 58:1368-1371. Link: https://goo.gl/yzhPfV
- Garg V, Giraddi GB, Roy S (2014) Comparison of efficacy of mandible and iliac bone as autogenous bone graft for orbital floor reconstruction. J maxillofac oral surg 14: 291-298. Link: https://goo.gl/LC261P
- Kaye B (1966) Orbital floor repair with antral wall bone grafts. Plast Recontr Surg 37: 62-65. Link: https://goo.gl/3uKLfU
- Lee HH, Alcaraz N, Reino A, Lewson W (1998) Reconstruction of orbital floor fractures with maxillary bone. Acta Otolaryngol Head Neck Surg 124: 56-59. Link: https://goo.gl/8aStKQ
- Hardesty RA, March JL (1987) A comparison of bone graft donor sites.The skull versus the iliac crest.Is there really a difference? Surg Forum x: 118-120. Link: https://goo.gl/ZnJHY3
- Lew Daniel (1997) Orbital floor reconstruction with autogenous mandibular symphyseal bone grafts. J oral maxillofac surg 55: 330-332. Link: https://goo.gl/wKAKpV
- Hammack BL, Enneking WF (1960) Comparative vascularization of autogenous& homogenous bone transplants. J Bone Joint Surg Am 42:811-817. Link: https://goo.gl/UV2N3t
- Marchac D (1978) Radical forehead remodeling for craniostenosis. Plast Reconstr Surg 61:823-835. Link: https://goo.gl/YhTMyZ
- Tessier P (1982) Autogenous bone grafts taken from the calvarium for facialand cranial applications. Clin Plast Surg 9: 531-538 Link: https://goo.gl/vZ6GdL
- Ilankovan VT, Jackson IT (1992) Experience in the use of calvarial bone grafts in orbital reconstruction. Br J Oral Maxillofac Surg 30: 92-96 Link: https://goo.gl/mYLuki
- Pensler J, McCarthy JG (1985)The calvarial donor site: an anatomic study in cadavers. Plast Reconstr Surg 75: 648-651 Link: https://goo.gl/59XUWh
- Zunz E, Blanc O, Leibovitch I, (2012) “Traumatic Orbital Floor Fractures: Repair with Autogenous Bone Grafts in a Tertiary Trauma Centre.” J Oral Maxillofac Surg 70: 584-592. Link: https://goo.gl/LP5zYB
- Kim DW, Choi SR, Park SH, Koo SH (2009) Versatile use of extended transconjunctival approach for orbital reconstruction. Ann Plast Surg 62: 374-380. Link: https://goo.gl/PYWZ17
- Siddique SA, Mathog RH (2002) A Comparison of parietal and iliac crest bone grafts for orbital reconstruction. J Oral Maxillofac Surg 60: 44-50. Link: https://goo.gl/obw7g7
- Zhu Z, Stevens MR, Wu H (2001) Use of autogenous cranial bone grafts for orbital floor reconstruction. Zhonghua Zheng Xing Wai Ke Za Zhi 17: 294-296. Link: https://goo.gl/zWqjHa
- Kline RM Jr, Wolfe SA (1995) Complications associated with the harvesting of cranial bone grafts. Plast Reconstr Surg 95: 5-13. Link: https://goo.gl/YuTJv4
- Baino F (2011) Biomaterials and implants for orbital floor repair. Acta Biomater 7: 3248-3266. Link: https://goo.gl/bpBiRU
- Young VL, Schuster RH, Harris LW (1990) Intracerebral hematoma complicating split calvarial bone-graft harvesting. PlastReconstrSurg 86: 763-765. Link: https://goo.gl/pfZKWB
- Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455-498. Link: https://goo.gl/WGaqbV
- Avashia YJ, Sastry A, Fan KL, Mir HS, Thaller SR (2012) Materials Used for Reconstruction After Orbital Floor Fracture. J Cranio Surg 23: 1991-1997. Link: https://goo.gl/R9tVCp
- Kraus M, Gatot A, Fliss DM (2001) Repair of traumatic inferior orbital wall defects with nasoseptal cartilage. J Oral Maxillofac Surg 59: 1397-1400. Link: https://goo.gl/iENvBu
- Laskin DM, Sarnat BG. (1953) the metabolism of fresh, transplanted, and preserved cartilage. Surg Gynecol Obstet 96: 493-499. Link: https://goo.gl/N7XHm1
- Castellani A, Negrini S, Zanetti U (2002) Treatment of orbital floor blowout fractures with conchal auricular cartilage graft: a report on 14 cases. J Oral Maxillofac Surg 60:1413-1417. Link: https://goo.gl/Q371zj
- Kruschewsky Lde S, Novais T, Daltro C, Castelo Branco B, Lessa M, et al. (2011) Fractured orbital wall reconstruction with an auricular cartilage graft or absorbable polyacid copolymer. J Craniofac Surg 22: 1256-1259. Link: https://goo.gl/qCGeQT
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
