International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Therapeutic applications of nanozymes and their role in cardiovascular disease
Naima Nashat1 and Zeshan Haider2*
2Centre of Agricultural Biochemistry and Biotechnology (CABB), University of Agriculture Faisalabad, Faisalabad, Pakistan
Cite this as
Nashat N, Haider Z (2021) Therapeutic applications of nanozymes and their role in cardiovascular disease. Int J Nanomater Nanotechnol Nanomed 7(1): 009-018. DOI: 10.17352/2455-3492.000039Enzyme mimetic is an increasing pull to overcome the limitations on natural enzymes. Enzyme mimicking is done with the help of nanozymes. Nanozymes contain nonmaterial which have enzyme-like activity; this similar activity attracts the researchers. Nanozymes studies showed that they are exceptional in many aspects, such as robustness to a harsh environment, long term storage, easy mass production, and high stability. In the field of biomedical technology, nanozymes have exhibited advantages to diagnose and treat disease. Nanozymes used to treat many therapeutics affect antiinflammatory, anti-ageing effects, neuroprotection, cytoprotection, dental biofilms, antioxidation, and antithrombotic effects. However, to broaden the applications of nanozymes, it is synergistically important to combine the activity of enzymes with properties of nanoscale such as optics, magnetic, mechanics, and electrics. Many types of enzymes have been reported, and their applications in Environmental remediation, therapeutics, and biosensor development. Compare with natural enzymes, current research on applications of nanozymes is tranquil somewhat limited. This uncertain issue will be the next borderline for promotes applications.
Introduction
Enzymes use as a catalyst in biology that converts substrates into products in any biochemical reaction which needs a catalyst. The first enzyme, urease, was said to be a protein in 1926, determined by James B. Since, enzymes well treated to be proteins that allow the enzymes to accomplish high activity as a catalyst. So, protein activity is missed when its exposure occurs at high temperature and high pH. Proteins are also affected by proteases present in the environment moreover natural enzymes have a high cost due to these practical applications of enzymes retard in industry [1].
However, enzyme mimetic is an increasing pull to overcome the limitations; enzyme mimicking is done with the help of nanozymes [2]. Nanozymes contain nonmaterial which have enzyme-like activity; this similar activity attracts the researchers. Nanozymes studies showed that they are extraordinary in many aspects, such as robustness to a harsh environment, long term storage, easy mass production, and high stability [3].
Nanozymes have different structures or sizes because nanomaterials are hard due to a porphyritic nucleus, and proteins are soft [4]. So the comparison with natural enzymes nanozymes is more stable because the nanomaterials they contain, are inorganic [2]. Nanozymes have inherent properties, and unique catalytic activities due to this nanozymes have great attention towards mimetic the natural enzymes until now 50 kinds of nanoparticles have been found. Catalytic activities of enzymes are Catalase (CAT), Peroxidase (POD), glucose oxidase [5], Superoxide Dismutase (SOD). Nanozymes are mainly divided into carbon-based, noble metal nanozymes, metal oxide nanozymes [1].
Applications of magnetic nanoparticles include cell separation, nucleic acid separation, wastewater treatment, biomedical separation, magnetic target drug delivery, catalysis [4]. However, to broaden the applications of nanozymes, it is synergistically important to combine the activity of enzymes with properties of nanoscale such as optics, magnetic, mechanics, and electrics [2]. Many types of enzymes have been reported, and their applications in Environmental remediation, therapeutics, and biosensor development [6].
The many latest developments in the field of nanotechnology have broadened the approach to investigate the novel enzymes. The possible catalytic mechanism for nanozymes has unravelled when combined with computer theoretical calculations, simulation. These studies improve the nanozymes catalytic activities. In biomedical technology, nanozymes have exhibited advantages in diagnosing and treating disease [7].
Therapeutic application of enzymes
Due to eliminating reactive nitrogen species (RNS) or reactive oxygen species (Mulder, Fayad, & biology) [8], nanozymes have been used for latent therapeutic [9]. Some interesting enzyme mimetic applications include CeO2 as Superoxide Dismutase (SOD) [10]. Their SOD mimicking activities mainly describe ROS effects, which converts the superoxide in H2O2 [3]. This section is based on the nanozymes therapeutic effect (Table 1) antiinflammatory, anti-ageing effects, neuroprotection, cytoprotection, dental biofilms, antioxidation, antithrombotic effects, etc.
Antiinflammatory effects
Inflammation plays a role in diabetes, physiology of arthritis, irritable bowel syndrome, heart disease, Alzheimer's disease, illnesses, and Parkinson's disease. The major constituent of the yellow spice turmeric, curcumin derived from the Curcuma longa. It is non-toxic and safe and has antiinflammatory properties [11]. A high level of nitric oxide [12] abnormally contributes to chronic inflammation and immunological disorder resulting in organ damage. Cerium oxide nanoceria has characteristics used in nanotherapeutics and decreases the moderator chronic inflammation [13]. Nanoceria can scavenge the reactive oxygen species [8] or free radicals and inhibit the mediators of inflammation from protecting the cell against the inflammatory diseases [4].
Catalase, glutathione peroxidase, and superoxide dismutase 1 (SOD1) are potent scavengers of ROS, in eye diseases, these enzymes are very effective because eye disease is related to oxidative stress. However, With the help of sodium hydroxide, acute corneal inflammation in animals is treated. Eye disease was treated by contingent ordination of eye drops; these drops contain many problems, so due to this reveal that nanoparticles improve the efficacy by drug carrier system or conquered the corneal diffusion barriers [14].
Antioxidant effects
ROS such as H2O2, O2•-, and •OH, are the by-products of metabolism. They can serve as second messengers at a low level and take part in many signalling processes [15]. Moreover, when the reactive oxide species are over-expressed, they lead to many unfavourable reactions; for example, over-expression of ROS produces oxidative damage to DNA, lipids, and proteins. They also induce cell apoptosis and many other diseases like cancers, atherosclerosis, kidney diseases, arthritis, and neurodegeneration [16]. Antioxidants protect the tissues from damages compel by the free radicals; It also improves the many other damaged tissues. Antioxidants are catalase, glutathione reductase, glutathione peroxidase, peroxiredoxins, and superoxide dismutase. Biological antioxidant required some elements which have no antioxidant activity but needed for the proper functioning of biological antioxidant; these include iron, selenium, manganese, zinc, and copper( [17]. The V2O5 has glutathione peroxidase (GPx) like antioxidant activity; it protects the cell from oxidative stress [18].
Superoxide Dismutase (SOD) enzyme is needed for antioxidants' defences, maintaining the level of O2- [4]. According to some new research, the Hollow PB nanozymes (HPBZs) contain multienzyme-like properties such as neuroprotection, and they scavenge the ROS( [19] (Figure 1). The over-production of ROS effect biomolecules leads to a wide range of human diseases, and induce tissue injury. Human skin which acts as a protective layer between the external environment and body is prone to pro-oxidant agents. ROS's over-production causes inflammatory and allergic skin disease, for example, psoriasis, urticaria, and atopic dermatitis [20].
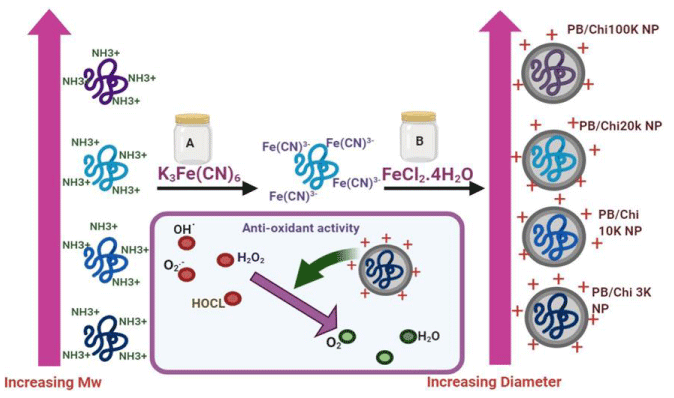
So, the Prussian blue nanoparticles have been reported about their antioxidant activity. The PB nanoparticles (PB NPS) a mixed-valence of iron cyanide containing hexacyanoferrate [Fe(CN)6]2+ complex and Fe3+ cations [21]. Due to their instability in biological buffers in vivo applications of PB nanoparticles are limited; the stability of these particles has been improved by coating with poly(vinylpyrrolidone), poly(diallyl dimethylammonium chloride), and polyethene glycol, from all these the chitosan, is best due to its excellent biocompatibility, good stabilization, biosafety, and biodegradability.
Hyperion Oh, et al. developed PB/Chi nanoparticles using many chitosan samples ranging from 3 to 100 kDa to improve the antioxidant activity and stability PB nanoparticles (Figure 2). Chitosan is a polysaccharide that is a permeation enhancer and mucoadhesive that facilitates NP retention in the administration [22]. The improvement in stability was examined by monitor change in the diameter of the nanoparticles and appearance. The antioxidant activities were examined by deoxyribose assays and 2,2-diphenyl 1-1-picrylhydrazyl(DPPH) [23].
Neuroprotection
Stroke is the third headmost cause of death which is also called cerebral ischemia. Bioenergetics failure occurs due to the reduction of oxygen and glucose delivery to the brain during a stroke. These conditions lead to stress, blood-brain barriers, and ultimately cell death occurs. Cerium oxide nanoparticles have the potential to treat the stroke. Cerium is a metal that belongs to the lanthanide series of the periodic table. When oxygen is combined with the cerium in a formulation of nanoparticles, they have also found antioxidant properties [24]. Cell line HT22 is the most well stood clonal cell system for analyzing the oxidative stress. The natural pro-oxidant for HT22 cells is the glutamate amino acid when glutamate is present, cysteine consumption is blocked, and oxytocic occurs. Reduction of C4+ to C3+ and loss of oxygen is retained by creating an oxygen vacancy. This is done with the help of ceria nanoparticles, and due to these reactions, catalytic application possible [25].
Antithrombosis effect
Bacterial infection and thrombus formation are two major harms. These harms lead to complications in biomedical devices such as heart valves, catheters, and vascular grafts. To develop the antimicrobial and antithrombotic is important to sustain the function of these biomedical devices [26]. Colloidal Mesoporous Silica (CMS) nanoparticles have engrossed enormous consideration as possible injectable drug delivery. In addition to their high Biodegradability and biocompatibility, CMS can selectively customize at their outer and inner surfaces to encapsulate molecules powerfully. They can be specifically functionalized on their outer surface [27]. The delivery of this multiple function CMS in the bloodstream prevents the thrombogenic effects. The coating of ethylene glycol addresses hemolytic behaviour and their fast degradation prevention. Moreover, the additional efforts are critical to delivering these manifold core-shell CMS to the blood and assurance sufficient circulation time to prevent the thrombotic effect. Heparin, anionic polysaccharide and highly sulfated, is best due to its anticoagulant property. Moreover, Heparin is conventionally injected intravenously or subcutaneously [28].
Heparin in free form involves an increased risk of bleeding due to these risks. Many other side effects occur. The example of this situation is this Heparin-induced thrombopenia even as diffusing all over the blood circulation system. Tissue targeted nanoceria the CMS with Heparin must be harmless and injectable into the bloodstream. Heparin and nanoceria prevent the clotting cascade's establishment only in the abrupt vicinity of nanocarriers and have no side effects [29].
Nitric oxide [12] is predictable as an antiplatelet agent. Nitric oxide [12] can prevent thrombus formation. Due to its ability to increase the cyclic Guanosine Monophosphate (cGMP) levels within the platelets, Nitric oxide [12] showed the antiplatelet agents. So, lowering the intracellular Ca2+ level required for the common passageway in the coagulation cascade, NO drug agents' release to accomplish the antiplatelet activity in vivo. These in vivo studies attract to mimic the natural role of NO release from the endothelial cells. Endothelial cells are healthy blood vessels[30].
NO was arising from the oxidation of L arginine and catalyzed by the enzyme nitric oxide synthase. Moreover, these enzymes use O2 as an oxidant and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) as a cofactor. When the cofactor supplies and L arginine concentration are inadequate, the NO reduced form nitroxyl (HNO) produced [30]. Analogous to NO the HNO has an anti-thrombogenic effect. The NO generating catalysts and non-releasing materials have some problems, HNO is the possible solution.
However, there is no report on the local production of HNO efficiently prevent thrombus formation. Some reports showed Graphene hemin glucose oxidase's conjugate use as a catalyst to generate HNO from L arginine and physiologically abundant glucose. These catalysts create a long-lasting antithrombotic covering [31].
Nanozymes role in cytoprotection
Platinum is used as an industrial catalyst in an extensive range. Some older studies about Platinum reported that the Pt NPs serve as both SOD mimics to scavenge H2O2, CAT, and O2•- [32]. Conventional methods also used poly (acrylic acid) (PAA) in the research process to improve their biocompatibility. To in situ synthesize Pt nanostructures; Nie, et al. used nanocarrier apoferritin (about) protein shell. These ferritin-platinum nanoparticles were non-toxic, stable with exceptional catalase-like activity; catalyze the breakdown of H2O2, and these nanoparticles are bioactive [32].
The non-cytotoxic properties of Pt NPs have been reported in previous research. All these studies showed that 10-4- 103 ng/cm2 (7.86×10-7 -7.86 mg/l) of 20-100 nm Pt NPs affected neither the cellular metabolism nor cell death [33]. Moreover, hybrids nanomaterials have been broadly invented. Graphene oxide [33] nanomaterials broadly use in research, due to their high conductivity, absorbing properties, large surface area, and outstanding biocompatibility [34].
However, the hybrid GO-Se nanomaterials have GPx like antioxidant properties for Cytoprotection. In the presence of GSH, the GPx catalyzes the breakdown of H2O2 to the harmless product. These nanomaterials catalyze the decomposition of H2O2 to the less reactive product. Comparison between Se NPs and GO-Se nanoparticles showed higher GPx mimic catalytic effectiveness. Moreover, GO-Se nanozymes are obsessed with a high capacity to scavenging the reactive oxygen species [35].
Liu and his co-workers constructed enzyme from the self-assembly of polymers with protein; this nanocomposite is used for cytoprotection. Protein (SP1) is a stress-responsive protein and has no sequence homology to other stress proteins. SOD and GPx's catalytic centre assembled into protein (SP1) and polymer nanoparticles (PD5). The electrooptic contact between polymer and protein can self-assemble and form nanowires with SOD and GPx like properties (Figure 3). These nanocomposites scavenge the overexpressed reactive oxygen species and maintain the intracellular balance [36].
Anti-aging effect
Ageing is determined by the combination of environmental factors and genetics. Caenorhabditis elegans have identified many genes such as daf-16, Age-1, Sir2, and daf-2. From which the daf-16, daf-2, Age-1 are associate with insulin-dependent signalling. Moreover, these genes are involved in oxidative stress responses and lead to stress resistance and longevity [37].
The redox reaction involves ageing; the detoxification of ROS regulates ageing. The ageing-related diseases are preventing by the help of ROS scavenging enzymes. Quick, et al. [37]reported that C60[C(COOH)2]3- based SOD mimic showed fascinating anti-ageing effects and also better mice cognition ability [37]. Comprehensive studies showed that the nanozymes lower the age-related mitochondrial superoxide and improve the biological functions of mitochondria. Moreover, these biological functions rescued the age-related cognitive destruction due to its presence in mitochondria [38].
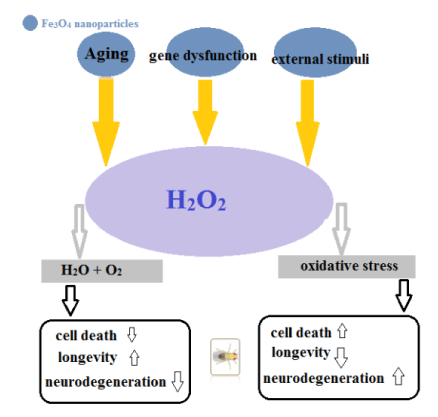
However, the drosophila melanogaster has been used to explain biological processes' mechanisms, including ageing, metabolism, and developments. Moreover, drosophila's short life cycle makes a suitable tool to study ageing mechanisms [39]. Zhang, et al. reported the Drosophila melanogaster ageing process (Figure 4).
They examined the effect of Fe3O4 NPs on drosophila life span and ageing, examined drosophila on different age stages. Results showed that the aged drosophila has a low climbing ability. The daily uptake of 200µg/mL Fe3O4 NPs reduced the ROS level and improved climbing ability [38].
Anti-bacterial effect
Bacteria produce many contagious diseases that have major health issues all over the world. Millions of peoples died due to bacterial related issues; anti-bacterial agents are primarily antibiotics [40]. Moreover, metal-containing inorganic salts, organic agents, and natural anti-bacterial agents are used as anti-bacterial materials. These antimicrobial agents have drawbacks (Table 2) [41]. So it is essential to induce stable, efficient, and novel antimicrobial agents to overcome bacterial diseases. The peroxidase constituent of cytochrome P450, take part in many essential living functions.
Peroxidase catalyzes the H2O2 to generate an elevated level of •OH and oxidize abundant organic substrates. The Qu and co-workers productively implement Graphene quantum dots (GQDs) with peroxidase type possessions to the anti-bacterial system. In a deficient concentration of H2O2, the GQDs catalyze the transformation of H2O2 to highly oxidized •OH and slaughter the bacteria efficiently. Moreover, the GQDs have an excellent anti-bacterial effect on Gram-positive and Gram-negative bacteria; these GQDs also used for wound disinfection( [42].
Dental biofilms
Nanozymes also use to deal with oral diseases associated with biofilms. Bacterial biofilms bacteria that stay on the surface of contact, polysaccharides, secreting proteins, and biomolecules. Biomolecules enfold the bacteria due to these bacteria aggregates. As a result of these biofilms, many human diseases are produced. The most costly and common biofilms associated with the oral disease are dental cavities and nanozymes to deal with these oral diseases [45]. When bacteria, especially Streptococcus mutants, use the food sugar to produce the polysaccharides and focus them on the upper layer of the teeth, the environment will gradually be more acidic due to this cavities biofilms formed because bacteria can be entrenched in the extracellular surface. Hence, it is hard to remove the oral biofilm [46].
Moreover, the acidic environments in the biofilm region can liquesce the analeptic component of teeth resulting in the commencement of dental cavities. Given the exclusive properties of enzymes, Koo's group used Fe3O4 nanoparticles with CAT NPS as the peroxidase mimics to prevent oral bacterial biofilm [2,47]. At the low pH environment, the oral biofilm CAT NPs could catalyze the production of •OH to kill the embedded bacteria and mutilate the biofilm matrix. CAT NPS alleviates the ossified of apatite in acidic pH and protects the teeth ideally.
Moreover, the CAT NP/H2O2 system keeps adequately hinder the formation and decadence of cavities, on mucosal tissue with no side effects. They also showed that ferumoxide exhibit properties of peroxidase in a pH reliant manner. These nanoparticles bind under the ultra-structure of biofilm due to this bacterial membrane disturb. They also used rodent models of early childhood caries and ex vivo biofilm; finding showed that acid damage of the enamel surface and contingent applied ferumoxide effectually suppresses biofilm amassing, also prevents the start of tooth cavitation. Ferumoxide NPs serve as an efficient therapy for biofilm-related oral diseases [48].
Nanozymes role in cardiovascular disease
In the United States, over 80 million peoples have one in three deaths recognized for Cardiovascular Disease (CVD). Post myocardial infarction [1] heart failure can cause common deaths due to cardiovascular diseases. Mechanisms showed that in the place of damaged myocardium collagenous scar formation, these scars are non-contractile and lead to ventricular dilation, infarct wall thinning and hypertrophy of the myocardium. If no particular treatment applied and left untreated, this failure progress due to this heart no longer supplies adequate blood to the body, which is distinct as heart failure [49]. Moreover, advanced therapies of CVD and current techniques for early detection are imperfect, and they also have less efficiency in preventing the diseases [8].
Nanotechnology involves the following constituents, human-made nature and the unique characteristics of new material and nanoscale dimensions of the whole system or its vital components. Cardiovascular nanomedicine aims to improve detection and address current CVD and therapy challenges by advancing in vivo and ex vivo biomarkers imaging and detection and improving tissue regeneration and drug delivery. Drugs are delivering with direct injection and nanocarriers [50].
Nanocarriers
Nanocarriers are nanomaterials with an affinity to wrap nanosized materials to transport, ideally release, and protect them at an addicted location or time. Nanocarrier size ranges from 1-100nm, many types of visitor molecules have been transferred with these nanocarriers, including imaging agents, metal nanoparticles, enzymes, dyes, and drugs [51]. Currently, many nanomaterials have been attempt and legalized in clinical trials: Liposomes [12] and micelles used in diseases as Drug Delivery Systems (DDS). Nanocarriers used in medicine to treat cardiovascular and other types of diseases differ in their chemical properties: nanotubes, polymeric nanoparticles, magnetic nanoparticles, liposomes, quantum dots, and dendrimers.
Liposomes
Liposomes size ranges from (80-300nm) they are lipid bilayer structure due to this use in medicine, and gene delivery. Liposomes are lipophilic and can pass target tissues/cell membranes [52]. These spherical vesicles have been most extensively investigated in nanomedicine with reapproval due to small toxicity for in vivo experiments. They have superior biocompatibility because liposome consists of phospholipids to form bilayer inside aqueous phase [53]. To protect the agents from degradation, they can also incorporate hydrophobic agents inward the lipid bilayer and hydrophilic therapeutic agents inside the aqueous place. The liposome's advantages, such as high stability in biological environments, high agent-loading efficiency, controllable release kinetics, the biodistribution of theragnostic agents, and biocompatibility provide liposome with efficient pharmacokinetics [54]. By the process of encapsulation, the drug is fused in the liposomes. Moreover, liposomes are employed as coordinators to deliver particles under the skin layers due to this frequency of administration reduce [55].
Micelles
Micelles consist of amphiphilic artificial molecules. To form monolayer micelles self-gather in aqueous solution with a hydrophobic phase, they incorporate hydrophobic agents. The micelles' size ranges from 10-100nm, and the inner space is more compassed than of liposomes [53]. These nanocarriers possessed of stimuli perceptive amphiphilic chunk copolymer are of pursuit due to their aptitude utilization in the field of discipline drug delivery [56].
Polymeric nanoparticles
Nanomedicine defines as the device of therapeutics and/or diagnostics on the nanoscale. Nanoscale gives benefits due to a huge degree of concurrent transfer and the prevention, treatment, and diagnosis of diseases in the biological systems [57]. Therapeutics based on polymeric nanoparticles are developed to advance the treatment and diagnosis of an extensive range of diseases. Leading in polymerization has implemented the engineering of leaded multifunctional polymeric NPs with accurate control, size, fictionalization, shape, and surface charge [58].
Advantage of these multifunctional polymeric NPs, where the rendition of multiple functional groups from nanoparticle manufacture empowers binding capabilities examined to linear polymers and higher cell recognition. Moreover, these polymers allow the encapsulation of cargo molecules that contain the drug and released at the targeted sites. In biosensing and photodynamic therapies fabrication of polymer, nanoparticles provide new avenues [58].
Poly(ethylene glycol) (PEG) is a hydrophilic, biocompatible, biologically inert polymer that has been permitted by the U.S. Food Drug Administration (FDA) for use in a wide range of application. The number and length of PEG cuffs per NPs have been shown to fabricate significant deviation in circulation time in blood vs clearance of organs by eliminating polymeric NPs [57]. Flourishing data supports the idea that NPS loaded coated drugs onto an angioplasty balloon surface is an assuring approach to moderately enhance and protract drug delivery to the wall of arteries [59]. Nanoparticles are made from poly (lactic-co-glycolic acid) (PLGA) which is a biodegradable polymer that has been used as a vehicle of drug delivery for continuous releases of both hydrophilic and hydrophobic drugs [22].
Carbon nanotubes: In nanotechnology, carbon nanotube has become the leading edge due to its unique thermal, mechanical and electrical properties. Their low weight and high mechanical strength combined with their stability and electron conductivity prepared them valuable materials for biomedical applications [60].
Moreover, single-walled carbon nanotube (SWCNT) has an exclusive 1-layer cylindrical structure that repose of a graphene sheet and nanometer-size diameter the length of several hundreds of nanometers. These single-walled carbon nanotubes are hydrophobic in aqueous media. They are used in life sciences and nanotechnology, carbon nanotube used as gene and drug delivery to the cells [61].
Nanoparticles local injection for myocardial infarction
Enormous inflammatory responses distinguish by the enrollment of macrophages and neutrophils to the flubbed myocardium. These sequential responses result in the curative of infarction. Enrollment leukocytes stash several proteinases such as protease, cathepsin B, and matrix metalloproteinases, to expedite the clearance away of necrotic debris. By the deposition of remodelling and new tissues, efficient repair of the myocardium is scantly completed. If this healing is imperfect, it may ultimately lead to the cleft of the heart failure and infarction [49].
The nanoparticles which are used in the study by Chang, et al. insulin growth factor (IGF-1) was complexed to poly(lactic-co-glycolic acid) (PLGA) by electro statistically employing polyethyleneimine (PEI) to established nanoparticles. These particles' effect was checked by injecting these particles into the border zone following myocardial infarction in mice. The PEI/PLGA combination was also more powerful in lowering left ventricular systolic/diastolic, reducing infarct area, thickening the infarct wall [50].
A Gene level approach examined by somasuntharam, et al. [62] brings Nox2-nicotinamide adenine dinucleotide phosphate (NADPH) oxidase small interfering RNA (siRNA) in polyketide situate nanoparticles. Nox2 is a known source of ROS and catalytic subunit of NADPH. Nox2 prevents cardiomyocytes apoptosis and improves cardiac function due to the complete knockout of Nox2 [62].
Atherosclerosis
Atherosclerosis is an analytical progression that involves the gathering of cholesterol underneath the intima of arteries. These progressions are pursued by local commencement of the immune system, deposition of connective tissues, and vascular smooth muscle cells [63]. Moreover, atherosclerotic plaque proceeds from the early atheromatous lesion. When the atheromatous plaque burst thromboembolic ischemia formed. These plaques are thin capped vulnerable through immune responses and inflammation and infiltration of macrophages with intraplaque haemorrhage, neovascular expansion of the vasa vasorum, and necrotic core enlargement [64]. Evidence showed that the ROS has a double role: oxidative stress as a harmful culprit and maintaining physiological vascular homeostasis as a signalling messenger. Dysfunction occurs when the ROS level beats the maximum limit due to this disease stat formed [65].
Atherosclerosis is a lipid and dietary accession disorder, in lesion formation lipid play a role they cannot report for all atherosclerosis corporations. Atherosclerosis begins with endothelial damage, and apolipoprotein B has attractions for the basal membrane exposed in the place, where the endothelium is damaged [12].
Angiogenic expansion of the vasa vasorum in the adventitia is the key feature of the atherosclerotic process; within atherosclerotic plaques, the extensive neovascular proliferation is prominent within lesions and linked with unstable stroke, myocardial infarction, and angina. To inhibit the proliferation of the vasa vasorum and quantifying angiogenesis research used rabbit model, they combined drug delivery and molecular imaging with targeted NPs. They used the drug fumagillin with an integrin targeted paramagnetic NPS αvβ3 [66].
Moreover, the cardiac magnetic resonance with αvβ3 targeted paramagnetic NPs used noninvasively showed that only 1-minute dose of αvβ3 with targeted fumagillin NPS lowered the angiogenesis of aortic for 3 weeks [64]. Many studies hypothesized that αvβ3 with targeted NPs serve as an efficient nanomedicine tool for drug delivery, monitoring atherosclerosis, and noninvasive characterization [66]. The application of nanozyme and their mimetic enzymes are given in Table 2.
Conclusion
Since the discovery of Fe3O4 nanoparticles as peroxidase mimics, nanozymes have attracted much attention. As natural enzyme mimics, nanozymes possess many advantages: low cost, easy preparation, excellent stability, and good durability. They have been widely applied in sensing, environmental treatment, anti-bacterial, cancer therapy, antioxidation, etc. This review summarized the classification, catalytic mechanism, activity regulation, and recent research progress of nanozymes in these fields in detail. Although nanozymes overcome many disadvantages of natural enzymes, several exciting challenges remain. Natural enzymes are widely used in fields, including medicine, food, industry, agriculture, environment, biotechnology, etc. Compared with natural enzymes, current research on applications of nanozymes are still rather limited. These unsolved issues will be the next frontier for further applications.
Authors’ contributions
Conceived and designed the experiments: Naima Nashat, and Zeshan Haider, Wrote the paper: Naima Nashat, and Zeshan Haider. Finally, finalized the final review paper by Zeshan Haider.
- Li Z, Feng K, Zhang W, Ma M, Gu N, et al. (2018) Catalytic mechanism and application of enzymes. Chinese Science Bulletin 63: 2128-2139.
- Gao L, Yan X (2016) Nanozymes: an emerging field bridging nanotechnology and biology. Sci China Life Sci 59: 400-402. Link: https://bit.ly/2NcxLk1
- Wang X, Hu Y, Wei H (2013) <Nanozymes in bionanotechnology from sensing to therapeutics and beyond. Royal society of chemistry.
- Golchin J, Golchin K, Alidadian N, Ghaderi S, Eslamkhah S, et al. (2017) Nanozyme applications in biology and medicine: an overview. Artif Cells Nanomed Biotechnol 45: 1-8. Link: https://bit.ly/2XQeK97
- Godin B, Sakamoto JH, Serda RE, Grattoni A, Bouamrani A, et al. (2010) Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol Sci 31: 199-205. Link: https://bit.ly/3bUdPwm
- Zhou Y, Liu B, Yang R, Liu J (2017) Filling in the Gaps between Nanozymes and Enzymes: Challenges and Opportunities. Bioconjug Chem 28: 2903-2909. Link: https://bit.ly/3ijWezm
- Wei H, Wang E (2013) Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev 42: 6060-6093. Link: https://bit.ly/3imB5o9
- Mulder WJ, Fayad ZA (2008) Nanomedicine captures cardiovascular disease. 28: 801.
- Wang X, Hu Y, Wei H (2016) Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorganic Chemistry Frontiers 3: 41-60. Link: https://rsc.li/3nOa8La
- Vernekar AA, Das T, Ghosh S, Mugesh G (2016) A Remarkably Efficient MnFe2 O4 -based Oxidase Nanozyme. Chem Asian J 11: 72-76. Link: https://bit.ly/3szMWUA
- Nair HB, Sung B, Yadav VR, Kannappan R, Chaturvedi MM, et al. (2010) Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem Pharmacol 80: 1833-1843. Link: https://bit.ly/3bIMxcu
- Lombardo D, Calandra P, Barreca D, Magazù S, Kiselev MAJN (2016) Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials (Basel) 6: 125. Link: https://bit.ly/35MlglJ
- Hirst SM, Karakoti AS, Tyler RD, Sriranganathan N, Seal S, et al. (2009) anti inflammatory properties of cerium oxidebase nanoparticles. Small 5: 2848-2856. Link: https://bit.ly/3oPSZlA
- Kost OA, Beznos OV, Davydova NG, Manickam DS, Nikolskaya II, et al. (2015) Superoxide Dismutase 1 Nanozyme for Treatment of Eye Inflammation. Oxid Med Cell Longev 2015: 5194239. Link: https://bit.ly/2XOd7ZG
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C (2006) Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28: 1091-1101. Link: https://bit.ly/2XPFQwU
- Thomas CE, Darley-Usmar V (2000) Forum on therapeutic applications of reactive oxygen and nitrogen species in human disease. Free Radic Biol Med 28: 1449-1450. Link: https://bit.ly/3suPt2t
- Singh S (2016) Cerium oxide-based nanozymes: Redox phenomenon at biointerfaces. Biointerphases 11: 04B202. Link: https://bit.ly/3oV5cFL
- Huang Y, Liu Z, Liu C, Ju E, Zhang Y, et al. (2016) Self-Assembly of Multi-nanozymes to Mimic an Intracellular Antioxidant Defense System. Angew Chem Int Ed Engl 55: 6646-6650. Link: https://bit.ly/38OZFe3
- Zhang W, Hu S, Yin JJ, He W, Lu W, et al. (2016) Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J Am Chem Soc 138: 5860-5865. Link: https://bit.ly/2M11NGC
- Li H, Zhang W, Ding L, Li XW, Wu Y, et al. (2019) Prussian blue-modified ferritin nanoparticles for effective tumor chemo-photothermal combination therapy via enhancing reactive oxygen species production. J Biomater Appl 33: 1202-1213. Link: https://bit.ly/2XPHsqs
- Zhang W, Wu Y, Dong HJ, Yin JJ, Zhang H, et al. (2018) Sparks fly between ascorbic acid and iron-based nanozymes: A study on Prussian blue nanoparticles. Colloids Surf B Biointerfaces 163: 379-384. Link: https://bit.ly/3sAbnBq
- Menon JU, RavikumaOh H, Lee JS, Sung D, Lee JH, Moh SH, et al. (2019) Synergistic antioxidant activity of size controllable chitosan-templated Prussian blue nanoparticle. Nanomedicine 14: 2567-2578. Link: https://bit.ly/39ILRRE
- Estevez AY, Pritchard S, Harper K, Aston JW, Lynch A, et al. (2011) Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic Biol Med 5r P, Pise A, Gyawali D, Hsia CC, et al. (2014) Polymeric nanoparticles for pulmonary protein and DNA delivery. Acta Biomater 10: 2643-2652. Link: https://bit.ly/38T0vH0
- 1: 1155-1163. Link: https://bit.ly/2Ncxg9D
- >Schubert D, Dargusch R, Raitano J, Chan SW (2006) Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem Biophys Res Commun 342: 86-91. Link: https://bit.ly/38VqygJ
- Cai W, Wu J, Xi C, Ashe AJ, Meyerhoff ME (2011) Carboxyl-ebselen-based layer-by-layer films as potential antithrombotic and antimicrobial coatings. Biomaterials 32: 7774-7784. Link: https://bit.ly/3szoMJK
- Slowing II, Trewyn BG, Giri S, Lin VSY (2007) Mesoporous Silica Nanoparticles for Drug Delivery and Biosensing Applications. Advanced Functional Materials 17: 1225-1236. Link: https://bit.ly/3oWCNit
- Cauda V, Schlossbauer A, Kecht J, Zürner A, Bein T (2009) Multiple core-shell functionalized colloidal mesoporous silica nanoparticles. Journal of the American Chemical Society 131: 11361-11370. Link: https://bit.ly/3nP0Uyd
- Argyo C, Cauda V, Engelke H, Radler J, Bein G, et al. (2012) Heparin-coated colloidal mesoporous silica nanoparticles efficiently bind to antithrombin as an anticoagulant drug-delivery system. Chemistry 18: 428-432. Link: https://bit.ly/2LSw0Yx
- Suchyta DJ, Handa H, Meyerhoff ME (2014) A nitric oxide-releasing heparin conjugate for delivery of a combined antiplatelet/anticoagulant agent. Mol Pharm 11: 645-650. Link: https://bit.ly/3qqX5B9
- Xue T, Peng B, Xue M, Zhong X, Chiu CY, et al. (2014) Integration of molecular and enzymatic catalysts on graphene for biomimetic generation of antithrombotic species. Nat Commun 5: 3200. Link: https://bit.ly/3qxZo5c
- Fan J, Yin JJ, Ning B, Wu X, Hu Y, et al. (2011) Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 32: 1611-1618. Link: https://bit.ly/3bLNZe5
- Nakanishi H, Hamasaki T, Kinjo T, Yan H, Nakamichi N, et al. (2013) Low Concentration Platinum Nanoparticles Effectively Scavenge Reactive Oxygen Species in Rat Skeletal L6 Cells. Nano Biomedicine and Engineering 5: 85. Link: https://bit.ly/2KrVDz6
- Cao Y, Ma Y, Zhang M, Wang H, Tu X, et al. (2014) Ultrasmall Graphene Oxide Supported Gold Nanoparticles as Adjuvants Improve Humoral and Cellular Immunity in Mice. Advanced Functional Materials 24: 6963-6971. Link: https://bit.ly/35N1yWY
- Huang Y, Liu C, Pu F, Liu Z, Ren J, et al. (2017) A GO-Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem Commun (Camb) 53: 3082-3085. Link: https://bit.ly/3il5GSS
- Sun H, Miao L, Li J, Fu S, An G, et al. (2015) Self-assembly of cricoid proteins induced by “soft nanoparticles”: an approach to design multienzyme-cooperative antioxidative systems. ACS Nano 9: 5461-5469. Link: https://bit.ly/3oT3QLK
- Quick KL, Ali SS, Arch R, Xiong C, Wozniak D, et al. (2008) A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol Aging 29: 117-128. Link: https://bit.ly/3qHyYOT .
- Zhang Y, Wang Z, Li X, Wang L, Yin M, et al. (2016) Dietary iron oxide nanoparticles delay aging and ameliorate neurodegeneration in drosophila. Advanced materials 28: 1387-1393. Link: https://bit.ly/39Bx9Mk
- Moloney A, Sattelle DB, Lomas DA, Crowther DC (2010) Alzheimer's disease: insights from Drosophila melanogaster models. Trends Biochem Sci 35: 228-235. Link: https://bit.ly/2M1hw8E
- Natsos G, Mouttotou N, Ahmad S, Kamran Z, Ioannidis A, et al. (2019) The genus Campylobacter: detection and isolation methods, species identification & typing techniques. Journal of the Hellenic Veterinary Medical Society 70: 1327-1338. Link: https://bit.ly/38Svoem
- Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358: 135-138. Link: https://bit.ly/2LZwwnA
- Sun H, Gao N, Dong K, Ren J, Qu X (2014) Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 8: 6202-6210. Link: https://bit.ly/3inlX9X
- Chakraborty A, Boer JC, Selomulya C, Plebanski M (2017) Amino acid functionalized inorganic nanoparticles as cutting-edge therapeutic and diagnostic agents. Bioconjugate Chem 29: 657-671. Link: https://bit.ly/3sv5dCp
- Giuliani A, Rinaldi AC (2011) Beyond natural antimicrobial peptides: multimeric peptides and other peptidomimetic approaches. Cell Mol Life Sci 68: 2255-2266. Link: https://bit.ly/38Nxnkd
- Jefferson K (2004) What drives bacteria to produce a biofilm? FEMS microbiology lett. 236: 163-173. Link: https://bit.ly/2M2tdw8
- Cerning J (1990) Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol Rev 7: 113-130. Link: https://bit.ly/39GoWXi
- Cormode DP, Gao L, Koo H (2018) Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol 36: 15-29. Link: https://bit.ly/38SeXPn
- Liu Y, Naha PC, Hwang G, Kim D, Huang Y, et al. (2018) Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat Commun 9: 2920. Link: https://bit.ly/38R1WFJ
- McCarthy JR (2010) Nanomedicine and cardiovascular disease. 3: 42-49.
- Suarez S, Almutairi A, Christman KJB (2015) Micro-and nanoparticles for treating cardiovascular disease. Biomater Sci 3: 564-580. Link: https://bit.ly/2LXE2j1
- Kurniasih IN, Keilitz J, Haag RJCSR (2015) Dendritic nanocarriers based on hyperbranched polymers. Chemical Society Reviews 44: 4145-4164. Link: https://rsc.li/2LErxsI
- Qamar SA, Asgher M, Khalid N, Sadaf M (2019) Nanobiotechnology in health sciences: Current applications and future perspectives. Biocatalysis and Agricultural Biotechnology 22. Link: https://bit.ly/2Y2OmJn
- Katsuki S, Matoba T, Koga Ji, Nakano K, Egashira KJF (2017) Antiinflammatory nanomedicine for cardiovascular disease. Front Cardiovasc Med 4: 87. Link: https://bit.ly/2Nc1hX8
- Xing H, Hwang K, Lu Y (2016) Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics 6: 1336-1352. Link: https://bit.ly/3qqY0BB
- Franzé S, Marengo A, Stella B, Minghetti P, Arpicco S, et al. (2018) Hyaluronan-decorated liposomes as drug delivery systems for cutaneous administration. Int J Pharm 535: 333-339. Link: https://bit.ly/3654COr
- Rosenbaum I, Harnoy AJ, Tirosh E, Buzhor M, Segal M, et al. (2015) Encapsulation and covalent binding of molecular payload in enzymatically activated micellar nanocarriers. J Am Chem Soc 137: 2276-2284. Link: https://bit.ly/39KF4H4
- Elsabahy M, Wooley KL (2012) Design of polymeric nanoparticles for biomedical delivery applications. Chem Soc Rev 41: 2545-2561. Link: https://bit.ly/2XM4Hlx
- Lam SJ, Wong EH, Boyer C, Qiao GG (2018) Antimicrobial polymeric nanoparticles. Progress in polymer science 76: 40-64. Link: https://bit.ly/39E0PZ5
- Iyer R, Kuriakose AE, Yaman S, Su LC, Shan D, et al. (2019) Nanoparticle eluting-angioplasty balloons to treat cardiovascular diseases. Int J Pharm 554: 212-223. Link: https://bit.ly/3bUeePo
- Martinelli V, Bosi S, Peña B, Baj G, Long CS, et al. (2018) 3D Carbon-Nanotube-Based Composites for Cardiac Tissue Engineering. ACS Applied Bio Materials 1: 1530-1537. Link: https://bit.ly/2LYDlG8
- Ohta T, Hashida Y, Yamashita F, Hashida M (2016) Development of novel drug and gene delivery carriers composed of single-walled carbon nanotubes and designed peptides with PEGylation. J Pharm Sci 105: 2815-2824. Link: https://bit.ly/39HV38N
- Somasuntharam I, Boopathy AV, Khan RS, Martinez MD, Brown ME, et al. (2013) Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction. Biomaterials 34: 7790-7798. Link: https://bit.ly/38QMkSy
- Schiener M, Hossain M, Viola JR, Ortega-Gomez A, Weber C, et al. (2014) Nanomedicine-based strategies for treatment of atherosclerosis. Trends Mol Med 20: 271-281. Link: https://bit.ly/2KnEgzi
- Winter PM, Caruthers SD, Zhang H, Williams TA, Wickline SA, et al. (2008) Antiangiogenic synergism of integrin-targeted fumagillin nanoparticles and atorvastatin in atherosclerosis. JACC Cardiovasc Imaging 1: 624-634. Link: https://bit.ly/3qz1uSH
- Kim KS, Song CG, Kang PMJA, signalling R (2019) Targeting oxidative stress using nanoparticles as a theranostic strategy for cardiovascular diseases. Antioxid Redox Signal 30: 733-746. Link: https://bit.ly/3oQZHIc
- Winter PM, Neubauer AM, Caruthers SD, Harris TD, Robertson JD, et al. (2006) Endothelial ανβ3 integrin–targeted fumagillin nanoparticles inhibit angiogenesis in atherosclerosis. Arterioscler Thromb Vasc Biol 26: 2103-2109. Link: https://bit.ly/3bNPubz
- Gao L, Fan K, Yan X (2017) Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications. Theranostics 7: 3207-3227. Link: https://bit.ly/3p4Axpi
- Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE (2007) Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res 73: 549-559. Link: https://bit.ly/38UjhO5 .
- Fiorani L, Passacantando M, Santucci S, Di Marco S, Bisti S, et al. (2015) Cerium Oxide Nanoparticles Reduce Microglial Activation and Neurodegenerative Events in Light Damaged Retina. PLoS One 10: e0140387. Link: https://bit.ly/3qtq9I2
- Das M, Patil S, Bhargava N, Kang JF, Riedel LM, et al. (2007) Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials 28: 1918-1925. Link: https://bit.ly/3bMdE6z
- Asati A, Santra S, Kaittanis C, Nath S, Perez JM (2009) The oxidase-like activity of polymer‐coated cerium oxide nanoparticles. Angew Chem Int Ed Engl 48: 2308-2312. Link: https://bit.ly/3swSTSb
- Mu J, Zhang L, Zhao M, Wang Y (2013) Co3O4 nanoparticles as an efficient catalase mimic: Properties, mechanism and its electrocatalytic sensing application for hydrogen peroxide. Journal of Molecular Catalysis A: Chemical 378: 30-37. Link: https://bit.ly/2XPr2yp
- Biparva P, Abedirad SM, Kazemi SY (2014) ZnO nanoparticles as an oxidase mimic-mediated flow-injection chemiluminescence system for sensitive determination of carvedilol. Talanta 130: 116-121. Link: https://bit.ly/3qtkUbr
- Xue T, Jiang S, Qu Y, Su Q, Cheng R, et al. (2012) Graphene‐supported hemin as a highly active biomimetic oxidation catalyst. Angew Chem Int Ed Engl 51: 3822-3825. Link: https://bit.ly/35NJADA
- Xia J, Li F, Ji S, Xu H (2017) Selenium-functionalized graphene oxide that can modulate the balance of reactive oxygen species. ACS Appl Mater Interfaces 9: 21413-21421. Link: https://bit.ly/35NCKxO
- Liu X, Wang Q, Zhao H, Zhang L, Su Y, et al. (2012) BSA-templated MnO 2 nanoparticles as both peroxidase and oxidase mimics. Analyst 137: 4552-4558. Link: https://bit.ly/39GLfvQ
- Singh N, Savanur MA, Srivastava S, D'Silva P, Mugesh G (2017) A redox modulatory Mn3O4 nanozyme with multi‐enzyme activity provides efficient cytoprotection to human cells in a Parkinson's disease model. Angew Chem Int Ed Engl 56: 14267-14271. Link: https://bit.ly/2LUAG01
- Zhou H, Han T, Wei Q, Zhang S (2016) Efficient enhancement of electrochemiluminescence from cadmium sulfide quantum dots by glucose oxidase mimicking gold nanoparticles for highly sensitive assay of methyltransferase activity. Anal Chem 88: 2976-2983. Link: https://bit.ly/2XPsmkR
- Xia X, Zhang J, Lu N, Kim MJ, Ghale K, et al. (2015) Pd–Ir core-shell nanocubes: a type of highly efficient and versatile peroxidase mimic. ACS Nano 9: 9994-10004. Link: https://bit.ly/39KoLKz
- Peng FF, Zhang Y, Gu N (2008) Size-dependent peroxidase-like catalytic activity of Fe3O4 nanoparticles. Chinese Chemical Letters 19: 730-733. Link: https://bit.ly/3bMYlKP
- Ragg R, Natalio F, Tahir MN, Janssen H, Kashyap A, et al. (2014) Molybdenum trioxide nanoparticles with intrinsic sulfite oxidase activity. ACS Nano 8: 5182-5189. Link: https://bit.ly/35RV6Ot
- Liu T, Shi S, Liang C, Shen S, Cheng L, et al. (2015) Iron oxide decorated MoS2 nanosheets with double PEGylation for chelator-free radiolabeling and multimodal imaging-guided photothermal therapy. ACS Nano 9: 950-960. Link: https://bit.ly/2LI6Qfx
- Ghosh S, Roy P, Karmodak N, Jemmis ED, Mugesh G (2018) Nanoisozymes: Crystal‐Facet‐Dependent Enzyme‐Mimetic Activity of V2O5 Nanomaterials. Angew Chem Int Ed Engl 130: 4600-4605. Link: https://bit.ly/2LvDHUT
- Natalio F, André R, Hartog AF, Stoll B, Jochum KP, et al. (2012) Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol 7: 530. Link: https://bit.ly/38T7cZp
- Huang W, Wu H, Li X, Chen T (2016) Facile One‐Pot Synthesis of Tellurium Nanorods as Antioxidant and Anticancer Agents. Chem Asian J 11: 2301-2311. Link: https://bit.ly/38VLjJf
- Gao L, Liu Y, Kim D, Li Y, Hwang G, et al. (2016) Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 101: 272-284. Link: https://bit.ly/3nRI3CD
- Katsuki S, Matoba T, Koga Ji, Nakano K, Egashira K (2017) Antiinflammatory nanomedicine for cardiovascular disease. Front Cardiovasc Med 4: 87. Link: https://bit.ly/38NqYpg

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
