International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Nanoparticulate system for cancer therapy: An updated review
Dharmendra Kumar* and Pramod Kumar Sharma
Cite this as
Kumar D, Sharma PK (2018) Nanoparticulate system for cancer therapy: An updated review. Int J Nanomater Nanotechnol Nanomed 4(2): 022-034. DOI: 10.17352/2455-3492.000027Nowadays, pharmaceutical nanotechnology has been developed as the most emerging branch in the field of pharmacy. “Nanotechnology refers to the nanosize formulation. These nanoformulations may be used in treatment of various life-threading diseases like cancer. Due to the advantages of their nano size and shape, nanoformulations have been shown to be favorable drug delivery systems and may be useful for encapsulating and conjugating of drugs, enabling most precise tumor targeting and controlled release. Nanoparticle drug delivery system have several advantages such as enhanced intracellular infiltration, hydrophobic solubility, and drug circulation time and also reduce nonspecific uptake and toxic effect for cancer therapy. A large number of Nanoparticle technologies have been developed for cancer treatment to improve the therapeutic efficacy and safety for anticancer drugs. In this paper, we review the most significant advancement in pharmaceutical nanotechnologies with methods of preparation and their use in drug delivery for cancer therapy.
Introduction
According to national nanotechnology initiative, Nanoparticles are structures of sizes ranging from 1 to 100 nm in at least one dimension. Nanoparticles properties like physicochemical and biological are more easily taken up by cell than larger molecules, so Nanoparticles may be more suitable as drug delivery system [1].
Now days Nanoparticulate system gained more importance than conventional dosage form in cancer therapy because conventional dosage form have more challenge to deliver the drug in adequate quantity to the tumor site. While Nanoparticulate system may be possibility to deliver chemotherapeutic drug at target site easily [2].
Chemotherapeutic drugs are toxic to cancer cell but their high toxicity and low specificity also destroyed the healthy cells. A possible strategy to overcome these problems or improve therapeutic efficacy and decrease their toxic effect is called Nanoparticles technology [3]. The main object of these nanotechnologies is to transport proper amount of drug to desirable site and decreases toxic effect of drugs on other tissues [4].
In this review, we discussed Nanoparticles technologies and also focused on parameter for material selection for Nanoparticle and their advantages. These technologies include Liposomes, Polymer drug conjugates, Polymeric Nanoparticles, Micelle, Dendrimer, Polymersome, Protein Nanoparticles, Biological Nanoparticles, Inorganic Nanoparticles and Hybrid Nanoparticles.
Advantages of nanoparticles technologies in cancer therapy
Various studies show that Nanoparticles have ability to target to cancer cells without damaging healthy cells. So now a day Nanoparticles technologies are considered as superior drug delivery system in cancer therapy than other conventional dosage form. Target and enter into selective tissue at molecular level.
Increase cellular uptake and drug localization.
Accurate and selective drug delivery to cancerous cell without interaction with healthy cells
Providing large surface area
Providing high absorption rate
Less amount of dose required.
Decrease drug resistance.
Decrease toxicity.
To improve the uptake of poorly soluble drugs
Nanoparticles can better deliver drugs to tiny areas within the body.
Nanoparticles overcome the resistance offered by the physiological barriers in the body [5-7].
Factors affecting the selection of material for nanoparticles preparation are
Need of Nanoparticles size.
Drug properties such as stability and aqueous solubility
Desired drug release profile
Required surface charge of Nanoparticles
Biocompatibility and biodegradability
Toxicity and antigenicity of product [8].
Cancer is one of the most common problems and serious health issue in this world. Human body contains millions of tiny cells; these tiny cells are living units of the body. Cancer is a complex disorder that results from multiple genetic changes and cellular abnormalities [9]. Genetic changes that cause cancer can be, Inherited from our parents, Person’s lifetime and Environmental exposures such as chemicals in tobacco, smoke, radiation, ultraviolet rays from the sun [10].
Nanoparticles technologies
Liposome Nanoparticles: Liposomes were the first Nanoparticles technology applied in medicine in 1961 [11]. Aim of liposomal drug delivery system to increase efficacy, decrease toxicity and easy administration [12]. Liposomal Nanoparticles are most used Nanoparticles for cancer therapy, these are easily and self-assembled from amphiphilic lipid and excipients. The lipid part form a bilayer based on hydrophobic interaction with hydrophilic head groups. Hydrophobic drug molecules can be encapsulated in lipid bilayer and hydrophilic drug molecules can be encapsulated in aqueous phase [13]. Drug release from liposomes depends on composition, pH, and osmotic gradient and surrounding environment [14]. Lipids are used in these formulations are approved by FDA, that are DSPE (1, 2-distearoyl-sn-glycero-3-phosphoethanolamine), HSPE (hydrogenated phosphatidylcholine from soybean lecithin), EggPG (egg yolk phosphatidylglycerol), DSPC (1, 2-distearoyl-glycero-3-phosphocholine). Liposome Nanoparticles have demonstrated multiple special benefits as drug delivery system, such as used to carry very potent drug to their low encapsulated load, instability in blood stream and poor solubility of many drugs. Many times researchers reported various challenges during the production of liposome are difficult reproducing formulation process, uniform particle size, efficient drug loading, and time consuming process.
Types of liposomes: On the basis of phospholipid bilayer and the size of liposomes, these are following types [15-16].
Multilamellar Vesicles (MLV) - these types of liposomes are contains multiple number of phospholipid bilayer member separated by aqueous phase. The size of multilamellar vesicle liposomes may up to 5 μm.
Small Unilamellar Vesicles (SUV) - these types of liposomes are contains single phospholipid bilayer member surrounding the aqueous phase. The of Small unilamellar vesicles liposome may be in the range of 20-100 nm.
Large Unilamellar Vesicle (LUV) - these types of liposomes are also contain single phospholipid bilayer member surrounding the aqueous phase. The of Small unilamellar vesicles liposome may be in the range of 100-250 nm.
Polymer drug conjugates nanoparticles
The concept of polymer conjugates for anticancer agent was proposed in 1975 [17]. Polymer drug conjugation achieved enhanced permeability and retention effect by tumor specific targeting [18].
Polymeric drug conjugation system is the most important and older polymeric drug delivery system. Polymer–drug conjugates are most advancement in the field of Nanoparticles technology and currently in clinical trials phase III. These Nanoparticles can deliver high dose of chemotherapeutic drugs because in which drug conjugates with polymer through side chain. The size of polymer conjugates is below 20 nm mostly. The way of conjugating the drug to the Nanoparticles and its strategy is most important in cancer therapy. A drug molecule may be encapsulated in Nanoparticles or covalently attached to surface of Nanoparticles. Covalent attaching strategy had more advantages than other ways [19]. On the basis of various studies found that, application of Nanoparticles to tumor may be improved by the conjugated of polymer and drug moiety. These conjugations may allow more specific recognition and preferential interaction of drug to targeted tumor site [20].
Polymeric nanoparticles
The purpose of polymeric Nanoparticles was to develop Nanoparticles for prolonged drug delivery system [21]. Polymeric Nanoparticles are flexible in design because of polymer properties such as biodegradable and non-biodegradable, synthetic and natural synthetic sources [22]. Commonly used polymers are poly (lactic acid) (PLA), dextran, and chitosan [23]. Polymer Nanoparticles can be used to improve the efficacy, toxicity, bioavailability, solubility and pharmacokinetics of a drug. These particles may reduce toxicity in tumors and improved therapeutic response [24]. Polymeric Nanoparticles may offer encapsulation and delivery of bio-molecules for genetic medicine, immunotherapy and gene editing. Polymeric Nanoparticles offer the various advantages in cancer therapy but during the development of these particles some challenges affect the safety and efficacy of the polymer formulations [25]. These challenges are process scalability, process reproducibility, particle size control and efficient drug loading. Drug can be encapsulated on polymeric Nanoparticles during polymerization step [26]. Drugs may be released from polymeric Nanoparticles by desorption, diffusion, or Nanoparticle erosion in target tissue [27].
Micelle nanoparticle
Micelles are self assemble Nanoparticles with hydrophobic core composed from lipid and polymers. Micelles are the best drug delivery system for hydrophobic drugs. Only those chemical have an amphiphilic nature can form micelles in aqueous solution [28]. Micelles are generated when hydrophilic portions surrounding by hydrophobic phase. Micelles are most favorable drug delivery system for poorly water soluble drugs [29-31]. Pharmacokinetics properties of micelles were influenced by size of micelles Nanoparticles, generally accepted range of micelles is 50 – 150 nm, but larger the Nanoparticles size can carry more drug load because of high encapsulation volume [32]. Transport properties of micelles may be influenced by shape of micelles Nanoparticles. Discs and rod shape micelles have more accepted blood circulation properties than spherical particles [33].
Dendrimer nanoparticles
The word dendrimers derived from the Greek word “DENDRON” means tree and “MEROS” means part, so its appearance likes TREE. This technology discovered by Tomalia and coworker in early 1980. Dendritic polymers are newly recognized polymeric structure after linear, cross linked and branch polymer [34-35]. Dendrimers are repetitively branched molecules within the range of 5-10nm. They can be modified as required to carry the drug for targeting site [36]. Dendimers serves suitable pharmacokinetic properties for systematic drug delivery. Structurally, dendrimers have three parts, namely a central core, tiers of multifunctional unit and terminal or end groups.
Dendrimers serves several properties those facilitated various biological applications as following [37-41].
Neutral and negative charge dendrimers are biocompatible while positive charge dendrimers may show toxic effects.
Structure of dendrimers may affect pharmacokinetics properties.
Retention and bio-distribution character may improve by increase water solubility and size of dendrimers by PEGylation.
Therapeutic agent can be attached to functional groups.
Can be modulated for target-specific drug delivery.
Feasibility to develop with defined molecular weight.
Good entrapment efficiency.
Offering surface for functionalization.
Very low polydispersity index.
Very low size (1–5 nm).
Polymersome nanoparticles
Structurally polymersomes are similar as liposomes but compositions are different, polymersome is composed of synthetic polymer/polypeptide amphiphiles and self-assembled. Liposomes drug delivery system is the most widely used drug delivery system for anticancer drug moieties but their short half-life and slow drug release required to develop new alternatives. Synthetic polymers are most promising candidates to exhibit longer half-life and better drug release [42-44], when polymer and liposomes technology works together to design Nanoparticles are called polymersomes Nanoparticles [45-47]. Resulted polymersome Nanoparticles have been shown long half-life, better drug release, enhanced stability and more side chain functioning [48]. As liposomes, hydrophobic membrane and aqueous core of polymersome enables to encapsulate to both hydrophobic and hydrophilic drug moieties. Polymersome Nanoparticles technologies have ability to deliver both hydrophilic and hydrophobic drug in alone or combination [49].
Protein nanoparticles
Protein Nanoparticles are generally with 130 nm size. These particles bound drug with albumin to enhance intrinsic targeting abilities and permeability with retention effect at tumor site [50]. Protein Nanoparticles have gained great attention in nanotechnology because of their low toxicity, biodegradability, metabolizable and easy amenable to surface modification for drug attachment [51]. Various types of proteins are used to prepare protein nanotechnology are water soluble proteins such as bovine, human serum albumin and insoluble proteins such as zein and gliadin [52-53]. The most important advantage of protein Nanoparticles as drug carrier system may target the drug by modified body distribution and improvement of cellular uptake of the substances [54].
Biological nanoparticles
Biological Nanoparticles can be developed from organic and inorganic compounds based on natural biomolecules. Biological Nanoparticles derive from single or multiple assemblies of protein subunits [55]. These are unicellular microorganism with various shapes and sizes. Biological Nanoparticles have capacity to bind with both hydrophilic and hydrophobic drug molecule [56]. Biological Nanoparticles are divided in two categories are: a. delivery of small drug molecules for cancer treatment, b. gene therapy and vaccine applications. These systems are modified by chemical or genetic modification to achieve tumor specific delivery [57].
Inorganic nanoparticles
Various type of Nanoparticles are used as drug delivery such as silica Nanoparticles [58], quantum dots [59-60], metal Nanoparticles [61], and lanthanide Nanoparticles [62-63], Inorganic Nanoparticles are generally metal based particles. These may synthesized with near monodispersity. These Nanoparticles have ability to energy convert into heat at some specific conditions [64]. Metallic Nanoparticles are used as drug delivery system since last few decades but now days this technology is a favorable drug delivery system for anticancer drugs because these technology have various advantages such as efficiency of drugs, biocompatibility, drug loading, nontoxic to normal cells and easily reached to targeted tumor sites. This technology used various metals to synthesized Nanoparticles like gold, silver, iron oxide. Gold Nanoparticles are synthesized in various size range but commonly used ranges are 2-100 nm. Cellular uptake of these particles is inversely prepositional to their size and larger particle i.e. 80-100 nm does not diffuse in to tumor site and stay near the blood vessels [65-66]. These particle sizes depend on the thiol/gold ratio during the synthesis, as the thiol amount increases particle size decreases [67-68]. In this era various types of gold Nanoparticles take place in research such as gold nanoshells, gold nanosphere, gold nanorods and gold nanocages [69]. Another newest inorganic Nanoparticles technology for cancer therapy was developed as silver Nanoparticles.
Hybrid nanoparticles
Hybrid Nanoparticles are the advancement of liposome and micelles. These are composed of two different materials that form core and corona structure. Core contains metallic or polymeric material while corona contains lipid layer that worked as protecting membrane. As we have discussed in earlier in part of liposome that the drug moieties are attached on the surface of liposome or incorporated into hydrophilic phase to enhance retention time of drug to cancer cell. But at this time liposome decorated with paramagnetic molecules and enable to detection of angiogenesis [70-71]. So in these cases hybrid Nanoparticles technologies is required. Various types of inorganic material such as gold Nanoparticles and iron oxide Nanoparticles able to improve image contrast [72]. That’s by gold Nanoparticles and iron oxide Nanoparticles are encapsulated in liposome, hydrophilic gold Nanoparticles are encapsulated in hydrophilic phase and hydrophobic gold Nanoparticles are inserted in hydrophobic membrane.
Advancement of nanoparticle preparation methods
The mode of preparation of Nanoparticles plays a vital role to achieve the properties of Nanoparticles. The selection of these methods depends on the physical and chemical properties of drug and polymer. Scientist worked from ancient time to prepare Nanoparticles via various methods and their modifications, these methods and their modifications are listed below.
Emulsion-Solvent evaporation method
This method is widely used method for preparation of Nanoparticles. This method consist two steps a. emulsification of polymer solution into water phase b. evaporation of solvent until Nanoparticle precipitation. Prepared Nanoparticles are collected by ultracentrifugation as washed with distilled water [73] (Figure 1).
Modified Emulsion-Solvent Evaporation Method (Table 1).
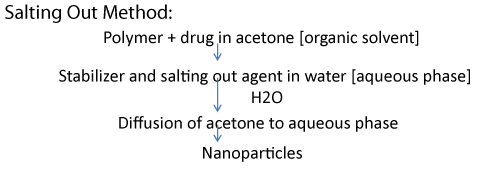
Salting out method (Figure 2)
Modified Salting Out Method (Table 2)
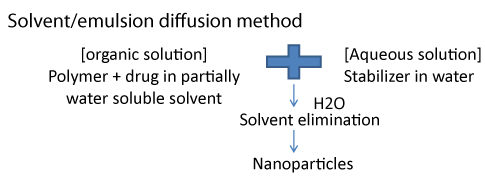
Solvent/emulsions diffusion method (Figure 3)
Modified Solvent/emulsions diffusion method (Table 3)
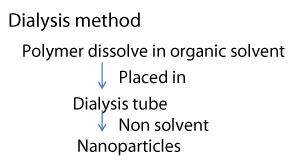
Dialysis method (Figure 4)
Modified Dialysis method (Table 4)
Precipitation method (Figure 5)
Modified Precipitation Method (Table 5)
Patent filled by inventors to prepared nanoparticles
Researchers developed new formulations and their methods to achieve their goals, latest worked done by various researchers listed here (Table 6).
Conclusion
As summarized above, new scientific approaches serves advanced technologies and overcome various challenges i.e toxicity, absorption, tumor site targeting, solubility, drug resistance, dose requirement etc. The ultimate goal of developing new technologies should be change the way of cancer treatment and overcome the challenges. Development of Nanoparticles drug delivery system is a future hope with great impact on cancer treatment approaches. Because researchers realize that Nanoparticles drug delivery may be stabilized to treat various type of cancer. Nanoparticles as drug delivery system are prepared to improve therapeutic and pharmacological properties of conventional drug delivery system. Drug molecule incorporated in Nanoparticles offers controlled release and possibilities to targeting to tumor site as well as protection of drug from degradation. Drug conjugations with Nanoparticles are more effective and selective and low toxic to healthy cells as well as required low therapeutic dose. Nowadays, various Nanoparticles based drug delivery is currently under preclinical evaluation phases. Some of Nanoparticle technologies have few limitations but these have possibility to improve with small modifications.
- Khan I, Saeed K, Khan I (2017) Nanoparticles: Properties, applications and toxicities. Arabian Journal of Chemistry. Link: https://tinyurl.com/ycul53lv
- Pillai G (2014) Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development. SOJ Pharm Pharm Sci 1:13-21. Link: https://tinyurl.com/ybgdgf9b
- Vieira DB, Gamarra LF (2016) Advances in the use of nanocarriers for cancer diagnosis and treatment. Einstein 14: 99-103. Link: https://tinyurl.com/y8mshypm
- Javad S (2014) Advanced drug delivery systems: Nanotechnology of health design: A review. Journal of Saudi Chemical Society 18: 85-99. Link: https://tinyurl.com/yajjd55k
- Lee CC, Gillies ER, Fox ME, Guillaudeu SJ, Fréchet JM, et al. (2006) A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc Nat Acad Sci U S A 103: 16649-16654. Link: https://tinyurl.com/y7sogeq8
- Lobenberg R, Maas J, Kreuter J (1998) Improved body distribution of 14 Clabelled AZT bound to Mataraza Nanoparticles in rats determined by radioluminography. J Drug Target 5: 171-179. Link: https://tinyurl.com/y8byc63z
- Dutta T, Jain NK (200) Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim Biophys Acta 1770: 681-686. Link: https://tinyurl.com/yaagtym3
- Kreuter J (1994) Nanoparticles in Colloidal Drug Delivery Systems. J Kreuter Ed., Marcel Dekker, New York, NY, USA, 219-342.
- DK Chanchal, S Alok, S Rashi, RK Bijauliya, RD Yadav, et al. (2018) Various medicinal plants used in the treatment of anticancer activity. IJPSR 9: 1424-1429. Link: https://tinyurl.com/y9a2ayed
- Sakarkar DM, Deshmukh VN (2011) Ethnopharmacological review of traditional medicinal plants for anticancer activity. Int J Pharm Tech Res 3: 298-308. Link: https://tinyurl.com/yadhwzna
- Bangham AD, Horne RW (1964) Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol 8: 660-668. Link: https://tinyurl.com/yddqtnxj
- Kim S (1993) Liposomes as carriers of cancer chemotherapy. Current status and future prospects. Drugs 46: 618-638. Link: https://tinyurl.com/yavgrhz4
- Kulkarni p, Yadav JD, Kumar AV (2011) Liposomes: a novel drug delivery system. Int J Curr Pharm Res 3: 10-18.
- dos Santos Giuberti C, de Oliveira Reis EC, Ribeiro Rocha TG, Leite EA, Lacerda RG, Ramaldes GA, et al. (2011) Study of the pilot production process of long-circulating and pH-sensitive liposomes containing cisplatin. J Liposome Res 21: 60-69. Link: https://tinyurl.com/yayp5fbo
- Pandey H, Rani R, Agarwal V (2016) Liposome and their applications in cancer therapy. Braz Arch Boil Technol 59: 1–10. Link: https://tinyurl.com/ycsk7sbh
- Vemuri S, Rhodes CT (1995) Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharm Acta Helv 70: 95-111. Link: https://tinyurl.com/yazf56gl
- Rings DH (1975) Structure and properties of pharmacologically active polymers. Journal of Polymer Science Polymer Symposium 51: 135-153. Link: https://tinyurl.com/yb36g4sn
- Matsumura Y, Maeda H (1986) A new concept for macromolecular therapeutics in cancer chemotherapy mechanism of tumoritropic accumulation of proteins and the antitumour agent SMANCS. Cancer Res 6: 6387-6392. Link: https://tinyurl.com/y84a92fb
- Agnieszka Z, Wilczewska (2012) Nanoparticles as drug delivery systems. Pharmacol Rep 64: 1020-1037. Link: https://tinyurl.com/yc2lmgku
- Long JT, Cheang TY, Zhuo SY, Zeng RF, Dai QS, et al. (2014) Anticancer drug-loaded multifunctional Nanoparticles to enhance the chemotherapeutic efficacy in lung cancer metastasis. J Nanobiotechnology 12: 37. Link: https://tinyurl.com/yccg5tgy
- Masood F (2016) Polymeric Nanoparticles for targeted drug delivery system for cancer therapy. Materials Science and Engineering 60: 569-578. Link: https://tinyurl.com/y7rb6dk7
- Soppimath S, Kumaresh (2001) Biodegradable polymeric Nanoparticles as drug delivery devices. J Control Release 70: 1-21. Link: https://tinyurl.com/y6wpdvfy
- Basu A, Kunduru KR, Doppalapudi S, Domb AJ, Khan W (2016) Poly (lactic acid) based hydrogels. Adv Drug Deliv Rev 107: 192-205. Link: https://tinyurl.com/y9xp4cx9
- Xiao-Yun Lu, Dao-Cheng Wu, Zheng-Jun Li, Guo-Qiang Chen (2011) Polymer Nanoparticles. Progress in Molecular Biology and Translational Science 104: 299-323. Link: https://tinyurl.com/ycjylque
- Desai N (2012) Challenges in development of Nanoparticle-based therapeutics. AAPS J 14: 282-295. Link: https://tinyurl.com/y7w8ofcp
- Mora-Huertas CE, Fessi H, Elaissari A (2010) Polymer-based nanocapsules for drug delivery. Int J Pharm 385: 113–142. Link: https://tinyurl.com/yczxgqmt
- Torchilin V (2008) Multifunctional pharmaceutical nanocarriers. Springer Science + Business Media, LLC, NY 33-66. Link: https://tinyurl.com/y7gvag92
- Torchilin VP (2004) Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol Life Sci 61: 2549-2559. Link: https://tinyurl.com/ya2ccu7z
- Gao Z, Lukyanov AN, Singhal A, Torchilin VP (2002) Diacyl lipid polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett 2: 979-982. Link: https://tinyurl.com/ycnzhmq5
- Mu L, Chrastina A, Levchenko T, Torchilin VP (2005) Micelles from poly(ethylene glycol) phosphatidyl ethanolamine conjugates (PEG-PE) as pharmaceutical nanocarriers for poorly soluble drug camptothecin. J Biomed Nanotechnol 1: 190-195. Link: https://tinyurl.com/yaa4bwtr
- Wang J, Mongayt DA, Lukyanov AN, Levchenko TS, Torchilin VP (2004) Preparation and in vitro synergistic anticancer effect of vitamin K3 and 1,8-diazabicyclo[5,4,0]undec-7-ene in poly(ethylene glycol)-diacyllipid micelles. Int J Pharm 272: 129-135. Link: https://tinyurl.com/y95yuqrp
- Dong H, Shu JY, Dube N, Ma Y, Tirrell MV (2012) 3-Helix Micelles Stabilized by Polymer Springs. J Am Chem Soc 134: 11807–11814. Link: https://tinyurl.com/yalvvfxf
- Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M (2007) Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2: 249-255. Link: https://tinyurl.com/yafjhyl8
- DA Tomalia, H Baker, J Dewald, M Hall, G Kallos, et al. (1985) A New class of polymers: starburst-dendritic macromolecules. Polym J 17: 117-132. Link: https://tinyurl.com/yak25qza
- Carmo DR, Silveira SFT, Laurentiz SR, Bicalho ML, Filho DL, et al. (2013) Synthesis and a Preliminary Characterization of Poly (Proppylene)Imine Hexadecylamine Dendrimer (DAB-Am-16) Modified with Methyl Acrylate. American Chemical Science Journal 3: 314-324. Link: https://tinyurl.com/yagrkrz5
- Tomalia DA, Naylor AM, Goddard JW (1990) Starburst dendrimers: molecular‐level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angewandte Chemie Int 29: 138-175. Link: https://tinyurl.com/ybkw3pr5
- Jain K, Kesharwani P, Gupta U, Jain NK (2010) Dendrimer toxicity: Let's meet the challenge. Int J Pharm 394: 122-142. Link: https://tinyurl.com/ycwjeu2e
- Khopade AJ, Shenoy DB, Khopade SA, Jain NK (2004) Phase structures of a hydrated anionic phospholipid composition containing cationic dendrimers and pegylated lipids. Langmuir 20: 7368-7373 Link: https://tinyurl.com/y8v62qxz
- Wijagkanalan W, Kawakami S, Hashida M (2011) Designing Dendrimers for Drug Delivery and Imaging: Pharmacokinetic Considerations. Pharmaceutical 28: 1500-1519. Link: https://tinyurl.com/yaq3nvff
- Milhem O, Myles C, mckeown N, Attwood D, Emanuele A (2000) Polyamidoamine Starburst dendrimers as solubility enhancers. Int J Pharm 197: 239-241. Link: https://tinyurl.com/y9p5tqaj
- Yiyun C, Tongwen X (2005) Dendrimers as potential drug carriers. Part I. Solubilization of non-steroidal antiinflammatory drugs in the presence of polyamidoamine dendrimers. Eur J Medicinal chem 40: 1188-1192. Link: https://tinyurl.com/y9n5jeba
- Moghimi SM, Peer D, Langer R (2011) Reshaping the future of nanopharmaceuticals: ad iudicium. ACSnano 5: 8454-8458. Link: https://tinyurl.com/ybp9s7nk
- Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW (2012) Treating metastatic cancer with nanotechnology. Nat Rev Cancer 12: 39-50. Link: https://tinyurl.com/yaperq6f
- Duncan R (2003) The dawning era of polymer therapeutics. Nat Rev Drug Discov 2: 347-360. Link: https://tinyurl.com/ya2u7vbw
- Discher DE, Eisenberg A (2002) Polymer vesicles. Science 297: 967-973. Link: https://tinyurl.com/yas8mgzl
- Caterina LoPresti, Hannah Lomas, Marzia Massignani, Thomas Smarta, Giuseppe Battaglia (2009) Polymersomes: nature inspired nanometer sized compartments. Journal of Materials Chemistry 19: 3576-3590. Link: https://tinyurl.com/y9ejsnzc
- Massignani M, Lomas H, Battaglia G (2010) Polymersomes: A synthetic biological approach to encapsulation and delivery. modern techniques for nano- and microreactors/-reactions 229: 115-154. Link: https://tinyurl.com/yb9ea3zq
- Lee JS, Ankone M, Pieters E, Schiffelers RM, Hennink WE, et al. (2011) Circulation kinetics and biodistribution of dual-labeled polymersomes with modulated surface charge in tumor-bearing mice: comparison with stealth liposomes. J Control Release 155: 282-288. Link: https://tinyurl.com/y7srveyf
- Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, et al. (2006) Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J Control Release 116: 150-158. Link: https://tinyurl.com/y9ks75k2
- Seki T, Fang J, Maeda H (2009) Tumor-Targeted Macromolecular Drug Delivery Based on the Enhanced Permeability and Retention Effect in Solid Tumor. Pharmaceutical Perspectives of Cancer Therapeutics. 93-120. Link: https://tinyurl.com/y76pr6kx
- Mohsen J, Z Babaei (2008) Protein Nanoparticle A unique system as drug delivery vehicles. African Journal of Biotechnology. 7: 4926-4934. Link: https://tinyurl.com/ycxs8xdu
- Weber C, Coester C, Kreuter J, Langer K (2000) Desolvation process and surface characterisation of protein Nanoparticles. Int J Pharm 194: 91-102. Link: https://tinyurl.com/y7fbo2gg
- Ezpeleta I, Irache JM, Stainmesse S (1996) Gliadin Nanoparticles for the controlled release of all-trans-retinoic acid. International Journal of Pharmaceutics 131: 191-200. Link: https://tinyurl.com/yddm5z3h
- Schafer V, Briesen H, Andreesen R, Steffan AM, Royer C, et al. (1992) Phagocytosis of Nanoparticles by human immunodeficiency virus infected macrophages a possibility for antiviral drug targeting. Pharm Res 9: 541-546. Link: https://tinyurl.com/yabwcw5l
- Li L, Liu J, Diao Z, Shu D, Guo P, et al. (2009) Evaluation of specific delivery of chimeric phi29 pRNA/siRNA Nanoparticles to multiple tumor cells. Mol Biosyst 5: 1361-1368. Link: https://tinyurl.com/y7gab6er
- Saboktakin M (2017) The biological and biomedical Nanoparticles applications. Int J Mol Biol Open Access 2: 76‒87.
- Cong-fei Xu, Jun Wang (2015) Delivery systems for siRNA drug development in cancer therapy. Asian Journal of Pharmaceutical Sciences. 10: 1-12. Link: https://tinyurl.com/y6vpl2mg
- Tan W, Wang K, He X, Zhao XJ, Drake T, et al. (2004) Bionanotechnology based on silica Nanoparticles. Med Res Rev 24: 621-638. Link: https://tinyurl.com/y9n7dcp2
- Mark Stroh, John P Zimmer, Dan G Duda, Tatyana S Levchenko, Kenneth S Cohen, et al. (2005) Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat Med 11: 678-682. Link: https://tinyurl.com/y7gj5255
- Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, et al. (2005) Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307: 538-544. Link: https://tinyurl.com/ybr34pab
- Daniel MC, Astruc D (2004) Gold Nanoparticles assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104: 293-346. Link: https://tinyurl.com/yd7x6zmp
- Nichkova M, Dosev D, Gee SJ, Hammock BD, Kennedy IM (2005) Microarray immunoassay for phenoxy benzoic acid using polymer encapsulated Eu: Gd2O3 Nanoparticles as fluorescent labels. Anal Chem 77: 6864–6873. Link: https://tinyurl.com/y7jlcvt4
- Chen Y, Chi Y, Wen H, Lu Z (2007) Sensitized luminescent terbium Nanoparticles: preparation and time-resolved fluorescence assay for DNA. Anal Chem 79: 960-965. Link: https://tinyurl.com/ycjd8z6f
- Johannsen M, Gneveckow U, Eckelt L, Feussner A, Waldöfner N, et al. (2005) Jordan A Clinical hyperthermia of prostate cancer using magnetic Nanoparticles presentation of a new interstitial technique. Int J Hyperthermia 21: 637-647. Link: https://tinyurl.com/y7ke6jur
- El-Sayed IH, Huang X, El-Sayed MA (2006) Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold Nanoparticles. Cancer Lett 2: 129-135. Link: https://tinyurl.com/ybmvn6uw
- Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD (2007) The structure of a thiol monolayer-protected gold Nanoparticle at 1.1 Å resolutions. Science 318: 430-433. Link: https://tinyurl.com/yb9xfwyn
- Bhattacharya S, Srivastava A (2003) Synthesis of gold Nanoparticles stabilized by metal-chelator and the controlled formation of close-packed aggregates by them. Proc Indian Acad Sci (Chem Sci) 115: 613-619. Link: https://tinyurl.com/ydafsk5x
- Li L, Fan M, Brown R, Van LJ, Wang J, et al. (2006) Synthesis, properties and environmental applications of nanoscale iron-based materials: A review. Environ Sci Technol 36: 405-431. Link: https://tinyurl.com/y7p4ccal
- Han G, Martin CT, Rotello VM (2006) Stability of gold Nanoparticles bound DNA towards biological chemical physical agents. Chem Biol Drug Des 67: 78-82. Link: https://tinyurl.com/yb48az62
- Michael J Sailor, Ji Park Ho (2012) Hybrid Nanoparticles for Detection and Treatment of Cancer. Adv Mater 24: 3779-802. Link: https://tinyurl.com/yatbfwm4
- Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, et al. (1998) Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med 4: 623-626. Link: https://tinyurl.com/y8lx6gzm
- Storrs RW, Tropper FDH, Song YCK, Kuniyoshi JK, Sipkins DA, et al. (1995) Paramagnetic Polymerized Liposomes: Synthesis, Characterization, and Applications for Magnetic Resonance Imaging. J Am Chem Soc 117: 7301-7306. Link: https://tinyurl.com/y9ygcnge
- Song CX, Labhasetwar V, Murphy H, Qu X, Humphrey WR, et al. (1997) Formulation and characterization of biodegradables Nanoparticles for intravascular local drug delivery. J Control Release 43: 197-212. Link: https://tinyurl.com/y8b9mdm6
- Vineeth P, Rao PR Vadaparthi, Kumar K, Dileep B Babu, Veerabhadra Rao A, Suresh babu K (2014) Influence of organic solvents on Nanoparticle formation and surfactants on release behaviour in-vitro using costunolide as model anticancer agent. International Journal of Pharmacy and Pharmaceutical Sciences 6: 638-645. Link: https://tinyurl.com/ycydj7lz
- Liu J, Qiu Z, Wang S, Zhou L, Zhang S (2010) A modified double-emulsion method for the preparation of daunorubicin-loaded polymeric Nanoparticle with enhanced in vitro anti-tumor activity. Biomed Mater 5: 065002. Link: https://tinyurl.com/ydejy7cp
- Ubrich N, Bouillot P, Pellerin C, Hoffman M, Maincent P (2004) Preparation and characterization of propanolol hydrochloride nano particles: A comparative study. J Control release 97: 291-300. Link: https://tinyurl.com/yczh4gbd
- Jaiswal J, Gupta SK, Kreuter J (2004) Preparation of biodegradable cyclosporine Nanoparticles by high-pressure emulsification solvent evaporation process. J. Control Release 96: 169-178. Link: https://tinyurl.com/ybaembov
- Vandervoort J, Ludwig A (2002) Biocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design study. Int J Pharm 238: 77-92. Link: https://tinyurl.com/y779txlz
- Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE (2001) Biodegradable polymeric Nanoparticles as drug delivery devices. J Control Release 70: 1-20. Link: https://tinyurl.com/y6wpdvfy
- Lambert G, Fattal E, Couvreur P (2001) Nanoparticulate system for the delivery of antisense oligonucleotides. Adv Drug Deliv Rev 47: 99-112. Link: https://tinyurl.com/ybesk4yd
- Jung T, Kamm W, Breitenbach A, Kaiserling E, Xiao JK, et al. (2011) Biodegradable nano particles for oral delivery of peptides. Journal of Applied Pharmaceutical Science 6: 228-234.
- Quintanar-Guerrero D, Allémann E, Fessi H, Doelker E (1998) Preparation techniques and mechanism of formation of biodegradable Nanoparticles from preformed polymers. Drug Dev Ind Pharm 24: 1113-1128. Link: https://tinyurl.com/yazl6yx3
- Allemann E, Gurny R, Doekler E (1993) Drug-loaded Nanoparticles preparation methods and drug targeting issues. Eur J Pharm Biopharm 39: 173-191. Link: https://tinyurl.com/ybfjn7gw
- Ahmed R Gardouh, Mamdouh M Ghorab, Shaded GS Abdel-Rahman (2012) Effect of Viscosity, Method of Preparation and Homogenization Speed on Physical Characteristics of Solid Lipid Nanoparticles. ARPN Journal of Science and Technology. 2: 996-1006. Link: https://tinyurl.com/y9xg8tl8
- Salam M Habib, Ayed S Amr, Imad M Hamadneh (2012) nanoencapsulation of alpha-linolenic acid with modified emulsion diffusion method. Journal of the American Oil Chemists' Society 89: 695–703. Link: https://tinyurl.com/yaklyqhs
- Sarmento B, Martins S, Ferreira D, Souto EB (2007) Oral insulin delivery by means of solid lipid Nanoparticles. Int J Nanomedicine 2: 743-749. Link: https://tinyurl.com/y97drfyj
- Lu W, Zhang Y, Tan YZ, Hu KL, Jiang XG (2005) Cationic albumin conjugated pegylated Nanoparticles as novel drug carrier for brain delivery. J. Control Release 107: 428-448. Link: https://tinyurl.com/yc8bsybk
- Vargas A, Pegaz B, Devefve E, Konan-Kouakou Y, Lange N, Ballini JP (2004) Improved photodynamic activity of porphyrin loaded into nano particles: an in vivo evaluation using chick embryos. Int J Pharm 286: 131-145. Link: https://tinyurl.com/y98lrjjk
- Shabouri MH (2002) Positively charged nano particles for improving the oral bioavailability of cyclosporine-A. Int J Pharm 249: 101-108. Link: https://tinyurl.com/ycq3guqa
- Yoo HS, Oh JE, Lee KH, Park TG (1999) Biodegradable nano particles containing PLGA conjugates for sustained release. Pharm Res 16: 1114-1118. Link: https://tinyurl.com/y9bkwc7w
- Hideki Murakam, Masao Kobayashi , Hirofumi Takeuchi, Yoshiaki Kawashim (1999) Preparation of poly(dl-lactide-co-glycolide) Nanoparticles by modified spontaneous emulsification solvent diffusion method. Int J Pharm 187:143-152. Link: https://tinyurl.com/yar4qevb
- Cen Chen, Yang Wei, Dan Tong Wang, Chao Long Chen, Qing Zhuang, et al. (2014) A modified spontaneous emulsification solvent diffusion method for the preparation of curcumin-loaded PLGA Nanoparticles with enhanced in vitro anti-tumor activity. Frontiers of Materials Science 8: 332-342. Link: https://tinyurl.com/y9g5kpks
- Darshana Jain S, Rajani Athawale B, Bajaj Amrita N, Shruti S Shrikhande, Peeyush N Goel, et al. (2014) Unraveling the cytotoxic potential of Temozolomide loaded into PLGA Nanoparticles. Daru 22: 18. Link: https://tinyurl.com/y8rcpue7
- Jain D, Athawale R, Bajaj A, Shrikhande S, Goel PN, et al. (2013) Studies on stabilization mechanism and stealth effect of poloxamer 188 onto PLGA Nanoparticles. Colloids Surf B Biointerfaces 109: 59-67. Link: https://tinyurl.com/ya4t8mku
- Zhang HZ, Gao FP, Liu LR, Li XM, Zhou ZM, et al. (2009) Pullulan acetate Nanoparticles prepared by solvent diffusion method for epirubicin chemotherapy. Colloids and Surfaces B Biointerfaces 71: 19-26. Link: https://tinyurl.com/yb7jt69a
- Oh I, Lee K, Kwon HY, Lee YB, Shin SC, et al. (1999) Release of adriamycin from poly(γ-benzyl-l glutamate) /poly(ethylene oxide) Nanoparticles. Int J Pharm 181: 107-115. Link: https://tinyurl.com/ycxg5pkd
- Kandpal ND, Sah S, Loshali R, Joshi R, Prasad J (2014) Co-precipitation method of synthesis and characterization of iron oxide Nanoparticles. Journal of scientific & industrial research 73: 87-90. Link: https://tinyurl.com/ybgozdqf
- Kumar SS (2012) Chemical Synthesis of Zinc Oxide Nano particles by Precipitation Method. International Journal of Engineering and Technical Research 1-4.
- Pu X, Sun J, Wang Y, Wang Y, Liu X, et al. (2009) Development of chemically stable 10-hydroxycamptothecin nano suspensions. International Journal of Pharmaceutics. 379: 167-173. Link: https://tinyurl.com/ybqxyfoe
- Bilati Ugo, Allémann Eric, Doelker Eric (2005) Development of a nano precipitation method intended for the entrapment of hydrophilic drugs into Nanoparticles. European Journal of Pharmaceutical Sciences 24: 67-75. Link: https://tinyurl.com/y7gd3rg9
- Némati F, Dubernet C, Fessi H, Colin A, Verdière DE, et al. (1996) Reversion of multidrug resistance using Nanoparticles in vitro: Influence of the nature of the polymer. International Journal of Pharmaceutics 138: 237-246. Link: https://tinyurl.com/ybbfjk78
- Chacón ML, Berges J, Molpeceres MR, Aberturas S, Guzman M (1996) Optimized preparation of poly D,L (lactic-glycolic) microspheres and Nanoparticles for oral administration. International Journal of Pharmaceutics 141: 81-91. Link: https://tinyurl.com/ybovb7ng
- Yang Duk, Chun Kim, Yeon Ju, Kyung Hee (2018) Composition for producing a metal Nanoparticle comprising ginseng extract and use thereof. KR20180030493A. Link: https://tinyurl.com/y7exl8ub
- Yang Duk, Chun Kim, Yeon joo, Abra Lager (2018) A composition for producing metal Nanoparticles comprising Siberian ginseng extracts and the use thereof. KR20180036951A Link: https://tinyurl.com/yb267cd8
- Hou Zhenqing, Song Liang, Yang Wang Li, Yange Zhang, Xiuming (2018) Methotrexate prodrugs and one kind of dual targeting method for preparing Nanoparticles. CN108014346A.
- Chul-hee Won, Min-hee D, Kim Sung Chan (2018) Synthesis of carbon Nanoparticle-polymer composite for delivery of bioactive materials and the uses thereof. KR20180014429A.
- Yuan Zhi, Zhang Yahui (2017) Self-targeted anti-cancer nano-particle and preparation method thereof. CN107596384A.
- Jingwei Shao, Zhichun Shen, Yuehuang Wu (2017) Self-assembled Nanoparticles based on low-generation PAMAM (polyamidoamine) dendrimer loaded anti-cancer drugs and application of self-assembled Nanoparticles in anti-tumor field. CN107281164A. Link: https://tinyurl.com/ybs9a8st
- Feng Lei, Dai Haiwei, Jiang Hanming, Jing Yu (2017) Nanoparticle for killing cancer cells and preparation method thereof. CN107041876A.
- Jingwei Shao, Aixiao Xu, Chen Sijia, Guo Yan (2017) Carrier-free co-assembled tumor targeting anti-cancer nano medicine as well as preparation method and application thereof. CN107158014A.
- Xu Qin, Zhang Baolin, Yuan Cancan, Lichao Su (2017) Folic acid mediated antitumor drug superparamagnetic tumor targeted Nanoparticle and preparation method thereof. CN107375235A.
- Donghang Xu, Jianqing Gao, Huzongquan Fu (2017) Preparing Nanoparticles. CN107823183A.
- Bin Li, Xiguang Ye, Xiaorong Lin, Zhongzheng Chen, Yuanyuan Zhang, et al. (2017) A method of selenium Nanoparticles prepared using a water extract of Camellia plants and prepared by nano selenium. CN107758628A.
- Wang Bing, Liang Junlong, Chen Ruru, Jin Li, Hu Qinli (2017) Preparation method of polymeric Nanoparticles based on double-layer synergistic controlled-release medicine delivery. CN107320459A.
- Ling Li, Chen Xu, Yana Liu, Zhennan Shi, Fan Lu (2017) Nanoparticles porous Prussian blue and its preparation method and application of the wrapped amino silica. CN107929756A.
- Gao Wei, Guihua Ye (2017) PH-sensitive targeted LPNs (lipid poly-L-histidine hybrid Nanoparticles) for encapsulating anti-tumor drugs. CN107551277A.
- Bing Wang, Junlong Liang, Ruru Chen, Jin Li, Yiwei Huang (2017) Preparation method of nanometer micro particles embedded with anti-cancer medicine loaded carbon quantum dots. CN107397958A.
- Jiandu Lei, Yanxue Liu, Yongli Cao, Zheng Duo, Luo Min, et al. (2017) One kind of modified pectin folate Nanoparticles dual targeting delivery method. CN107970453A.
- Chang Cai, Min Liu, Yanna Zhao, Han Jun (2017) A pharmaceutical - phospholipid / albumin complexes and preparation of Nanoparticles. CN107753435A.
- Jieqiao Pan, Hong Yan, Xiaoli Liao, Yang Li, Zhihui Li, et al. (2017) Preparation of polyoxometallate supported anticancer nanometer preparation. CN107137721A.
- Mingjuan Xu, Leilei Xia, Wang Ye, Zhang Caihong, Shengyu Cai, et al. (2017) Applications of cuprous oxide Nanoparticles in preparation of drug for treating gynecological tumors. CN107050051A.
- Jiandu Lei, Yanxue Liu, Yongli Cao, Zheng Shuo, Min Luo, et al. (2017) A novel dual targeting pectin - preparing multi-arm polyethylene glycol anticancer drug combined. CN107737347A.
- Yanqing Guan, Shiwei Du, Lingkun Zhang (2017) Preparation method and application of photosensitive magnetic Nanoparticle system capable of inhibiting growth of breast cancer cells. CN106668871A. Link: https://tinyurl.com/y8qttguh
- Jiang Hulin, Xing Lei, Yang Chenxi (2017) Amphipathic non-steroidal anti-inflammation platinum Nanoparticle and preparation method thereof. CN107296794A.
- Qiang Yi, Kang Ying, Kang Ma, Gu Zhongwei Yi (2017) Drug-loaded hybrid Nanoparticle and preparation method thereof. CN107243000A.
- White AW, Almassy R, Calvert AH, Curtin NJ, Griffin RJ, et al. (2000) Resistance-Modifying Agents, Synthesis and Biological Properties of Benzimidazole Inhibitors of the DNA Repair Enzyme Poly(ADP-ribose) Polymerase. J Med Chem 43: 4084–97. Link: https://tinyurl.com/ya9tq9x8
- Musella A, Bardhi E, Marchetti C, Vertechy L, Santangelo G, et al. (2018) Rucaparib an emerging parp inhibitor for treatment of recurrent ovarian cancer Cancer Treat Rev 66: 7-14. Link: https://tinyurl.com/y9w3ffgy
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, et al. (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. New England Journal of Medicine. 369: 134-144. Link: https://tinyurl.com/ya5dfkuu
- (2010) PARP inhibitor, MK-4827 shows anti-tumor activity in first trial in humans. Link: https://tinyurl.com/ycodww82
- (2016) Novartis LEE011 (ribociclib) granted FDA Priority Review for first-line treatment of HR+/HER2- advanced breast cancer", Novartis. Link: https://tinyurl.com/y77d9yvn
- Huang WS, Liu S, Zou D, Thomas M, Wang Y, et al. (2016) Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J Med Chem 59: 4948-4964. Link: https://tinyurl.com/yawawymg
- Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D (2002) Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 1: 433-443 Link: https://tinyurl.com/y94a7p4w
- (2017) Research, Center for Drug Evaluation and. "Approved Drugs - Durvalumab (Imfinzi)". Link: https://tinyurl.com/74cm93d
- (2008) Why San Diego Has Biotech", Fikes, Bradley, J. San Diego Metropolitan. April 1999.
- Seyfizadeh N, Seyfizadeh N, Hasenkamp J, Huerta-Yepez S (2016) A molecular perspective on rituximab A monoclonal antibody for B cell non Hodgkin lymphoma and other affections. Crit Rev Oncol Hematol 97: 275-290. Link: https://tinyurl.com/ybc9dxkq
- Baselga J, Coleman RE, Cortés J, Janni W (2017) Advances in the management of HER2-positive early breast cancer. Crit Rev Oncol Hematol 119: 113-122. Link: https://tinyurl.com/y97dnoql
- (2011) Puma Acquires Global Rights to Pfizer's Phase III Breast Cancer Drug Neratinib. GEN. Link: https://tinyurl.com/y8aynjcz
- (2017) Daunorubicin hydrochloride. The American Society of Health-System Pharmacists.
- Kim ES (2017) Enasidenib First Global Approval. Drugs 77: 1705-1711. Link: https://tinyurl.com/y9wq8pk3
- (2017) BESPONSA 1 mg powder for concentrate for solution for infusion. UK Electronic Medicines Compendium. Link: https://tinyurl.com/ybtv9o2h
- BLA 125646 Tisagenlecleucel - Novartis Briefing document to FDA ODAC. Link: https://tinyurl.com/ybnlxenv
- Los M, Roodhart JM, Voest EE (2007) Target practice lessons from phase III trials with bevacizumab and vatalanib in the treatment of advanced colorectal cancer. Oncologist 12: 443-450. Link: https://tinyurl.com/y9dhy8fs
- Palmer AM, Stephenson FA, Williams RJ (2007) Society for Medicines Research 40th anniversary symposium. Drug News Perspect 20: 191-196. Link: https://tinyurl.com/y7wvajz6
- FDA prescribing information for Aliqopa. Link: https://tinyurl.com/ycr2vbzm
- Gemtuzumab ozogamicin Link: https://tinyurl.com/yb78awpu
- (2017) FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. Link: https://tinyurl.com/ycduqskq

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
