International Journal of Immunotherapy and Cancer Research
Rosuvastatin inhibit the cell proliferation and the expression of angiogenic factors Vascular Endothelial Growth Factor and Matrix-Metalloproteinase-9 in DU-145 human prostate cancer cells
Abdolkhaleg Deezagi1*, Armaghan Jamalnia2 and Hanieh Jaefary2
2Department of Biochemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran
Cite this as Deezagi A, Jamalnia A, Jaefary H (2021) Rosuvastatin inhibit the cell proliferation and the expression of angiogenic factors Vascular Endothelial Growth Factor and Matrix-Metalloproteinase-9 in DU-145 human prostate cancer cells. Int J Immunother Cancer Res 7(1): 008-014. DOI: 10.17352/2455-8591.000031
Background: Pathophysiology angiogenesis refers to uncontrollable endothelium capillary proliferation, it can be seen in diseases such as growth and metastasis of tumors. The aim of this was to study the effects of Rosuvastatin on the cell growth and expression of the Vascular Endothelial Growth Factor (VEGF) and matrix metal proteases (MMP-9) genes in human prostate cancer DU-145 cells.
Methods: DU145 cells were cultured in RPMI medium containing 10% FBS, then treated with Rosuvastatin (5-100μM) for 96 hr. The cell proliferation was analyzed by cell counting, MTT and trypan blue tests. Nitric oxide production was assayed by Greis test. The wound healing scratching test was employed to analyze the cell migration. The VEGF and MMP-9 gene expression was analyzed by RT-PCR.
Results and conclusion: The Rosuvastatin can cause a higher rate of inhibition in cell proliferation and growth which accomponish by inhibition of cell migration in scratching assay. Considerably, decrease in cell growth and migration was observed in half of the DU145 cells, treated by 25 µM rosuvastatin. NO assay showed that high concentrations of the medication are able to reduce NO production and release. Moreover, VEGF-A and MMP-9 genes expression could be inhibited by 50 µM of Rosuvastatin.
Introduction
Prostate cancer can be considered malignant tumor which grows external part of the gland to develop gradually in the internal part, then, is transmitted to all parts of the body via blood flow. Although there is no diagnosed symptom in the cancer at the primary stage, the tumor steadily grows to put pressure on urinal, consequently [1]. Tumor tissues are able to adsorb nutrients and oxygen up to one to two millimeters radii, then it is compulsory to form new feeding vessels network [2]. During oncogenesis and cancer progression the cancerous cells can induce to form new vessel from the available arterioles network as similar to normal angiogenesis. It means that more tumor size increases, more the environment becomes hypoxic and oxidized to express and produce increasingly several kinds of growth factors for developing local blood vessels, as the consequence [3]. Angiogenesis can be stimulated in tumor by many involving factors such as growth factors, whether specific or non-specific for endothelial tissue, could be considered as the angiogenesis inducers in tumor [4].
Vascular endothelial growth factors (VEGFs) plays the main and crucial role to induce angiogenesis. Five members have been reported for VEGFs’ family. Some tumor cells release VEGF A protein to affect tumor microenvironment, containing stromal surrounding cells for stimulating VEGF F promoter to express the gene. So, there is a collaboration and correlation between normal and cancerous cells [5,6]. Not only VEGFs can induce the proliferation of the endothelial cells, but also increase their permeability. The aspect can be effective to start angiogenesis, because well-developed and grown vessels have to be unstable, before branching [7].
Also, extracellular matrix metalloproteinases (MMPs) which are playing remarkable roles in the constructive trends of the body structures and forming, effectively involve in tumor angiogenesis and metastasis [8]. Digestion of the basic membrane components such as collagen and fibronectin, by MMPs, is the most important stage of the polyphase pathway to cause metastasis. Many studies have demonstrated that a high level of MMPs, particularly MMP-2 and MMP-9 which are released by epithelial cells, relates to increased metastasis in prostate cancer[9]. It is considered that MMP-2 and MMP-9 are profoundly expressed by stromal cells, whilst other MMPs are biosynthesized by tumor cells [9]. In contrast, MMPs digest plasminogen to release angiostatin as an inhibitor of angiogenesis. The inhibitor, then, binds to its specific receptor on the surface of the endothelial cells. Endostatin, a collagen fragment released by the protolithic activity of the elastase, can also inhibit MMPs [10].
Statins are the competitive inhibitors of the 3‑hydroxy‑3‑methylglutaryl‑coenzyme A (HMG‑CoA) reductase that are widely applied to treat cardiovascular disorders, particularly hyperlipidemia, at the present time. Recent studies demonstrate that statins can significantly act as antioxidant and anti-inflammatory compounds, independent on the antilipidemic effects [11,12]. Rosuvastatin is a new generation and artificial type of the statins. There are many studies to report the anticancer activities of the medications on several types of the cancer cells[13]. Erbaş, et al. evaluated the rosuvastatin effects on the activity of the arginase, polyamines including putrescine, spermidine and spermine, and ornithine levels, in cancer tissue. They found that rosuvastatin can protectively affect breast cancer, as well as inhibition of arginase activity and ornithine and consequently polyamine levels [14]. It is also reported that rosuvastatin can individually inhibit colon adenocarcinomas development. It is also more effective for excluding polyamines and improving the activity of the natural killer cells [15]. El Sayed, et al. could find some signals which are play roles in the antitomur effects of resuvastatin to influence positively on hepatocellular carcinoma by inhibiting p-focal adhesion kinase/p-rous sarcoma oncogene cellular homolog cascade [16]. A recent study showed that polymeric nanocapsules loaded with resuvastatin have anticancer activity on hepatocellular carcinoma by enhancing apoptosis [17]. Also, Kheiri, et al. recently used resuvastatin- loaded nanoparticles to suppress MCF-7 cell lines of the breast cancer [18] The literature reviews show that blocking angiogenesis by some small chemical inhibitors might have very limited effect on cancerous cells growth. Interest in finding anti-angiogenesis agents for cancer prevention and inhibition is attractive. Understanding the molecular mechanism and their biological actions are necessary too. Because of some limitation and disadvantage of each agent to inhibition of these processes are under investigation. Therefore, the aim of this work was to investigate the effect of Rosuvastatin on the growth, proliferation and migration of human prostate cancer DU-145 cells and correlation of them by VEGF and MMP-9 genes expression.
Materials and methods
Cell preparation and culture
DU-145 cells is a standard cell line of the prostate cancer, used in remedial studies [19]. The cell line was provided from in National Institute for Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran, and prepared for the experiment which is granted by the organization. The cells were cultured on RPMI medium (Bio – IDEA, Modena, Italy), containing FBS 10% and added 120mg Penicillin, 200mg Streptomycin and 3.7g sodium bicarbonate. The cells’ growth and proliferation was monitored by optical microscope. The cells were trypsinized and passaged every three or four days, when the cells confluency reached to 90%.
Resuvastatin treatment
2.0 ×105 DU-145 cells were seeded in each wells of 6 well plates. 2.0 ×104 of cells were seeded in each microwells of 96 flat bottom micro wells for MTT assay (Nunc, Roskilde, Denmark). The cells were incubated overnight for adherence. A stock solution of Rosuvastatin (5mM) (Aria Daru, Tehran, Iran) was prepared in Dimethyl Sulfoxide (DMSO) (Merck, Dermashtd, Germany). The cells were treated by different concentration of Rosuvastatin (0, 5, 10, 25,50 and 100µM) in RPMI medium, supplemented with 10% of FCS and incubated for 96 hr at 37°C. In some experiments DMSO (as a solvent) was added to medium alone (as a cell free system) and/or in above condition as controls. After 96 hr, the cells were collected and the total cell number, viability and MTT cell proliferation assays were done as below. Cell number and viability was enumerated using a Neobar hemocytometer.
MTT assay
MTT assay approach was utilized to measure cells proliferation for analysis of the rosuvastatin effects on the cells. Breifly, 10µl MTT was added to each well, then the cells were incubated for 4 hours. Then the supernatants were aspirated. The diformazan dyes which utilized by the cell pelelts were sulibilised by adding 100 µL of DMSO. They were shacked for 15s by the shaker of the ELISA reader and analyzed by the Elisa reader at 580nm.
Measurement of the generation of Nitric Oxide (NO)
The amount of NO generation from DU-145 cells were assayed after treatment by Rosuvastatin. Cell-free supernatants were recovered after incubation. Produced NO was quantitatively measured as NO3- (Nitrate: a stable metabolite of NO) and NO2- (Nitrite) concentrations by using enzymatic and colorimetric NO assays[19]. In this method, we used reduction of nitrate by commercially Aspergilus niger nitrate reductase and measurement of the product (Nitrite) by Griess reagent in micro titer plate. In brief, 50 µl of cells condition medium or standards were added to each well. Just prior to each assay freshly made solutions of NADPH ( 0.02 M ) (Sigma, St. Louis, MO, USA)10µl, mixed solution of Glucose-6- Phosphate 50mM (Sigma, St. Louis, MO, USA)and Glucose-6- Phosphate Dehydrogenase (100 U/ml) 23 µl (Sigma, St. Louis, MO, USA), Nitrate Reductase 0.1 U/ml, 10 µl (Sigma, St. Louis, MO, USA)and 7µl of Tris buffer 1.0 M pH 7.5 were prepared separate and added to related wells. Reactant were mixed finely and incubated at room temperature for 30 min. After that 100µl of freshly prepared Greiss reagent, (1% Sulfanilamide (Sigma, St. Louis, MO, USA) and 0.1% Naphtylenediamine (Sigma, St. Louis, MO, USA) in 2.8% of ortho-phosphoric acid ) was added to each well and incubated at room temperature for 10 min Finally the absorbance were measured at 550nm by ELISA reader ( Labsystems Multiskan, Roden, Netherlands).
Gene expression
Rosuvastatin treated DU-145 cells were collected and total RNAs were extracted by RNX-Plus Kit (SinaClon Co., Tehran, Iran), according to the manufacture’s protocol. Then the quality of the nucleic acids was measured by Nano-drop (Beckman Coulter Inc. CA, USA) at A 260/280 and A230/ 280. After that, RT-PCR, and consequently Real-time PCR, were employed by the designed specific primers as described in Table 1 to analyze VEGF and MMP-9 gene. First-strand cDNA was synthesized from 1 μg of total RNA using Maurine Maloney Leukemia virus (M-MLV, Fermentas) and reverse transcriptase (Fermentas Gmbh, Leon-Rot, Germany) with oligo-dT primer (Fermentas Gmbh, Leon-Rot, Germany), according to the manufacturer’s instructions.
The expected sizes of the RT-PCR product were 121 bp for GAPDH and 250 bp for VEGF. The thermal cycling conditions for amplification of those fragments were as follows: 94 °C for 10 min., followed by 35 cycles at 94 °C for 50s; 54 °C for 30s; 72 °C for 60s. This was followed by re-extension at 72 °C for 10 min. The PCR products were separated on a 2% agarose gel (using 0.5× TBE buffer) and visualized by ethidium bromide staining. For quantitation each band of gel was scanned by image analysis program (ImageJ. Exe). The expression of the target genes was quantified and then normalized by an endogenous reference housekeeping gene (GAPDH) relative to the calibrator (untreated cells). Relative intensity of each gene mRNA and fold of change referred to untreated control cells.
RT-PCR and real-time quantitative detection of the VEGF and MMP-9 mRNAs
Quantitative real-time PCR was carried out on cDNAs prepared for conventional RT-PCR. We used the ABI PRISMTM 7700 Sequence Detector System (PE Applied Biosystems) and the fluorescent dye SYBR® Green and the amount of PCR products was determined based on the fluorescence produced during the extension step of each cycle in a closed tube. To normalize the amount of total RNA present in each reaction, we used GAPDH as housekeeping gene an internal control. The threshold cycle (Ct) value for each sample was proportional to the log of the initial amount of input cDNA. Relative expression levels of VEGF and MMP-9 in each treatment group were derived from normalizing the Ct value of genes against that of an endogenous reference and a calibrator, where by GAPDH was used as a normalizer and untreated DU-145 cells as a calibrator. Quantitative data were analyzed and relative quantification of VEGF and MMP-9 mRNAs were derived by the 2-ΔΔCT methods, as described by Livak [20].
Quantitative ELIZA for VEGF
The amount of secreted VEGF in response to Rosuvastatin by DU-145 cells was assayed in supernatants by using commercial Calbiochem human VEGF ELISA kits. (Calbiochem-Merck-Bioscience, Darmstadt, Germany) according to the procedure manual of the kits. The method is a ‘‘sandwich’’ enzyme immunoassay employing monoclonal and polyclonal antibodies. Quantitation is achieved by construction of a standard curve using known concentrations of VEGF calibrators of the kit.
Scratching assay
Cell migration was measured using the wound healing assay as described in our previous work [21]. Briefly, equal numbers of DU-145 cells (5×105) were plated and kept overnight in 6-well plates. After that the monolayer of cells was carefully wounded by manual scratching with a micro-pipette tip head. The cells were treated with different concentration of Rosuvastatin and incubated for 96 hr. The wounded regions were photographed at different times interval (0-96hr) using a phase contrast microscope (Nikon). The photographs of the cells were analyzed using a Cell science software program. The distance between the two sides of scratch layers of images taken from migration of all concentrations at specified times was determined by the Cell science software.
Statistical analysis
The data were statistically analyzed by One-way ANOVA test via Graphed software, and the graphs were drawn by Microsoft Office Excel 2016.
Results
Cell morphology
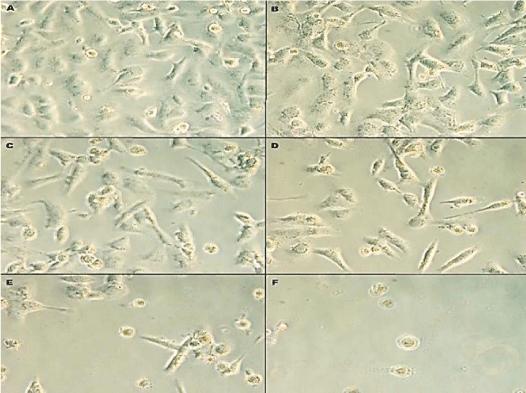
The response of human prostate cancer cells line DU-145 to Rosuvastatin were initially assayed by cells morphology and behavior, cell growth, viability and MTT assay. The cells morphology was observed by microscope to assay qualitatively the effects of Rosuvastatin on DU145 cells after 96 hr treatment of cells. The phenotypic changes and morphology of the cells in response to Rosuvastatin was shown in Figure 1. With increasing of Rosuvastatin concentration the cell density, confluency and adherence were decreased. In untreated control cells, the cells fully confluenced, stretched, spindle-shaped, and without appendages which are pressed together (B). The cells treated by 10,25,50 and 100µM of Rosuvastatin respectively (C,D,E and F). The morphology of treated cells was changed. The cells confluency were decreased and cell proliferation were suppressed by increase of Rosuvastatin concentration.
Cell proliferation
The cells, viability and growth were determined by using trypan blue exclusion test, cell counting and MTT cell proliferation assays. The result of cell counting show that Rosuvastatin inhibited cell growth potential and cell proliferation parameters up to 10% in the presence of Rosuvastatin 50-100µM in comparison of untreated control cells (R2 = 0.96) (p<0.05) (Figure 2a). In MTT assay, the absorbance values of 4 independent tests were averaged and growth curves were constructed (Figure 2b). The results indicate that, Rosuvastatin inhibited cell proliferation of DU-145 cells in a linear dose dependent manner (R2 = 0.97). The cells viability was decreased from 98% to 45% in presence of 5 to 100 µM of Rosuvastatin (Figure 2c). The results indicated that cells were still viable (>70%) after treatment with 25 µM of Rosuvastatin.
Nitric oxide production
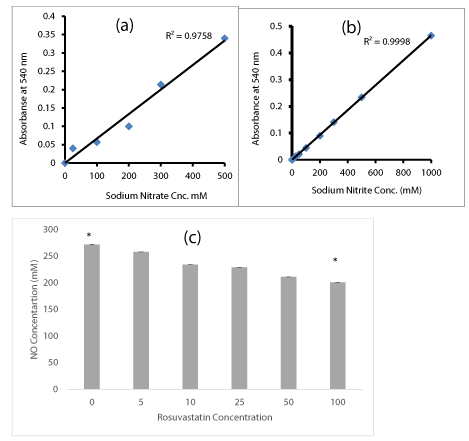
The response of DU-145 cells to Rosuvastatin and Nitric Oxide production were assayed in cells supernatant. The supernatants were assessed for yield of released NO. The assays were carried out as explained in methods those factors should tightly controlled to prevent failure of the assay. An assay failure was defined as the inability to generate a standard curve in the range of 0.05-2.0 AU. Initially the standard calibration curves of Sodium Nitrite and Sodium Nitrate were plotted. The measured absorbance was plotted against the sodium Nitrate (Figure 3a) or Sodium Nitrite (Figure 3b) concentrations. A higher degree of correlation was observed in standard curves. When the DU-145 cells were treated by Rosuvastatin, the amount of released NO in culture medium was quantified by measurement of NO metabolites (Nitrate and Nitrite) as described in methods. DU-145 cells those treated with Rosuvastatin specially in higher doses, (50-100 µM ), significantly reduced the production and release of NO to 20-30% in comparison to untreated cells.
Gene expression and VEGF secretion
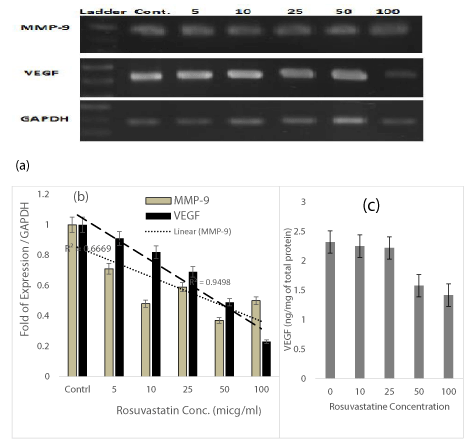
So as to examine level of VEGF and MMP-9 mRNA expression in DU-145 cells in response to Rosuvastatin RT-PCR and real-time PCR were performed using RNA from untreated and treated cells. The quantity of the target was normalized by an endogenous reference (GAPDH) relative to the calibrator (untreated cells). This method revealed the expression of VEGF mRNA, was suppressed from 10-80% in presence of 5-100 µM of Rosuvastatin in comparison to untreated control cells(R2=0.95) (Figure 4b). The results presented in Figure 4a show that Rosuvastatin treatment of DU-145 cells indicated to inhibition of the MMP-9 gene expression from 30-60% in presence of 5-100 µM of Rosuvastatin in comparison to untreated control cells too (R2=0.67) (Figure 4b).
VEGF content in supernatants were quantitavely measured by using VEGF ELISA test too. The VGEF content of samples were calculated from the standard curve of calibrators of the kit. Results were shown in Figure 4c. The VGEF content was decreased about 25-35% in presence of 50 – 100 µM of Rosuvastatin.
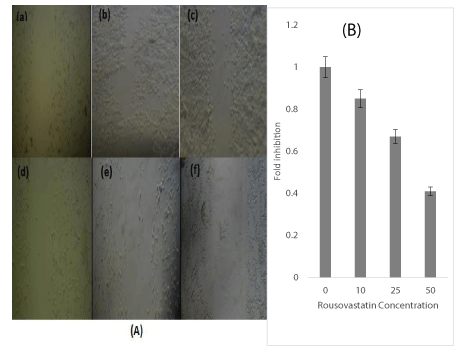
The effect of Rosuvastatin on the proliferation and migration of DU-145 by scratching assay. The monolayer of treated and untreated cells was carefully wounded by manual scratching with a micro-pipette tip head as described in the Materials and Methods. A) The wounded regions were photographed after 96hr using a phase contrast microscope. a, wounded region at time zero b,c,d,e and f (untreated, 10 ,25,50 and 100 µM of Rosuvastatin respectively) the proliferation and migration of cells after 96 hr incubation in presence of 0, 10 ,25,50 and 100 µM of Rosuvastatin respectively. The two sides of scratch layers of images taken from migration of all concentrations was determined by the Cell science software. The average migration distance in each photograph was calculated(B). *indicated Rosuvastatin concentrations in comparison with untreated control cells(p<0.05). All experiments were repeated 3 times in duplicates. The results are mean ± 1.0 SD for three separate experiments.Cell scratching and migration
The effect of Rosuvastatin on proliferation and migration of DU-145 cells were done by scratching assay as described in the Materials and Methods. The wounded regions were observed at different times interval (0-96hr). The photographs of migrations after 96 hr were shown in figure 5a. The distance between the two sides of scratch layers of images taken from migration of all concentrations was determined by the Cell science software. The average migration distance in each photograph was calculated. The percentage difference for each concentration at the same specified time was calculated and compared to untreated control cells. The calculated data were presented as a graph generated (Figure 5b). The cell migration was inhibited about 20-60% in presence of 10 -50 µM of Rosuvastatin (p<0.05) in comparison to untreated cells
Discussion
Prostate cancer is considered as the second common type of cancer after skin cancer, as well as the second fatal cancer in males after lung cancer, in developed countries. Different mediator molecules can play roles to induce angiogenesis in the tumor. Some vascular growth factors such as VEGF are specific for epithelial tissue, whilst the other factors such as MMPs are able to generally influence a wide range of tissues[22]. These activator factors can be released by the tumor cells, surrounding tissues, and macrophages, and fibroblasts entering into the tissue. It has been demonstrated that VEGF enhances vascular permeability, as the prominent and fundamental stage for angiogenesis in tumors and wounds. So, the vascular permeability induces the formation of fibrin gel, as a substrate for the growth and development of the tumor and epithelial cells [23]. MMPs are endopeptidases, depended on zinc, to play remarkable roles in the degradation of the cell surface receptors, cellular proliferation, migration, and differentiation, apoptosis and also angiogenesis. The function of these metalloproteases is regulated by a complex including activators, inhibitors, and cell surface receptors [24]. It has been shown that the inhibitors of MMPs can negatively influence angiogenesis, significantly. VEGF_A and MMP-9 genes can positively stimulate the expression and release each other [25].
The angiogenesis plays important role in the invasion and progression of solid tumors. For growing of tumor the cancerous cells must to promote angiogenesis. Cholesterol is one of the players in blood vessels formation especially in androgen dependent organs such as breast, prostate and ovary. It has been probably suggested that statins exert anti-angiogenic effects to prevent metastasis by down-regulating of angiogenic factors, inhibition of cell adhesion to the extracellular matrix and inhibition of endothelial cells proliferation[26]. Our result show that Rosuvastatin changed the morphology of the DU-145 cells from a narrow spindle fibroblast like cells. These alterations inhibited the cells in losing their adhesiveness and could probably dissolving the extracellular matrix, thereby cause inhibition of the invasiveness and migration in scratching assay. The regulation of mevalonate by statins leads to the reduction of cholesterol level and its, metabolites such as isoprenoids, farnesyl pyrophosphate and ‘geranyl geranyl pyrophosphate, which are found in multi-protein portions (e.g. Ras, Rho, i, E and Roc) which some of cellular processes such as cell signaling, differentiation, reproduction, and apoptosis modulate by these intermediates[27]. Our result shown that Rosuvastatin inhibit the migration of DU-145 cells in scratching assay. This property of Rosuvastatin is probably due to the pleiotropic effects of statins on cancer and the inhibition of the RhoA / Rho kinase / NF-jB pathway in breast cancer cells and the minimization of genes involved in cell invasion, such as tissue plasminogen activator urokinase, metalloproteinases and show chemo-sensitizing effects by impairing Ras family GTPase signaling [28].
Nowadays, there are many increasing studies to report statin effects, as well as cardiovascular treating advantages, such as anti-inflammation, immune system regulation, and antioxidant activity. Anti-tumor in vitro experiments on model animals have indicated that statins are able to inhibit cancer cell proliferation and induce apoptosis, then reduce tumor growth. Thus, statins have been considered as a potential medications for cancer treatment, over the last decades. Recent findings show that high concentrations of statins are able to permeate indirectly into the tumor cells to inhibit angiogenesis, however, side effects should be considered [29]. There are some in vitro and in vivo studies to show that fluvastatin and lovastatin can prevent metastasis and growth of the pancreatic cancer cells, as well as atorvastatin inhibiting metastasis in melanoma cells [30].
As the results of the present study, raising rosuvastatin dose can effectively inhibit the growth of DU145 cells, so that, 100 µM rosuvastatin extremely shows an inhibitory effect on the growth and proliferation of these cells. The medication is able to restrict tumor development vascular because rosuvastatin can reconstruct vessels in systemic malignancies. The drug can also influence as an inhibitor on tumor growth and development of malignant prostate cancer. In a recent study, it has been reported that long-term consumption of high doses of rosuvastatin can decrease the risk of breast cancer and, similarly, inhibit breast cancer cell invasion and metastasis [31]. The results of the present study demonstrated that although higher doses of rosuvastatin indicate more inhibitory effects on the expression of the VEFG gene, as an important factor in angiogenesis, there was no inhibitory effect of the drug on the expression of the MMP-9 gene as the other factor contributing in angiogenesis. Many studies have been indicated dual effects of the different doses of the statins on angiogenesis in tumor. These medications, for instance, reduces VEFG production and inhibit the capillary tube formation. As the findings of this study, statins are interestingly able to both enhance and inhibit angiogenesis. In other words, lower concentrations of rosuvastatin enhance angiogenesis, in contrast, inhibitory effects on the angiogenesis could be observed in higher doses of the drug. Therefore, dose plays a key role for enhancing or inhibiting angiogenesis. In another study, it has been reported that there is no same sensitivity to atorvastatin among six cancer cell lines, related to brain, colon, uterus, skin, prostate, and breast.
Conclusion
According to the results, it is concluded that higher concentrations of the Rosuvastatin can cause a higher rate of inhibition in cell proliferation and growth which accomponish by inhibition of cell migration in scratching assay. Considerably, decrease in cell growth was observed in half of the DU145 cells, treated by 25 µM rosuvastatin. NO assay showed that high concentrations of the medication are able to reduce NO production and release. Moreover, VEGF-A and MMP-9 genes expression could be inhibited by 50 µM of Rosuvastatin.
This work was supported by research funds (No. 540) from National Institute of Genetic Engineering and Biotechnology, Tehran- Iran.
Ethical approval
The study protocol was approved by the Research Ethics Committee at National institute of Genetic Engineering and Biotechnology (Tehran-Iran).
- Litwin MS, Tan H (2017) The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 317: 2532–2542. Link: https://bit.ly/3bffvQ8
- Roozbahani M, Jamshidian H, Mahmoudi E, Arshi A (2018) Angiogenesis: A review of molecular mechanism. Scientific Journal of Iran Blood Transfus Organ 15: 59-70.
- Zuazo-Gaztelu I, Casanovas O (2018) Unraveling the role of angiogenesis in cancer ecosystems. Front Oncol 8: 248. Link: https://bit.ly/3hd9dEv
- Ramezani T, Baharara J (2014) A review on Angiogenesis in tumor. Journal Cell Tissue 5: 89-100. Link: https://bit.ly/3fahDcW
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, et al. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacological Rev 56: 549-580. https://bit.ly/3xSSnk2
- Costache MI, Ioana M, Iordache S, Ene D, Costache CA, et al. (2015) VEGF expression in pancreatic cancer and other malignancies: a review of the literature. Rom J Intern Med 53: 199-208. https://bit.ly/3uBtkjp
- Álvarez-Aznar A, Muhl L, Gaengel K (2017) VEGF receptor tyrosine kinases: key regulators of vascular function. Curr Top Dev Biol 123: 433-482. Link: https://bit.ly/3ttjPl2
- Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M (2016) Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int J Mol Sci 17: 868. https://bit.ly/2RFF31Q
- Baci D, Bruno A, Cascini C, Gallazzi M, Mortara L, et al. (2019) Acetyl-L-Carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells: rationale for prevention and interception strategies. J Exp Clin Cancer Res 38: 464. Link: https://bit.ly/3uBfkX4
- Stansborough RL, Bateman EH, Al-Dasooqi N, Bowen JM, Wignall A, et al. (2018) Vascular endothelial growth factor (VEGF), transforming growth factor beta (TGFβ), angiostatin, and endostatin are increased in radiotherapy-induced gastrointestinal toxicity. Int J Radiat Biol 94: 645-655. Link: https://bit.ly/2RJjAF9
- Liu A, Wu Q, Guo J, Ares I, Rodríguez JL, et al. (2019) Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacol Ther 195: 54-84. Link: https://bit.ly/3tyc0dX
- Alegret M, Silvestre J (2006) "Pleiotropic effects of statins and related pharmacological experimental approaches." Methods Find Exp Clin Pharmacol 28: 627-656. Link: https://bit.ly/3y8jYy3
- Shin SK, Cho JH, Kim EJ, Kim EK, Park DK, et al. (2017) Anti-inflammatory and anti-apoptotic effects of rosuvastatin by regulation of oxidative stress in a dextran sulfate sodium-induced colitis model. World J Gastroenterol 23: 4559-4568. Link: https://bit.ly/3ewJqp2
- Erbaş H, Bal O, Çakır E (2015) Effect of rosuvastatin on arginase enzyme activity and polyamine production in experimental breast cancer. Balkan Medical Journal 32: 89. Link:
- Janakiram N, Mohammed A, Bryant T, et al. (2016) Potentiating NK cell activity by combination of Rosuvastatin and Difluoromethylornithine for effective chemopreventive efficacy against Colon Cancer. Sci Rep 6: 37046. https://doi.org/10.1038/srep37046.
- El Sayed I, Helmy MW, El-Abhar HS (2018) Inhibition of SRC/FAK cue: A novel pathway for the synergistic effect of rosuvastatin on the anti-cancer effect of dasatinib in hepatocellular carcinoma. Life sciences 213: 248-257.
- Aldalaen S, El-Gogary RI, Nasr M (2019) Fabrication of rosuvastatin-loaded polymeric nanocapsules: a promising modality for treating hepatic cancer delineated by apoptotic and cell cycle arrest assessment. Drug development and industrial pharmacy 45: 55-62.
- Kheiri MH, Alimohammadi N, Danafar H (2019) Preparation of biocompatible copolymeric micelles as a carrier of atorvastatin and rosuvastatin for potential anticancer activity study. Pharm Dev Technol 24: 303-313. Link: https://bit.ly/2RJUOVK
- Livak KJ, Schmittgen TD (2001) Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 22DDCT Method. Methods 25: 402–408. Link: https://bit.ly/3xYGQQ9
- Deezagi A, Shomali S (2018) Prostaglandin F-2α Stimulates The Secretion of Vascular Endothelial Growth Factor and Induces Cell Proliferation and Migration of Adipose Tissue Derived Mesenchymal Stem Cells. Cell J 20: 259–266. Link: https://bit.ly/3f5oHYj
- Viallard C, Larrivée B (2017) Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogenesis 20: 409-426. Link: https://bit.ly/3bcI0y0
- Sivaraman Siveen K, Prabhu K, Krishnankutty R, Kuttikrishnan S, Tsakou M, et al. (2017) Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: Potential and challenges. Curr Vasc Pharmacol 15: 339-351. Link: https://bit.ly/2SzpMA1
- Klein T, Bischoff R (2011), Physiology and pathophysiology of matrix metalloproteases. Amino Acids 41: 271-290. Link: https://bit.ly/3uBBQ1Q
- Quintero-Fabián S, Arreola R, Becerril-Villanueva E, César Torres-Romero J, Arana-Argáez V, et al. (2019) Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol 9: 1370. Link: https://bit.ly/3xUS0Wp
- Sopková J, Vidomanová E, Strnádel J, Škovierová H, Halašová E (2017) The role of statins as therapeutic agents in cancer. Gen Physiol Biophys 36: 501-511. Link: https://bit.ly/3evwGii
- Choi HD, Baik JJ, Kim SH, Yu SN, Chun SH, et al. (2016) Rosuvastatin induces ROS-mediated apoptosis in human prostate cancer PC-3 cells. Journal of Life Science 26: 398-405.
- Ahmadi M, Amiri S, Pecic S, Macha F, Rosik J, et al. (2020) Pleiotropic effects of statins: A focus on cancer. Biochim Biophys Acta Mol Basis Dis 1866: 165968. Link: https://bit.ly/3evTbUr
- Alfaqih MA, Allott EH, Hamilton RJ, Freeman MR, Freedland SJ (2017) The current evidence on statin use and prostate cancer prevention: are we there yet?. Nat Rev Urol 14: 107-119. Link: https://bit.ly/2RaZOm2
- Alexandrova R, Dinev D, Glavcheva M, Danova J, Yetik-Anacak G, et al. (2019) Briefly about Anticancer Properties of Statins. Survival 23: 24. Link: https://bit.ly/33IQHMp
- Beckwitt CH, Brufsky A, Oltvai ZN, Wells A (2018) Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res 20: 144. Link: https://bit.ly/3tyOTjp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
