International Journal of Immunotherapy and Cancer Research
Tadalafil inhibits elevated glutamic oxaloacetic transaminase during alcohol aflatoxin induced hepatocellular carcinoma in rats
Divya Rawat and Raj Kumar Koiri*
Cite this as
Rawat D, Koiri RK (2020) Tadalafil inhibits elevated glutamic oxaloacetic transaminase during alcohol aflatoxin induced hepatocellular carcinoma in rats. Int J Immunother Cancer Res 6(1): 010-013. DOI: 10.17352/2455-8591.000022Hepatocellular carcinoma is a type of primary liver cancer and dietary exposure to aflatoxins is one of major causative factor for the development of HCC. Present study was planned to assess the role of PDE5 inhibitor tadalafil on glutamic oxaloacetic transaminase during aflatoxin induced HCC. Rats of control group received normal food and water ad libitum. Alcohol and HCC group received chronic dose of alcohol via drinking water for two weeks. After two weeks HCC group received single dose of crude aflatoxin and allowed to develop HCC for six weeks. After six weeks, HCC rats were post-treated with tadalafil via drinking water for 10 days. Result revealed that PDE5 inhibitor tadalafil treatment reduced glutamic oxaloacetic transaminase level in liver and serum towards normal in HCC rats suggesting its therapeutic potential.
Abbreviations
BSA: Bovine Serum Albumin; GOT: Glutamic Oxaloacetic Transaminase; HCC: Hepatocellular Carcinoma; NAD: Nicotinamide Adenine Dinucleotide; NADH: Nicotinamide Adenine Dinucleotide Reduced; PDA: Pancreatic Ductal Adenocarcinoma; PDE5: Phosphodiesterase 5; ROS: Reactive Oxygen Species
Introduction
Hepatocellular carcinoma, a type of primary liver cancer is the fifth most common type of cancer worldwide. Risk factors for developing HCC includes hepatitis B & C virus, alcoholism and consumption of aflatoxin contaminated food [1]. Aflatoxins are fungal secondary metabolites produced by Aspergillus flavus and Aspergillus parasiticus. Aflatoxin B1 (AFB1) is a non-viral risk factor for HCC in humans. About 5-28% of global HCC cases are attributed by aflatoxin exposure. In a study it was found that AFB1 exposure is associated with increased risk for HCC in habitual alcohol consumption in Hepatitis C Virus (HCV) infected persons. Thus we could say AFB1 exposure contributes to the development of HCC in participants with high risk factors for cirrhosis including alcohol and HCV infection [2]. Glutamic Oxaloacetic Transaminase (GOT) (E.C.2.6.1.1) is also known as Aspartate Amino Transferase (AAT) which catalyzes the bidirectional conversion of L-aspartate and α-ketoglutarate to oxaloacetate and L-glutamate. GOT is widely distributed in heart and liver tissue and is used for assessing myocardial and hepatic damage [3]. There are different isoforms of GOT such as GOT1 which regulates cellular metabolism via utilization of carbohydrates and amino acids to meet the nutrient demands. It was observed that in Pancreatic Ductal Cell Carcinoma (PDAC) cells, glutamine-derived aspartate is converted to OAA (oxaloacetate) by GOT1 and it was involved into the maintenance of redox homeostasis through conversion of OAA to pyruvate.
Liver is the primary metabolic center regulating glucose, lipid and amino acid metabolism. Many hepatic pathologies including HCC are associated with altered metabolism and increased anaplerosis [4]. Altered energy metabolism is a hallmark of cancer cells. Glutamate-oxaloacetate transaminase plays essential role in amino acid metabolism, malate-aspartate shuttle and tricarboxylic acid cycle. These pathways are associated with energy homeostasis during catabolism and anabolism. Recent studies suggest that GOT1 associated pathways are involved in cancer cell metabolism, which supports their redox balance and proliferation. Oxaloacetate has been reported as blood-based glutamate scavenger as it increases deamination of glutamate to 2-ketoglutarate via GOT. Recently GOT1 has been represented as therapeutic target in cancer.
Sorafenib (Nexavar) a multikinase inhibitor is currently the only systemic treatment approved in the treatment of terminal HCC. In preclinical models sorafenib exhibited antiangiogenic effects on cell growth and induction of apoptosis via downregulation of MCl-1 (antiapoptotic protein). In mouse xenograft models of HCC, sorafenib was found to regress angiogenesis via dose dependent manner by blocking Raf/MEK/ERK pathway and extracellular receptor tyrosine kinases [5]. New drugs like tyrosine kinse inhibitors lenvatinib and cabozantinib in the firstline, PDL1 inhibitors (programmed cell death ligand 1 inhibitors) – nivoluman, pembrolizumab, regorafenib and monoclonal antibody ramucirumab in the second line has been demonstrated to improve the clinical outcomes in HCC patients, however, median survival remains approximately 1 year thus major therapeutic breakthrough are still needed [6].
Tadalafil is a phosphodiesterase 5-inhibitor (PDE5i) that has been developed for the management of pulmonary arterial hypertension and erectile dysfunction. Moreover, it has been used for cardiovascular diseases and cancer [7]. Many studies have demonstrated PDE5 inhibitors as protective against ischemisa/reperfusion (I/R) injury, diabetic cardiomyopathy and doxorubicin cardiotoxicity. Mechanistically, PDE5 inhibitors work through increased expression of nitric oxide synthases, protein kinase G activation and phosphorylation of glycogen synthase kinase-3β. The present study for the first time reports the therapeutic efficacy of tadalafil in alcohol-aflatoxin induced HCC.
Materials and methods
Sodium acetate buffer, L-aspartic acid, α-ketoglutarate, pyridoxal-5-phosphate and fast blue B salt were purchased from HiMedia Laboratories, Pvt. Ltd, Mumbai, India and were of high analytical grade. Tadalafil was obtained from Macleods Pharmaceuticals Pvt Ltd.
Experimental design
Male albino rats weighing 150±25 gm were used in this experiment. Rats were maintained at the animal facility at standard laboratory conditions. This work was approved by SIPS animal ethical committee (SIPS/EC/2015/65). Rats were randomly divided into four groups-
Group 1: Normal control (N) group received standard diet and water ad libitum.
Group 2: Alcohol Control (AC) group received 5% alcohol via drinking water for two weeks.
Group 3: Hepatocellular Carcinoma (HCC) group received 5% alcohol and single intraperitoneal dose of aflatoxin and left to allow development of HCC for six weeks.
Group 4: HCC rats treated with tadalafil (HCC +T); after six weeks of HCC development, HCC rats were post-treated with 10 mg/kg bw dose of tadalafil via drinking water for 10 days.
Preparation of liver homogenate
After completion of treatment 3 rats from each group were sacrificed by cervical dislocation and liver was dissected out. Tissues were immediately washed with ice-cold normal saline stored at -80oC until required for biochemical study. 10% liver homogenate was prepared by homogenization in ice chilled 20 mM Tris buffer (pH 7.4) and supernatant was obtained after centrifugation at 16,000 x g for 30minutes. Protein concentration was determined using BSA as standard [8].
Glutamic oxaloacetic transaminase activity by non-denaturing PAGE followed by substrate staining
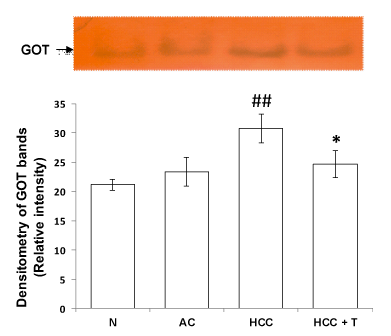
For GOT assay, supernatant containing 80 μg protein was loaded in each lane of 10% non denaturing PAGE. After completion of electrophoresis, gels were subjected to substrate specific staining solution as described earlier [9]. Briefly, 100 ml staining reaction mixture consisted of 0.2 M sodium acetate buffer pH (5.0), 500mg L-aspartic acid, 70mg α-ketoglutaric acid, 10mg pyridoxal-5-phosphate, 300mg fast blue B salt. Gel was incubated in staining solution in the dark at 37oC until dark brown bands appeared. Gels were scanned and bands were quantified by gel densitometry software.
Analysis of serum glutamate-oxaloacetate-transaminase
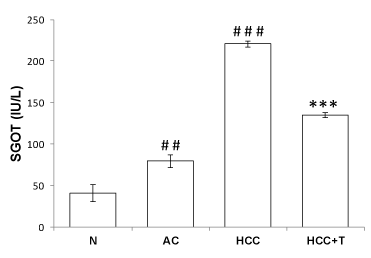
Using commercially available kit by ERBA Diagnostics Mannheim, Germany, the activity of GOT was determined as recommended by International Federation of Clinical Chemistry (1976). Decrease in absorption at 340 nm was observed as NADH is converted into NAD. The rate of decrease in absorbance is measured which is proportional to GOT activity. Suitably diluted volume of serum was added to GOT reagent and the contents of the cuvette were mixed and incubated at 37oC for 5 min. Decrease in OD was recorded at 1 min interval for 5 min against blank at 340 nm using UV spectrophotometer. Activity was expressed in terms of absorbance change /min into international unit (IU).
Statistical Analysis
Experimental data was expressed as mean±standard deviation. Student’s t-test was applied for determining the level of significance between the control and experimental groups. p<0.05 was considered significant.
Result
Tadalafil declined the elevated level of GOT in liver of hepatocellular carcinoma rats
Transaminases are enzymes that are involved in the catabolism of amino acids and are involved in the catalytic process of interconversion of a pair of amino acids and a pair of α-keto acids. Glutamic oxaloacetic transaminase (GOT) is known to act as a link between carbohydrate and protein metabolism. During the development and progression of hepatocellular carcinoma, level of GOT increased significantly in HCC as compared to normal control rats (p<0.01). Treatment of HCC rats with PDE5 inhibitor tadalafil led to significant decline in the level of GOT (p<0.05) in liver of HCC rats in (Figure 1).
Enzymatic assay of GOT shows elevated level of GOT in serum of alcohol (p<0.01) and HCC rats (p<0.001), thereby confirming damage to hepatocytes and impaired function of liver. Treatment of HCC rats with tadalafil restored the GOT level towards the normal level in serum, suuggesting that it imparts hepatoprotection (Figure 2).
Discussion
Aminotransferases catalyzes nitrogen redistribution between amino acids and their corresponding oxoacids which participate in proetin metabolism and gluconeogenesis. Two distinct forms of aminotransferases are known out of which one is soluble and other is mitochondrial isoform. Selective measurement of these isoforms has been reported to have clinical significance as their distribution in tissues and relative concentration depends upon pathological condition. GOT has been used as biomarker for liver toxicity since long time. During amino acid degradation and their biosynthesis first step is transamination reaction. An amino group is transferred from a donor amino acid to acceptor α-keto acid. Reversible transamination reaction occur in hepatic cells and when liver encounters any pathological condition these reactions clinically signify damage to hepatocytes. Transamination reactions are catalyzed by pyridoxal phosphate-dependent enzymes known as amino transferases. The α-amino nitrogen from amino acid catabolism is channeled through glutamate thus making it key source of nitrogen for biosynthesis and excretion. Similar but less prevalently oxidative deamination occurs via conversion of amino acid to its corresponding α-keto acid.
Studies suggests that GOT1 plays key role in pancreatic ductal adenocarcinoma (PDA) suggesting it as a good drug target in cancer [10]. It has been demonstrated that inhibition of GOT1 sensitizes cancer cells to glucose deficiency, which may be counteracted by oxaloacetate and phosphoenol pyruvayte and metabolic intermediates downstream to GOT1 [11]. In pancreatic ductal adenocarcinoma of humans, oncogene KRAS improves glutamaine dependency by using glutamine-derived aspartate to produce oxaloacetate via GOT1 in cytoplasm. Oxaloacetate in turn is converted to malate and then pyruvate to maintain high NADPH/NADP+ ratio for redox homeostasis. Hence disrupting these reactions led to disruption of glutamine metabolism [12]. Previous reports have also suggested that GOT1 null 143B osteosarcoma cells showed accumulation of NADH and decreased NADH/NAD+ ratio when exposed to nutrient depletion [11]. These findings suggest that aminotransferases play important role in energy homoeostasis.
Cancer cells show increased glutamine flux, accumulated glutamine by glutaminolysis which is necessory to meet the energy demands of cancer cells. Myc-transformed cells were found to depend upon α-keto glutarate synthesis and glutamate dehydrogenase. Further aspartate and alaniane transaminase reversibly convert glutamate to α-ketoglutarate and oxaloacetate to asparate or pyruvate to alanine respectively. These reactions occur despite the presence of glutamate dehydrogenase in the mitochondria of cancer cells which catalyzes conversion of glutamate to α-keto glutarate. In breast cancer having mutated Myc, undergo apoptosis when aspartate transaminase is inhibited. Cancer cells involve transaminase reactions to produce α-keto glutarate [13].
Similarly, disruption of redox homeostasis was observed when aspartate transaminase is knocked down in pancreatic cancer resulting in growth inhibition both in vitro and in vivo. In various studies inhibition of transaminases showed absence of toxicity in non-neoplastic cells suggesting important role of aminotransferases as drug target in cancer. Inhibition of aspartate aminotransferase interferes in redox homeostais has been reported to increase reactive oxygen species (ROS) during chemotherapy and radiation in cancer [9]. In agreement with this, in the present study increased activity of GOT in HCC group was observed and post treatment of HCC rats with PDE5 inhibitor tadalafil led to a significant decline in the level of GOT in HCC rats (Figures 1,2). It is speculated that inhibition of GOT may interfere in redox homeostasis to increase ROS level in the treatment of HCC.
Conclusion
It is worthy to note that this is the first study highlighting the role of tadalafil in balancing glutamic oxaloacetic transaminase level which is elevated in aflatoxin induced HCC. Since glutamic oxaloacetic transaminase is known to play a key role in mobilizing L-amino acids for gluconeogenesis and function as links between carbohydrate and protein metabolism under altered metabolism of cancer cells, its inhibition by PDE5 inhibitor tadalafil may serve as drug target to treat cancer.
DR is grateful to UGC for fellowship. This work was financially supported by a project from UGC-Faculty Research Promotion Scheme (F.30-12/2014/BSR) and SERB (SERB/LS-329/2013), Govt. of India, sanctioned to RKK. The authors are thankful to the Department of Zoology, Dr. Harisingh Gour Vishwavidyalaya, Sagar (MP), India for providing infrastructural facilities and financial support.
- Rawat D, Shrivastava S, Naik RA, Chhonker SK, Mehrotra A, et al. (2018) An overview of natural plant products in the treatment of hepatocellular carcinoma. Anticancer Agents Med Chem 18: 1838-1859. Link: http://bit.ly/2wIPqYd
- Chu YJ, Yang HI, Wu HC, Lee MH, Liu J, et al. (2018) Aflatoxin B(1) exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. Eur J Cancer 94: 37-46. Link: http://bit.ly/32tG12v
- Chen SH, Giblett ER (1971) Genetic variation of soluble glutamic-oxaloacetic transaminase in man. Am J Hum Genet 23: 419-424. Link: http://bit.ly/2PCLGhN
- Egnatchik RA, Leamy AK, Sacco SA, Cheah YE, Shiota M, et al. (2019) Glutamate-oxaloacetate transaminase activity promotes palmitate lipotoxicity in rat hepatocytes by enhancing anaplerosis and citric acid cycle flux. J Biol Chem 294: 3081-3090. Link: http://bit.ly/2usXonH
- Niu L, Liu L, Yang S, Ren J, Lai PBS, et al. (2017) New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer 1868: 564-570. Link: http://bit.ly/3a8NGGl
- Llovet JM, Montal R, Sia D, Finn RS (2018) Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 15: 599-616. Link: http://bit.ly/2HVmEWB
- Das A, Durrant D, Salloum FN, Xi L, Kukreja RC (2015) PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther 147: 12-21. Link: http://bit.ly/2HPC0fv
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193: 265-275. Link: http://bit.ly/2Vw1LJV
- Lone Y, Bhide M, Koiri RK (2017) Amelioratory effect of coenzyme Q10 on potential human carcinogen microcystin-LR induced toxicity in mice. Food Chem Toxicol 102: 176-185. Link: http://bit.ly/3c5WZs6
- Yoshida T, Yamasaki S, Kaneko O, Taoka N, Tomimoto Y, et al. (2020) A covalent small molecule inhibitor of glutamate-oxaloacetate transaminase 1 impairs pancreatic cancer growth. Biochem Biophys Res Commun 633-638. Link: http://bit.ly/37Yqx7Z
- Zhou X, Curbo S, Li F, Krishnan S, Karlsson A (2018) Inhibition of glutamate oxaloacetate transaminase 1 in cancer cell lines results in altered metabolism with increased dependency of glucose. BMC Cancer 18: 559-559. Link: http://bit.ly/3c2IyVQ
- Hirschey MD, DeBerardinis RJ, Diehl AME, Drew JE, Frezza C, et al. (2015) Dysregulated metabolism contributes to oncogenesis. Semin Cancer Biol 35: S129-S150. Link: http://bit.ly/2HVp9Iu
- Moreadith R, Lehninger A (1984) The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD (P)+-dependent malic enzyme. J Biol Chem 259: 6215-6221. Link: http://bit.ly/2HTMSsB
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
