International Journal of Immunotherapy and Cancer Research
A Very Rare Case of Polycythemia Vera Patient Who Developed Other Four Cancers
Romeo-Gabriel Mihaila*
Cite this as
Mihaila RG (2017) A Very Rare Case of Polycythemia Vera Patient Who Developed Other Four Cancers. Int J Immunother Cancer Res 3(1): 012-014. DOI: 10.17352/2455-8591.000013We find more and more often patients with two or more synchronous or metachronous cancers, who raise various issues on the risk factors and pathways. A polycythemia vera male patient, diagnosed at 44 years, evolved towards secondary myeloid metaplasia which was then transformed into secondary acute myeloid leukemia. He also presented breast adenocarcinoma and a basal cell carcinoma of the nasal pyramid and of the internal angle of the left orbital region during those 13 years of evolution. Pathogenetic mechanisms are discussed, as well as the followed treatments and their effectiveness. The appearance of the four new malignancies influenced his quality of life and have reduced his survival.
Introduction
The prevalence of polycythemia vera (PV) is assessed to be between 44 and 57 cases per 100,000 subjects [1]. A study conducted in Norway found that the incidence of PV doubled and tripled during the years 2007-2012 versus the 12 prior years [2]. The discovery of Jak2 V617F mutation and 2008 WHO guidelines (which consider that the mutation is a major criterion for PV diagnosis) can explain this increase (through a better possibility to support the diagnosis) [2]. It occurs more frequently in men. The median age of patients at the moment of diagnosis is about 61 years [3-6]. Median survival of patients with PV is estimated to be 14 years [7]. Regardless of age, patients have the same predisposition of progression to secondary myelofibrosis or acute leukemia [5,6], that could compromise their survival [7].
Case Report
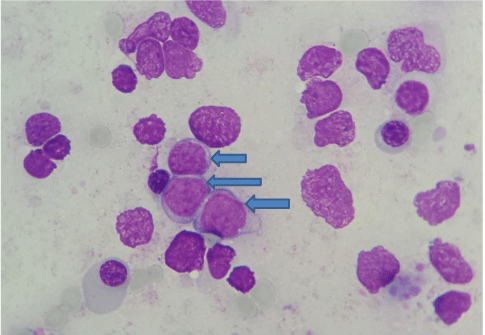
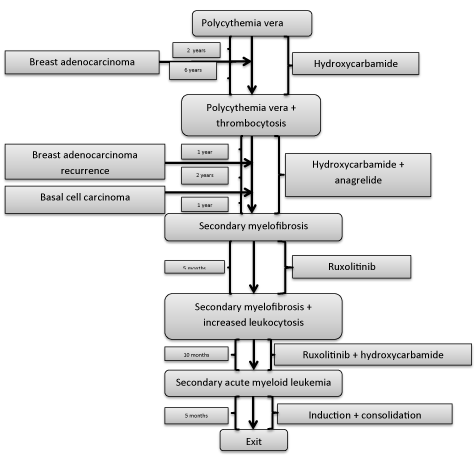
The patient’s family has given the written consent for publishing this case report. The patient was diagnosed with polycythemia vera (PV) at 44 years. Repeated blood emissions were not well tolerated so that the cytoreductive therapy with hydroxycarbamide (1.5 – 2.5 g/day, 5 day/week) was added. Jak2 V617F mutation was positive in homozygous variant five years later, when it could be determined. A thrombocytosis of 1,032,000/mm3 appeared after another 3 years that required the addition of anagrelide (1.5 mg/day). After an evolution of 12 years, he began to present a progressive mild anemia with blood hemoglobin levels of 10.4 – 9.1 g/dl. The bone marrow biopsy diagnosed a secondary myelofibrosis (MF): a bone marrow cellularity of 45%, with thickened reticulin fibers and extended collagen fibrosis (a grade 3 fibrosis), and cellular elements disposed on the fiber tracts, represented by fibroblasts, disorganized precursors with reduced maturation of erythroid and granulocytic series and numerous megakaryocytes placed in large groups, many of them - dysplastic, with hypo- and hyperlobated nuclei. Spleen began to grow until 16.5/10.5 cm in the next months and he became asthenic as he was anemic. A ruxolitinib treatment was given to him (2x20 mg/day), but in 5 months a leukocytosis between 18,340 and 44,480/mm3 appeared under associated treatment with hydroxycarbamide. The proportion of blast cells in peripheral blood and bone marrow progressively increased from 3% to 19%. A secondary acute myeloid leukemia type 2 FAB was diagnosed 13 years since the onset of PV and ten months after that of MF, when the patient had 40% blast cells in peripheral blood and 34% in mone marrow (Figure 1). Flowcytometry established that the blast cells were CD34+, CD33+ and CD13+. Echocardiography showed a normal left ventricular ejection fraction. He was treated with an induction cycle of chemotherapy with epirubicin 50 mg/day, 3 days, and cytarabine 200 mg/day, 7 days. The patient still had 9% of blasts at the exit from medullary aplasia. Unfortunately, his sister was not HLA-compatible. He continued with idarubicine 20 mg/day for 3 days, and cytarabine 2x3 g/day for 3 days, after that he still had 7% of blast cells in bone marrow. During the medullary aplasia he had an episode of paroxysmal atrial fibrillation, converted to sinus rhythm with amiodarone, which was continued then. The next cycle consisted in idarubicine 20 mg/day for 3 days, and cytarabine 2x2.5 g/day for 4 days, followed by complete remission at the exit from medullary aplasia (4% of blast cells in the bone marrow). Then, he received a next cycle with the same drugs and doses, with complete remission persistance. The last cycle was identical, but during medullary aplasia he developed cardiovascular complications. They responded initially to the treatment, but their subsequent evolution was unfavorable and led to death. We mention that during the periods of medullary aplasia he presented numerous gram-negative infections, hypokalemia and a crisis of loss of consciousness that required complex therapies.
A breast adenocarcinoma occurred after only two years since the PV discovery. It was operated, but it recurred 7 years later, when lymph nodes were observed in the right axyllary region. The patient was reoperated and received chemotherapy: four cycles with epirubicine and cyclophosphamide and anohter four with docetaxel, epirubicine and cyclophosphamide, followed by tamoxifen 40 mg/day. A basal cell carcinoma of the nasal pyramid and of the internal angle of the left orbital region was operated one year later.
Discussion
PV started in our patient at a much younger age than that of which it usually occurs. He was treated with hydroxicarbamide, who constitutes the first line of cytoreductive therapy in PV, as blood emissions were not well tolerated. Interferon and pegylated-interferon-α-2a, are a second-line option for PV patients [8] and clearly bring clinical benefits in PV patients [9], although the exact role of interferon-α continues to be undefined [8]. Unfortunately, they are not reimbursed for this indication in Romania.
It is known that PV can progress to myelofibrosis [10] in some patients. It seems that a high platelet counts (as in our patient which required anagrelide) and the presence of splenomegaly are significant predictors for the progression of PV towards post-PV MF [11]. It is estimated that PV can progress to secondary MF to about 30% of patients after a evolution of 2-13 years [12]. In our patient this transformation appeared after a evolution of 12 years. The aggressive clinical phenotype of MF explains the poor survival of patients [13].
As MF has a complex pathogenesis and variable clinical features [13], it can progress to acute leukemia sometime. It is estimated that secondary acute leukemia occurs in approximately 10% of patients with PV [12]. The risk of transformation into acute leukemia is 5% after treatment with hydroxycarbamide [14]. The transformation of post-PV MF in acute leukemia occurred very quickly in our patient - after just ten months.
Could ruxolitinib be involved in this transformation in our patient? Ruxolitinib is a Janus kinase 1 and 2 inhibitor that proved to be able to improve constitutional symptoms and reduce splenomegaly in most primary or post-PV myelofibrotic patients, and prolong their overall survival [15], but this last effect seems to be limited, as that on mutation load, and marrow fibrosis [13]. Given in PV patients resistant or intolerant to hydroxicarbamidum [9], ruxolitinib seems to can improve microvascular involvement [16], unfortunately, in Romania it is not reimbursed for this indication. Although it inhibits Jak-STAT mechanism, which is the main signaling pathway constitutively activated and involved in survival and proliferation of malignant cells from Ph-negative chronic myeloproliferative neoplasms, there were some worries on its potential role in altering the natural evolution of PV, but they have not been confirmed so far [16].
May Jak2 V617F mutation be involved in the disease progression in our patient? Jak2 V617F was discovered in 2005, can be found in 95% of PV patients [9], and is a driver mutation involved in the pathway of Ph-negative chronic myeloproliferative neoplasms [17]. It is discussed today that the acquisition of disease-initiating mutations in hematopoietic stem cells of some of these patients may be the result of an inherent genomic instability [18]. Jak2 V617F allele burden is higher in post-PV myelofibrotic patients compared with those having PV – finding that suggests a role for the accumulation of mutated alleles in the progression of PV through secondary MF [19] (Figure 2).
But it has been found that there are also mutations in epigenetic regulators and RNA splicing genes which have an important role in the disease progression [17]. When the presence of these mutations could be studied, we will better understand why some patients progress faster than others and some of them do not progress toward MF or acute leukemia transformation.
MF requires a personalized approach and only allogeneic stem cells transplantation can cure the disease [13]. Unfortunately, this intensifying therapeutic procedure has a non-negligible transplant-related mortality [9].
Second primary malignancies are relatively common in patients with PV. Their cumulative incidence of was 13.1% at 10 years in a study which included 3941 PV patients [7]. The standardized incidence ratio for acute myeloid leukemia was 12.24, in the same study [7]. The breast adenocarcinoma appeared in our patient after only two years of hydroxicarbamide therapy, so that its involvement in the pathogenesis of its second cancer seems to be unlikely. Hydroxicarbamide can produce various skin lesions, including spinocellular carcinoma after a long period of administration [20]. The basal cell carcinoma occurred in our patient after 11 years of its administration can be the result of its mutagenesis effect.
What explanation could be for the appearance of the two nonhematologic cancers in our patient? Besides their genomic instability, PV patients have a state of chronic inflammation which may be involved not only in the faster atherosclerosis development, but also in the emergence of a second cancer [21]. Adding statins and interferon-α2 to their treatment may be a prophylactic measure against this possible evolution [21].
- Mehta J, Wang H, Iqbal SU, Mesa R (2014) Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma 55: 595–600. Link: https://goo.gl/l4xwOi
- Roaldsnes C, Holst R, Frederiksen H, Ghanima W (2016) Myeloproliferative neoplasms: trends in incidence, prevalence and survival in Norway. Eur J Haematol 98: 85-93. Link: https://goo.gl/OYvQtI
- Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, et al. (2010) A prospective study of 338 patients with polycythemia vera: The impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia 24: 1574–1579. Link: https://goo.gl/KejOrS
- Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, et al. (2013) Survival and prognosis among 1545 patients with contemporary polycythemia vera: An international study. Leukemia 27: 1874–1881. Link: https://goo.gl/VM81AM
- Stein BL, Saraf S, Sobol U, Halpern A, Shammo J, et al. (2013) Age-related differences in disease characteristics and clinical outcomes in polycythemia vera. Leuk Lymphoma 54: 1989–1995. Link: https://goo.gl/RifQy1
- Stein BL, Oh ST, Berenzon D, Hobbs GS, Kremyanskaya M, et al. (2015) Polycythemia Vera: An Appraisal of the Biology and Management 10 Years After the Discovery of JAK2 V617F. J Clin Oncol 33: 3953-3960. Link: https://goo.gl/9z01yn
- Khanal N, Giri S, Upadhyay S, Shostrom VK, Pathak R, et al. (2016) Risk of second primary malignancies and survival of adult patients with polycythemia vera: A United States population-based retrospective study. Leuk Lymphoma 57: 129-133. Link: https://goo.gl/7HQAFk
- Aruch D, Mascarenhas J (2016) Contemporary approach to essential thrombocythemia and polycythemia vera. Curr Opin Hematol 23: 150-160. Link: https://goo.gl/PQPJxQ
- Soret J, Kiladjian JJ (2016) Place du ruxolitinib dans la stratégie thérapeutique des syndromes myéloprolifératifs. Bull Cancer 103: S29-S38. Link: https://goo.gl/M2C8bb
- Parnes A, Ravi A (2016) Polycythemia and Thrombocytosis. Prim Care 43: 589-605. Link: https://goo.gl/k8mEEa
- Bai J, Ai L, Zhang L, Yang FC, Zhou Y, et al. (2015) Incidence and risk factors for myelofibrotic transformation among 272 Chinese patients with JAK2-mutated polycythemia vera. Am J Hematol 90: 1116-1121. Link: https://goo.gl/KL9eyU
- Murray J (2005) Myeloproliferative disorders. Clin Med 5: 328-332.
- Palandri F, Auteri G, Baccarani M (2016) New strategies in myelofibrosis: the evolving paradigm of disease pathogenesis, prognostication and treatment. Hematol Oncol. Link: https://goo.gl/yMi5Xm
- Petrov L, Cucuianu A, Ghiurtz A (1997) Manual de Hematologie Clinică. Casa Cărții de Știință, Cluj-Napoca, 1997.
- Bose P, Verstovsek S (2016) Myelofibrosis: an update on drug therapy in 2016. Expert Opin Pharmacother 17: 2375-2389. Link: https://goo.gl/oVKmor
- Blum S, Martins F, Alberio L (2016) Ruxolitinib in the treatment of polycythemia vera: patient selection and special considerations. J Blood Med 7: 205-215. Link: https://goo.gl/axFcbG
- Takenaka K (2016) Myeloproliferative neoplasms: updates on molecular pathophysiology, diagnosis and treatment strategies. Rinsho Ketsueki 57: 1944-1955. Link: https://goo.gl/hoHkNL
- Scott LM, Rebel VI (2012) JAK2 and genomic instability in the myeloproliferative neoplasms: a case of the chicken or the egg? Am J Hematol 87: 1028-1036. Link: https://goo.gl/FOYP4a
- Rotunno G, Pacilli A, Artusi V, Rumi E, Maffioli M, et al. (2016) Epidemiology and clinical relevance of mutations in postpolycythemia vera and postessential thrombocythemia myelofibrosis: A study on 359 patients of the AGIMM group. Am J Hematol 91: 681-686. Link: https://goo.gl/iizvXh
- França ER, Teixeira MA, Matias Kde F, Antunes DE, Braz Rde A, et al. (2011) Cutaneous effects after prolongaded use of hydroxyurea in Polycythemia Vera. An Bras Dermatol 86: 751-754. Link: https://goo.gl/Dezsje
- Hasselbalch HC (2012) Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood 119: 3219-3225. Link: https://goo.gl/xuV0VT
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
