International Journal of Immunotherapy and Cancer Research
Immunosuppressive and Cytotoxic Potential of Flavonoids from Medicinal Plants: Preliminary Investigation for Anticancer Activity
Amit Gupta* and Revati N Chavan
Cite this as
Gupta A, Chavan RN (2017) Immunosuppressive and Cytotoxic Potential of Flavonoids from Medicinal Plants: Preliminary Investigation for Anticancer Activity. Int J Immunother Cancer Res 3(1): 007-011. DOI: 10.17352/2455-8591.000012Introduction: Attention related to scientific and research interest towards naturally derived compounds from medicinal plant products as they are considered to have less toxic side effects as compared to current treatments such as chemotherapy. Medicinal plant produces naturally occurring secondary metabolites especially flavonoids which are being investigated for their anticancer activities leading to the development of new clinical drugs.
Objective: In this regard, immunological studies were conducted to investigate its immunosuppressive and cytotoxic activity of flavonoids from the leaves of Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia on human whole blood and peripheral blood mononuclear cells (PBMC) against hepatitis B vaccine containing surface antigen (HBsAg)
Methods: for these studies, our group evaluated variable doses of flavonoids extracted from the leaves of Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia and estimated blood counts (lymphocytes, monocytes and granulocytes count using flow cytometry); nitric oxide production (using Griess reagent assay) and proliferation assay against HBsAg.
Results: The results of these studies claimed that these flavonoids showed sudden decline in monocytes and granulocytes count; nitric oxide production and also showed its cytotoxicity in the form of proliferation at higher doses as compared to control.
Conclusion: Overall, these flavonoids showed immunosuppressive as well as cytotoxic effect against HBsAg and also probable candidate for anticancer agent.
Introduction
Cancer is one of the major public health concern in all over the world. Several medicinal plants were screened and determined some anti-cancer agents i.e. taxol, vinblastine, vincristine, campothecin derivatives etc. Other promising anti-cancer agents that are reported i.e. flavopiridol, roscovitine, betulinic acid, silvestrol etc., [1]. In addition, there is another group that derived from medicinal plant products in the form of secondary metabolites i.e. flavonoids and their synthetic analogs are generally used in the treatment of ovarian, breast, cervical, pancreatic and prostate cancer [1-3].
Flavonoids (formed from the aromatic amino acids i.e. phenylalanine, tyrosine, and malonate) are one of the important constituent in the human diet, although they are generally considered as non-nutrients [4]. Generally, these flavonoid intakes can range between 50 and 800 mg a day, depending on the diet – consumption of vegetables, fruit, red wine, tea, unfiltered beer, etc. The absorption of the dietary flavonoids liberated from the food by chewing will depend on its physicochemical properties (i.e. molecular size, configuration, lipophilicity, solubility etc.) [4-6].
Generally, these flavonoid should be absorbed in small intestine or has to go to the colon before absorption and also depends upon the structure of flavonoid. Most flavonoids, except for the subclass of catechins, are present in medicinal plants but they are actually bound to sugars as b-glycosides. As per the literature, flavonoid glycosides converted into aglycan form and they are easily absorbed in small intestine [6-8]. The hydrophilic flavonoid glucosides such as quercetin are transported across the small intestine by the intestinal Na+-dependent glucose cotransporter (SGLT1). More than 4000 varieties of flavonoids isolated from various medicinal plants which is already identified. The most important effect of these flavonoids is the scavenging of oxygen-derived free radicals that is reported. In vitro experimental systems also showed that flavonoids possess anti-allergic, anti-viral and anti-carcinogenic properties [7,8]. The flavonoid is bound to albumin and then transported to the liver. The liver can extend the conjugation of the flavonoid by adding sulfate group, methyl group, or both. The addition of these groups which increases the circulatory elimination time and probably also decreases its toxicity. According to the literature, lot of flavonoids are reported from these medicinal plant products e.g. only one flavonoid was isolated from the leaves of Juniperus procera and could be identified as; 3′,4′,3,7-tetrahydroxyflavone [9], new flavonoid were also isolated from the stem bark of Choerospondias axillaries, the fruit of which was used for the treatment of cardiovascular diseases [10] etc.
One of the medicinal plants, Calotropis gigantea (Rui; family Asclepiadacea) have showed special attraction because of major phytochemical i.e. Calotropin that are present and showed various immunopharmacological activities [11,12] i.e. anti-inflammatory, antimicrobial, anti-diabetic etc. Adhatoda vasica (Adulsa; family Acanthaceae), medicinal plant products especially leaves showed various medicinal properties and it will protect our immune system against number of infectious diseases [13,14] e.g. cold, cough, asthma, malaria, bleeding disorder, reduces blood sugar level etc. Artocarpus integrifolia (Phanas; family Moraceae), medicinal plant which is reported as an important source or requirement of edible fruit and is widely used in traditional medicines. In Ayurveda, Artocarpus integrifolia possessed antimicrobial, anti-peptic ulcer, cardiovascular (anti-diabetic), anti-oxidant and immunomodulatory properties [15]. This is the first preliminary study related to anticancer activity of flavonoids isolated from medicinal plants especially leaves in order to determined its immunomodulatory (immunostimulator/immunosuppressor) effect against specific protein antigen.
Materials and Methods
Sample collection
Fresh leaves of these medicinal plants (Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia) were collected from nakshatra garden, Vidya Pratishthan, Baramati, Maharashtra India.
Extraction of flavonoids
For flavonoid extraction, using 10 g of fresh plant leaves of Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia were squashed with liquid nitrogen to prepare plant leaves powder. Afterwards, leaves powder of these medicinal plants were reflux with methanol (80 %; 100 ml) for 2 h at 100 ºC. After incubation, cool down the solution (another 1 h incubation) and filter it using whatman filter paper. Filtrate solution were collected containing constituents of plant powdered solution and then add ethyl acetate (20 ml) and distilled water (40 ml) in the ratio of 1:2. Incubate for overnight and then observed two separating layers i.e. upper layer containing ethyl acetate (evaporate it) and lower layer containing dried extracts (flavonoids) that are reported or settled at the bottom. For determining the flavonoid content qualitatively in these medicinal plants, small quantity of lead acetate solution is added and yellow precipitation if appeared it indicates the presence of flavonoid [4]. All three medicinal plants showed positive results related to flavonoid content.
Estimation of blood counts
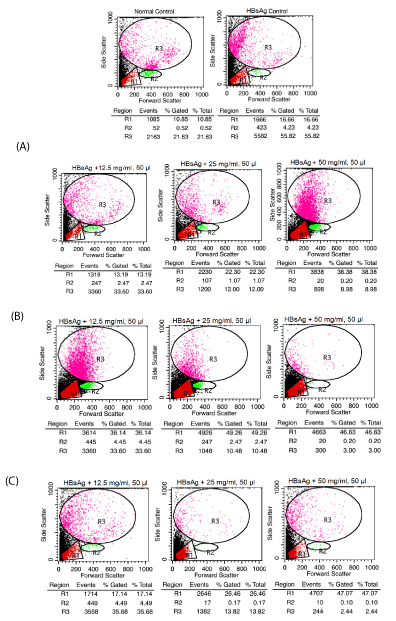
For these studies, exposure of variable doses of flavonoids (12.5 – 50 mg/ml; 50 µl) on human whole blood (n = 5; EDTA human blood samples collected from Mangal Pathology lab, Baramati) along with or without HBsAg (20 µg/ml; 10 µl) using forward and side scatter representing different phenotypes i.e. lymphocytes (R1), monocytes (R2) and granulocytes (R3) count that are analyzed using cell quest software (BD FACS Calibur). For these studies, addition of ACK lysing solution (1X, 2 ml) after incubation of these flavonoids extracted from the leaves of Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia with or without HBsAg and then followed by incubation for another 10 min. Afterwards, the supernatant was aspirated after centrifuging and washed two times with PBS. Finally, pellet dissolved in PBS and observed the cells through flow cytometer [11-15].
Cytotoxicity assay and estimation of nitric oxide production
Briefly, human PBMC (106 cells/ml; 100 µl; separated through Ficoll–Hypaque gradient centrifugation) were plated in 96 well plate and pre-incubated with HBsAg (20 µg/ml; 10 µl) and then treated with variable concentration of flavonoids (12.5 – 50 mg/ml; 50 µl). After addition of specific antigen and test samples, incubate the plate for another 24h at 37ºC (carbon dioxide incubator). The plates were centrifuged at 2500 rpm for 10 minutes and then supernatant (100 µl) was collected for estimation of nitric oxide [11-15].
In cytotoxicity assay, similar volume of fresh medium were added and again incubated the plate for another 24 h. After incubation, add MTT (5 mg/ml; 10 µl) solution and again incubate it for another 3-4 h. Thereafter, centrifuging the plate, discard the supernatant and appearance of formazon crystals settled at the bottom. These crystals were dissolved in dimethyl sulphoxide and the optical density was measured at 570 nm [11-14].
For these studies, PBMC cell culture supernatant was mixed with similar volume of Griess reagent (1% sulfanilamide and 0.1% naphthylethylene diamine dihydrochloride in 2.5% phosphoric acid) and incubated the plates at room temperature for another 10 minutes and absorbance at 540 nm was measured by spectrophotometer. The fresh culture medium (RPMI containing 10 % fetal bovine serum) was used as a blank. The nitrite quantity was determined from a sodium nitrite standard curve.
Statistical analysis
Values are expressed as Mean ± S.E. The difference between control and flavonoids extracted from Calotropis gigantea, Adhatoda vasica and A. integrifolia which is determined through Bonferroni multiple comparison test (one way ANOVA test).
Results
Estimation of lymphocytes, monocytes and granulocytes count
The result of these studies showed that these flavonoids extracted from the leaves of Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia showed dramatically decreased in monocytes as well as granulocytes count as compared to control (Figure 1). In other words, these flavonoids showed immunosuppressive and cytotoxic effect at higher doses against specific protein antigen.
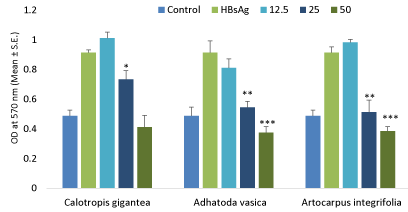
Cytotoxicity assay
The effect of variable doses of flavonoids on determining its cytotoxicity in human PBMC’s against specific protein antigen i.e. HBsAg as shown in Figure 2. The results of these studies showed that these flavonoids showed declined in proliferation at higher doses after treating with PBMC as compared to control.
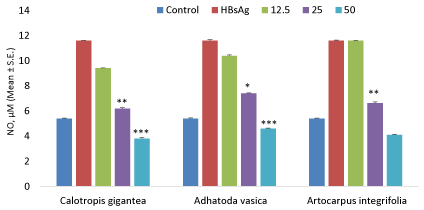
Nitric oxide production
The effect of variable doses of flavonoids on nitric oxide production in human PBMC’s as shown in Figure 3. The results showed that these flavonoids showed declined in nitric oxide at higher doses after treating with PBMC as compared to control.
Discussion
Medicinal plants have been found as important contributors to the pharmaceutical, agriculture and food industries. In recent times, researchers focused on medicinal plants to explore its various immunobiological activities all over the world. These medicinal plants are generally composed of primary (carbohydrates, proteins, fats etc.) as well as secondary metabolites (alkaloids, flavonoids, terpenoids etc.). These secondary metabolites especially flavonoids are considered to be phenolic compounds which are normally present in the pigments of fruit, vegetables, green tea and red wine [16,17]. The first flavonoid isolated from citrus fruits and was discovered by the Nobel Prize winner Albert Szent Gyorgyi who called them vitamin P. These flavonoids have shown series of important reactions which can interact with drug transport, interfere with cycline-dependent cell cycle regulation, inhibit protein glycosylation or affect the function of platelets [16,17].
Cancer chemoprevention, by the use of natural and dietary agents especially flavonoids that can reverse, suppress or prevent carcinogenic progression, has become an appealing strategy to combat the dogma associated with increasing cases of cancers worldwide. Number of epidemiological studies were reported and conducted that long-term consumption of diet rich in foods and vegetables containing flavonoids reduces the risk of chronic diseases especially cancer [1, 16, 17].
As per the literature survey, consumption of isoflavones are directly associated with decreased risk of estrogen related cancers and vascular diseases whereas total flavonoids including flavanones, and flavonols were inversely proportional to oral and laryngeal cancers [18, 19]. In addition, reduced risk rate of colorectal cancer was detected in high intake of flavones, isoflavones, anthocyanidins and flavonols. In contrast, there is no association between flavonoids and prostate cancer reported or emerged whereas inverse association was found between proanthocyanidins and colorectal cancer [20]. In this regard, we focused on various medicinal plants pertaining to flavonoids isolated from medicinal plants and determined its immunopharmacological activity against specific protein antigen.
Modulation (stimulatory or suppressive) of the immune system by these cytostatic agents is emerging as a major area in Immunopharmacology, especially in those cases where undesired immunosuppression is the result of therapy. These studies were conducted in human whole blood and peripheral blood mononuclear cells in order to estimate its immunosuppressive and cytotoxic activity. In this study, there is declined in the number of monocytes and granulocytes count in human whole blood after treating with variable doses of flavonoids which is confirmed through flow cytometric analysis and the results showed that these flavonoids from these medicinal plants showed immunosuppressive and cytotoxic activity against specific protein antigen i.e. HBsAg as compared to control and standard.
As per literature, nitric oxide production is mainly released by human PBMC and played a considerable role especially in the pathophysiology of hormonal immune system [21]. Actually, diseases related to animal or human can be characterized or diagnosed through lack or excess of nitric oxide production from human PBMC or animal peritoneal macrophages. In some circumstances, protection against decline in constitutive nitric oxide production or stimulation in the vasculature may intercept the development of vascular disease, while inhibition of uncontrolled nitric oxide production could also be a therapeutic target [21]. From these studies, these medicinal plants i.e. Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia showed inhibition of proliferation and nitric oxide production at higher doses as compared to control. In other words, these flavonoids showed some drastic decreased in nitric oxide production at higher doses and showed its therapeutic effect. In addition, these flavonoids of these medicinal plants showed inhibitory effects on HBsAg lymphocyte proliferation. From these results, it is confirmed that flavonoids from Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia showed immunosuppressive and cytotoxic effect against HBsAg.
Conclusion
The present study has shown the immunosuppressive and cytotoxic potential of flavonoid extracted from the leaves of Calotropis gigantea, Adhatoda vasica and Artocarpus integrifolia by inhibiting cellular immunity in the form of nitric oxide production, blood counts and proliferation assay. These findings also suggested that these flavonoids from medicinal plants suppress the immune system and also be one of the probable candidate like other chemotherapeutic agents. Further detailed studies of these flavonoids are required which might establish a possible mechanism for anticancer activity.
- Ochwang’I DO, Kimwele CN, Oduma JA, Gathumbi PK, Mbaria JM, et al. (2014) Medicinal plants used in treatment and management of cancer in Kakamega County Kenya. J Ethnopharmacology 151: 1040–1055. Link: https://goo.gl/rWw3eb
- Romagnolo DF, Selmin OI (2012) Flavonoids and cancer prevention: a review of the evidence. J Nutr Gerontol Geriatr 31: 206–238. Link: https://goo.gl/vr9CdO
- Costa-Lotufo LV, Khan MTH, Ather A, Wilke DV, Jimenez PC, et al. (2005) Studies of the anticancer potential of plants used in Bangladeshi folk medicine. J Ethnopharmacology 99: 21–30. Link: https://goo.gl/xCesVs
- Gupta A, Chaphalkar SR (2015) Immunosuppressive activity of flavonoids isolated from Terminalia arjuna, Prosopis spicigera and Mimusops elengi. Int J Res Pharmacy and Science 5: 14–17. Link: https://goo.gl/YgJ10d
- Gupta A, Chaphalkar SR (2015) Anti-inflammatory activity of flavonoids from medicinal plants against hepatitis B vaccine antigen on human peripheral blood mononuclear cells. Asian J Pharmac Clinical Res 3: 728–732. Link: https://goo.gl/Jmy2T0
- Spencer JP (2008) Flavonoids: modulators of brain function. British J Nutrition 99: 60–77. Link: https://goo.gl/ipkieq
- Kumar S, Pathania AS, Saxena AK, Vishwakarma RA, Ali A, et al. (2013) The anticancer potential of flavonoids isolated from the stem bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signaling pathway in human leukaemia HL-60 cells. Chemico Biol Interactions 205: 128–137. Link: https://goo.gl/lmNJ8y
- Agati G, Azzarello E, Pollastri S, Tattini M (2012) Flavonoids as antioxidants in plants: Location and functional significance. Plant Science 196: 67–76. Link: https://goo.gl/3h2Uhk
- Mujwah AA, Mohammed MA, Ahmed MH (2010) First isolation of a flavonoid from Juniperus procera using ethyl acetate extract. Arabian J Chem 3: 85-88. Link: https://goo.gl/UNUy7R
- Li CW, Cui CB (2014) One new and nine Known Flavonoids from Choerospondias axillaries and their in vitro antitumor, anti-hypoxia and antibacterial activities. Molecules 19: 21363–21377. Link: https://goo.gl/Nrl3Qq
- Gupta A, Chaphalkar SR (2016) Antidiabetic activity of Calotropis gigantea in human whole blood. J Disease Global Health 6: 107–112.
- Gupta A, Chaphalkar SR (2016) Inhibition of antigen specific T cell population using Calotropis gigantea and Terminalia arjuna. J Biology nature 5: 14–19. Link: https://goo.gl/V2WfOk
- Gupta A, Chitte R, Chaphalkar SR (2016) Comparative evaluation of aqueous extract and protease from Adhatoda vasica for determining its immunosuppressive activity. Indo American J Pharmaceutical Sci 3: 821-825. Link: https://goo.gl/nPZoMF
- Gupta A, Chaphalkar SR (2015) Immunosuppressive activity of saponin from the leaves of Adhatoda vasica. Int J Institutional Pharmacy and Life Sciences 5: 137–145. Link:
- Gupta A, Chaphalkar SR (2015) Immunosuppressive activity of crude saponins from the leaves of Calotropis gigantea, Calamus rotang and Artocarpus integrifolia. Int J Pharma sciences research 6: 1–5. Link: https://goo.gl/HC6r5i
- Braca A, Sortino C, Politi M, Morelli I, Mendez J (2002) Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol 79: 379–381. Link: https://goo.gl/FfbgWt
- Heber D (2014) Vegetables, fruits and phytoestrogens in the prevention of diseases. J Postgrad Med 50: 145–149. Link: https://goo.gl/ojPyiX
- Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, et al. (2000) Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130: 2243–2250. Link: https://goo.gl/8AnWPC
- Birt DF, Hendrich S, Wang W (2001) Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther 90: 157–177. Link: https://goo.gl/M2bGQe
- Batra P, Sharma AK (2013) Anti-cancer potential of flavonoids: recent trends and future perspectives. Biotech 3: 439–459. Link: https://goo.gl/e674sp
- Makchuchit S, Itharat A, Tewtrakul S (2010) Antioxidant and nitric oxide inhibition activities of Thai medicinal plants. J Med Assoc Thai 93: 227-235. Link: https://goo.gl/f0Y3wh
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
