International Journal of Dermatology and Clinical Research
Androgen receptor, oxidative stress and inflammation at the crossroads of skin diseases
Yiumo Michael Chan1*, Yen-ting Liu1 and Hardy W Chan2
2Helios Bioelectronics Inc, Taipei, Taiwan
Cite this as
Chan YM, Yen-ting L, Chan HW (2022) Androgen receptor, oxidative stress and Inflammation at the crossroads of skin diseases. Int J Dermatol Clin Res 8(1): 012-015. DOI: 10.17352/2455-8605.000045Copyright
© 2022 Chan YM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.To the Editor
Skin is the largest organ of the human body and acts as a protective barrier as our first line of defense. The development of effective treatments for skin diseases represents one of the biggest challenges in drug discovery. The pathogenesis of many skin disorders is complex and multifactorial. Here, we present evidence that a new class of compounds that can enhance the degradation of androgen receptors and activate antioxidant response might be attractive therapeutics for various skin diseases associated with androgen/androgen receptors, oxidative stress, and inflammation.
Androgen is a natural male sex hormone and binds to the Androgen Receptor (AR), a well-characterized nuclear hormone receptor that functions as a ligand-dependent transcription factor [1]. Androgen and AR are not only critical in regulating the development and functions of the reproductive systems in both males and females but also in skin biology as AR is expressed in various types of cells in the skin, such as epithelial cells of sebaceous glands, dermal papillae, interfollicular epidermal keratinocytes, and dermal fibroblasts [2]. Several skin diseases are thought to be androgen-dependent and/or associated with a dysregulation in androgen receptor signaling pathways, including acne vulgaris, androgenetic alopecia, hirsutism, and hidradenitis suppurativa [3]. Both AR antagonists and selective AR degraders are being developed to target the androgen-AR axis in these conditions as well as prostate cancer and neurodegenerative diseases [4,5].
ASC-J9 is a new class of compound originally developed for the treatment of prostate cancer. The compound is a structural analog of curcumin and was shown to have a higher capacity to degrade AR than other nuclear receptors [6]. ASC-J9 can also degrade the mutant AR protein in castration-resistant prostate cancer [7] and spinal and bulbar muscular atrophy (SBMA) [8]. Preliminary studies using topical ASC-J9 treatment in fuzzy rats demonstrated it could reduce sebum production and the size of sebaceous glands [3]. In view of its potential clinical benefits in skin diseases, a topical cream formulation of ASC-J9 has been developed to treat facial acne. A global Phase 2 proof-of-concept study (ClinicalTrials.gov Identifier: NCT01289574) showed promising results in reducing inflammatory lesions in patients with moderate to severe acne (manuscript in preparation). In addition, it has been suggested AR might suppress cutaneous wound healing by enhancing local TNF-α expression mediated by infiltrating macrophages. Topical treatment of ASC-J9 accelerated wound healing and reduced local TNF-α expression in mice [9]. A recent study further demonstrated that ASC-J9 blocked extracellular matrix production by inhibiting STAT3 signaling in fibroblasts isolated from patients with keloid, a type of scar resulting from abnormal wound healing [10].
Another curcumin analog, ASC-JM17, was identified through an assay screening for AR degradation capability. This compound was more effective in enhancing the degradation of AR compared to ASC-J9 and curcumin (Figure 1). In addition, ASC-JM17 was shown to be an effective Nrf2 (nuclear factor erythroid 2-related factor 2) activator to induce antioxidant response through up-regulation of antioxidant enzymes, such as HO-1 (heme oxygenase-1) and NQO1 (NAD[P]H quinone dehydrogenase 1) [11,12]. Treatment of ASC-JM17 in an SBMA transgenic mouse model effectively degraded the mutant AR protein in muscles and improved motor functions of the SBMA mice [11]. ASC-JM17 is developed as an oral formulation. A first-in-human study has demonstrated that the drug was safe and well-tolerated and has a favorable drug-like pharmacokinetic profile (ClinicalTrials.gov Identifier: NCT04392830). The first-in-patient study in SBMA patients is currently under development.
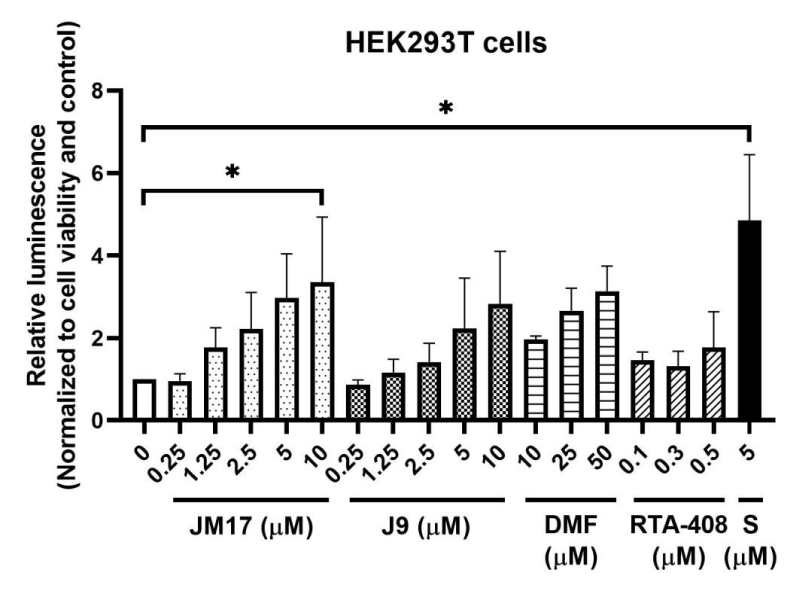
Nrf2 is the master transcription factor to regulate cellular redox [13] and there is ample evidence in the literature to support the critical role of oxidative stress and the Nrf2 pathway in the development of many disorders, including skin diseases [14]. For example, a well-known Nrf2 activator, dimethyl fumarate (DMF) was an approved treatment for psoriasis in the EU and for relapsing-remitting multiple sclerosis in the US [15]. Our results demonstrated that both ASC-JM17 and ASC-J9, and other known Nrf2 activators, sulforaphane and RTA-408 enhanced the stability of the Nrf2 protein, which is essential to Nrf2-mediated antioxidant responses since Nrf2 protein is rapidly targeted for proteasome-mediated degradation under basal condition (Figure 2). Sulforaphane, a naturally occurring compound, is a potent Nrf2 activator that was shown to increase transcription of Nrf2 and accumulation of Nrf2 protein [16]. RTA-408 (omaveloxolone) is a synthetic triterpenoid that was shown to activate and stabilize Nrf2 protein and inhibit ROS production [17]. Both compounds are under development for treatment of various diseases associated with oxidative stress and inflammation [18].
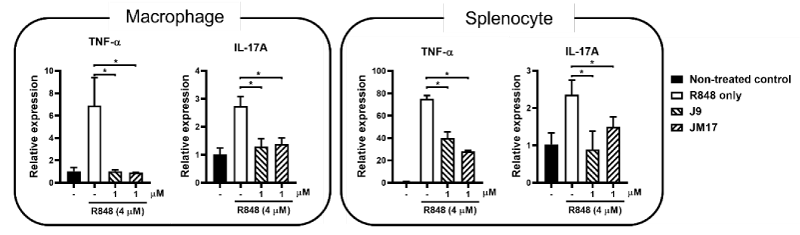
As the primary protective barrier of human body, skin is constantly under the insult of oxidative stress from the environment. Proper homeostasis of the skin regulated by Nrf2 is therefore essential to restore the redox balance and perhaps, also the skin microbiota. Interestingly, both ASC-JM17 and ASC-J9 were shown to suppress expression of proinflammatory cytokines in resiquimod-stimulated macrophages and splenocytes (Figure 3). Resiquimod (R848) is a potent immunostimulant of the antiviral Toll-like receptor (TLR) 7/8 signaling pathway and can mimic the Th1- and Th17-dependent immune responses that are associated with psoriasis [19]. In this experiment, TNF-α and IL-17A were selected as markers of Th1 and Th17 responses, respectively, to assess the effects of ASC-JM17 and ASC-J9 on their transcription regulation. Since many skin diseases, such as acne and psoriasis are often associated with excessive and abnormal inflammatory responses, the ability to activate antioxidant response and suppress inflammatory signal may be added benefits to the drug profile.
In summary, the pathophysiology of skin diseases likely involves multiple cellular pathways, acting in synergistical and/or compensatory manners. Treatment of androgen-dependent skin diseases might need to go beyond targeting the androgen-AR axis. The new class of androgen receptor degradation enhancers with pleotropic activities in reducing oxidative stress and inflammation offers an attractive approach in treating a variety of skin conditions, including androgen-associated skin diseases, inflammatory skin diseases, wound healing, and even aging. The development of different formulations might provide additional advantages in optimizing the route of administration for different indications based on the pathophysiology and target engagement strategy.
CWR22RV1 prostate cancer cell line was obtained from American Type Culture Collection (ATCC) (CRL-2505) and treated with ASC-JM17, ASC-J9, or curcumin at the specified concentrations for 24h. Total cell lysates were extracted with RIPA buffer (25 mM Tris-HCl pH 7.6, 137 mM NaCl, 1 mM EDTA pH 8.0, 1 mM EGTA pH 8.0, 1% Triton X-100, 2 mM sodium pyrophosphate, 25 mM β-glycerol phosphate) with protease inhibitor (04693132001, Roche) and phosphatase inhibitor (04906837001, Roche). Protein levels of full-length AR, AR variants from alternative splicing, and the internal control GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) were assessed by Western Blots using antibodies against AR (06-680, Millipore) and GAPDH (GTX100118, GeneTex).
HEK293T cells (ATCC, CRL-3216) were co-transfected with Nrf2-Luc and Keap1 constructs (N1391, Promega) via PolyJet transfected reagent (SL100688, SignaGen Laboratories) to monitor the stability of Nrf2-Luc fusion protein. Under basal condition, Nrf2 is targeted for degradation through ubiquitin-proteasome system mediated by the adaptor protein KEAP1. Luminescence signal was detected 4h after treatment of different Nrf2 activators at the specified concentrations, indicating increased stability of the Nrf2-Luc protein. The values of luminescence were normalized to cell viability and expressed as ratio normalized to no treatment control (mean±SEM). *p<0.05, Dunnett’s test using ANOVA for multiple comparison. DMF, dimethyl fumarate; RTA-408, omaveloxolone; S, sulforaphane.
Mouse bone marrow derived macrophages and splenocytes were isolated from C57BL/6J mice of 6 to 8 weeks old following the procedures described in previous study [20]. Bone marrow derived macrophages were prepared from bone marrow cells washed out of tibias and femurs and cultured in 70% Dulbecco’s modified Eagle’s medium (DMEM) and 30% L929-conditioned medium supplemented with 10% FBS, L-glutamine, antibiotics, and 10 mM HEPES. Splenocytes were isolated from spleens and cultured in RPMI1670 completed medium. Cells were co-treated with R484 and ASC-JM17 or ASC-J9 for 24h and the expression of the cytokines were measured by qPCR with specific forward and reverse primers.
TNF-α: forward 5’-AGCCCCCAGTCTGTATCCTT-3’
TNF-α: reverse 5’-CTCCCTTTGCAGAACTCAGG-3’;
IL-17A: forward 5’-TCTCTGATGCTGTTGCTGCT-3’
IL-17A: reverse 5’-ACGTGGAACGGTTGAGGTAG-3’;
GAPDH (internal control): forward
5’-ACCCAGAAGACTGTGGATGG-3’
GAPDH (internal control): reverse
5’-CACATTGGGGGTAGGAACAC-3’.
Data are expressed as relative expression to unstimulated (non-treated) control (mean ± SEM). * p < 0.05, unpaired two-tailed Student’s t-test.
- Davey RA, Grossmann M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin Biochem Rev. 2016 Feb;37(1):3-15. PMID: 27057074; PMCID: PMC4810760.
- Choudhry R, Hodgins MB, Van der Kwast TH, Brinkmann AO, Boersma WJ. Localization of androgen receptors in human skin by immunohistochemistry: implications for the hormonal regulation of hair growth, sebaceous glands and sweat glands. J Endocrinol. 1992 Jun;133(3):467-75. doi: 10.1677/joe.0.1330467. PMID: 1613448.
- Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res. 2012 Sep;304(7):499-510. doi: 10.1007/s00403-012-1265-x. Epub 2012 Jul 25. PMID: 22829074; PMCID: PMC3763909.
- Del Rosso JQ, Kircik LH, Stein Gold L, Thiboutot D. Androgens, Androgen Receptors, and the Skin: From the Laboratory to the Clinic With Emphasis on Clinical and Therapeutic Implications. J Drugs Dermatol. 2020 Mar 1;19(3):30-35. PMID: 32550699.
- Kargbo RB. PROTAC Compounds Targeting Androgen Receptor for Cancer Therapeutics: Prostate Cancer and Kennedy's Disease. ACS Med Chem Lett. 2020 May 18;11(6):1092-1093. doi: 10.1021/acsmedchemlett.0c00236. PMID: 32550986; PMCID: PMC7294551.
- Lai KP, Huang CK, Chang YJ, Chung CY, Yamashita S, Li L, Lee SO, Yeh S, Chang C. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am J Pathol. 2013 Feb;182(2):460-73. doi: 10.1016/j.ajpath.2012.10.029. Epub 2012 Dec 4. PMID: 23219429; PMCID: PMC3562731.
- Wang R, Lin W, Lin C, Li L, Sun Y, Chang C. ASC-J9(®) suppresses castration resistant prostate cancer progression via degrading the enzalutamide-induced androgen receptor mutant AR-F876L. Cancer Lett. 2016 Aug 28;379(1):154-60. doi: 10.1016/j.canlet.2016.05.018. Epub 2016 May 24. PMID: 27233475.
- Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007 Mar;13(3):348-53. doi: 10.1038/nm1547. Epub 2007 Mar 4. PMID: 17334372.
- Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009 Dec;119(12):3739-51. doi: 10.1172/JCI39335. Epub 2009 Nov 9. PMID: 19907077; PMCID: PMC2786793.
- Hong YK, Wu CH, Lin YC, Huang YL, Hung KS, Pai TP, Liu YT, Chen TC, Chan H, Hsu CK. ASC-J9 Blocks Cell Proliferation and Extracellular Matrix Production of Keloid Fibroblasts through Inhibiting STAT3 Signaling. Int J Mol Sci. 2022 May 16;23(10):5549. doi: 10.3390/ijms23105549. PMID: 35628356; PMCID: PMC9141592.
- Bott LC, Badders NM, Chen KL, Harmison GG, Bautista E, Shih CC, Katsuno M, Sobue G, Taylor JP, Dantuma NP, Fischbeck KH, Rinaldi C. A small-molecule Nrf1 and Nrf2 activator mitigates polyglutamine toxicity in spinal and bulbar muscular atrophy. Hum Mol Genet. 2016 May 15;25(10):1979-1989. doi: 10.1093/hmg/ddw073. Epub 2016 Mar 8. PMID: 26962150; PMCID: PMC5062587.
- Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018 Dec 10;29(17):1727-1745. doi: 10.1089/ars.2017.7342. Epub 2017 Oct 20. PMID: 28899199; PMCID: PMC6208165.
- Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021 Sep;20(9):689-709. doi: 10.1038/s41573-021-00233-1. Epub 2021 Jun 30. Erratum in: Nat Rev Drug Discov. 2021 Aug;20(8):652. PMID: 34194012; PMCID: PMC8243062.
- Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006 Dec;126(12):2565-75. doi: 10.1038/sj.jid.5700340. PMID: 17108903.
- Brück J, Dringen R, Amasuno A, Pau-Charles I, Ghoreschi K. A review of the mechanisms of action of dimethylfumarate in the treatment of psoriasis. Exp Dermatol. 2018 Jun;27(6):611-624. doi: 10.1111/exd.13548. Epub 2018 Apr 25. PMID: 29603404.
- Kubo E, Chhunchha B, Singh P, Sasaki H, Singh DP. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci Rep. 2017 Oct 26;7(1):14130. doi: 10.1038/s41598-017-14520-8. PMID: 29074861; PMCID: PMC5658327.
- Shekh-Ahmad T, Eckel R, Dayalan Naidu S, Higgins M, Yamamoto M, Dinkova-Kostova AT, Kovac S, Abramov AY, Walker MC. KEAP1 inhibition is neuroprotective and suppresses the development of epilepsy. Brain. 2018 May 1;141(5):1390-1403. doi: 10.1093/brain/awy071. PMID: 29538645.
- Robledinos-Antón N, Fernández-Ginés R, Manda G, Cuadrado A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev. 2019 Jul 14;2019:9372182. doi: 10.1155/2019/9372182. PMID: 31396308; PMCID: PMC6664516.
- Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002 Feb;3(2):196-200. doi: 10.1038/ni758. Epub 2002 Jan 22. PMID: 11812998.
- Tseng JC, Yang JX, Liu YL, Su YW, Lee AY, Chen YW, Liu KJ, Luo Y, Hong YR, Chuang TH. Sharpening up tumor microenvironment to enhance the efficacy of immune checkpoint blockade on head and neck cancer using a CpG-oligodeoxynucleotide. Cancer Immunol Immunother. 2022 May;71(5):1115-1128. doi: 10.1007/s00262-021-03062-8. Epub 2021 Sep 28. PMID: 34581869; PMCID: PMC9016021.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
