International Journal of Dermatology and Clinical Research
Pilot study of the efficacy of treatment of urticaria by using two traditional Vietnamese herbs administered systemically
Bach Thị Như Quỳnh3*, Pham Van Thức1, Nguyen Thị Thuỳ Dung2, Ninh Thị Kim Thu2, Nguyen Thị Hien1, Floch Katell3, Nguyen Thị Hồng Liên2 and Carre Jean-Luc3*
2Faculty of Traditional Medicine of Haiphong, University of Medicine and Pharmacy of Haiphong, Vietnam
3Faculty of Medicine and Health Sciences of Brest - University of Western Brittany, Brest, France
Cite this as
Như Quỳnh BT, Thức PV, Thuỳ Dung NT, Kim Thu NT, Carré JL, et al. (2022) Pilot study of the efficacy of treatment of urticaria by using two traditional Vietnamese herbs administered systemically. Int J Dermatol Clin Res 8(1): 007-011. DOI: 10.17352/2455-8605.000044Copyright
© 2022 Như Quỳnh BT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction
Among the many plants used in traditional medicine in many countries, Averrhoa carambola L. and lonicera japonica Thunb are regularly the subjects of publications describing the pharmacological effects of their extracts. Lonicera japonica Thunb is particularly studied. Its antioxidant properties are frequently cited [1], but publications describing its anti-inflammatory effects are really numerous [2], on a wide variety of organs (respiratory system [3], locomotor system [4], or skin [5,6]. Meta-analyses have made it possible to list hundreds of components present in lonicera japonica Thunb and to match them with different pharmacological activities [7]. The scientific literature also provides information on the pharmacological effects of Averrhoa carambola L. General effects on blood sugar and weight [8], or antioxidant activity [9] have been observed, and meta-analyses have also attempted to identify the molecules present in this plant and to link them to pharmacological activities [10,11]. However, many articles describe the toxic effects of this plant, and its renal and neurological effects [12,13].
In the traditional Vietnamese pharmacopeia, aqueous decoctions of the leaves of these two plants, Averrhoa carambola L. and lonicera japonica Thunb are regularly used, alone or in combination, by the local or general route.
These treatments can be used in acute or chronic urticaria, in order to treat pruritus, with an expected success of 100% improvement of symptoms in 80% of patients after 10 days of therapy according to Prof. P.V.Thuc from the University of Medicine and Pharmacy of Haiphong.
Recent literature allows finding an anti-allergic effect of some extracts of lonicera japonica Thunb [14] or a therapeutic effect on atopic eczema in association with other plants [15] or on a mouse model of induced asthma [16]. On the other hand, the literature review did not find any therapeutic effect on the immune system, but allergic reactions to the consumption of Averrhoa carambola L. fruit [17].
We have therefore conducted a pilot study, intended to highlight the clinical effect of these two plants observed in the practice of traditional Vietnamese medicine. The subsequent objective would be to set up biological research with a double aim: to explore the molecular mechanisms of pruritus impacted by these treatments on the one hand, and to identify one (or more) active principle(s) from these plants on the other hand.
The present study was carried out in Haiphong (Vietnam), where both products are authorized for medical use. This study was approved by the Medical Ethics Committee of Hai Phong University of Medicine and Pharmacy in December 2019. The principal investigator was Prof. Pham Van Thuc, whose allergology consultation enabled him to include eligible patients with chronic urticaria.
Material and methods
Patients of both sexes, aged 18 to 70 years, with chronic urticaria were eligible. Inclusion criteria were as follows: well-demarcated and highly irritating erythematous and/or papulo-oedematous skin lesions, localized or all over the body, with or without an identified cause. Patients with contact dermatitis, insect bites, simple erythema, psoriasis, patients receiving antihistamines or corticosteroids, and patients who refused to participate in the study were excluded.
After inclusion, patients were tested to assess their pruritus (patient assessment on the visual analog pruritus scale [18,19] allowing to assign a score between 0 and 10 to each assessment and pruritus score on the 5D itch scale questionnaire [20]. They were then divided into 4 randomized groups of 30 patients (treatment with Averrhoa carambola L., treatment with lonicera japonica Thunb, and treatment with both extracts and placebo).
The treatment consisted of a local application on the affected area 3 times a day (morning, afternoon, and evening) and an ingestion 3 times a day of 10 mL.
After 10 days of treatment, a new examination was performed including the same self-evaluation of pruritus. The file was then closed and recorded as is.
The evolution of pruritus during treatment was assessed by the difference between the two scores on the visual analog scale of pruritus.
Anxiety, reflecting the impact of pruritus on the patients’ quality of life, was assessed by the anxiety self-report inventory [21], which assigns 1 point to each item (10 positive and 10 negative items).
The evolution of anxiety during treatment was assessed by the difference between the two scores (at inclusion and at 10 days of treatment), each consisting of the difference between the number of positive and negative items.
After 10 days of treatment, patients completed a subjective questionnaire, Patients’ Global Impression of Change (PGIC), reflecting their experience of pruritus after treatment, and the status of their condition at the end of the protocol. Each item is assigned a point, ranging from significantly worse pruritus [1] to significantly better pruritus [7,22].
The preparation of the herbal treatments was traditionally carried out by the pharmacy of the Faculty of Traditional Medicine of Haiphong, Vietnam, using fresh (same day) leaves of carambola (Averrhoa carambola L.) harvested in Hai Phong and then washed by soaking 5 times in fresh water.
A decoction of 2 kg of carambola leaves is made in 3 liters (L) of water heated for 90 minutes at 100 °C, maintaining the volume at 3 L during the whole operation by adding water regularly. The liquid obtained (extract 1) is filtered on cotton gauze. The operation is repeated on the same leaves for 90 minutes with 3 L of water. The liquid obtained (extract 2) is also filtered.
The final solution obtained by combining extracts 1 and 2 is filtered again after an overnight rest, then concentrated by heating at 100°C with constant stirring to a volume of 1 L.
The preparation is identical for lonicera japonica Thunb.
The placebo is prepared as follows: 300 g of whole unpeeled ripe apples (Fructus Ziziphi jujube), dried, and then well washed in water are placed in 1 L of water. The whole is heated to 100°C and then left to boil until the liquid is reduced to 500 mL. After coarse filtration, 4 black tea bags are added and the liquid is boiled again for 5 minutes. After about 10 minutes of infusion, the preparation is filtered to remove impurities.
The concentrated preparations are yellow-brown in color, with a pungent and bitter taste. They were finally modified by the addition of sucrose to obtain a flavor and a color that could not be discriminated by physicians or patients in order to respect the double-blind protocol of the study. Sucrose is added to each preparation at a rate of 1:1 (g/mL) and the whole is heated for 5 hours at 60°C until the sucrose is completely dissolved and a homogeneous liquid is obtained, the syrup of starfruit leaves, lonicera, or placebo.
After preparation, the extracts can be stored in the refrigerator at 4 degrees Celsius and can be used for 10 days. They can also be stored in the freezer (< 0 °C) for use for 1 month. These recommendations on the duration of use of the treatments were communicated by the pharmacists of the Faculty of Traditional Medicine of Haifong.
The descriptive statistical analysis concerns the differences in the means of the pruritus evaluation scores (+/- standard deviation), performed by ANOVA on Excel.
Results
Considering the results expected by Pr Thuc (80% of patients with complete improvement of symptoms), the number of patients included in each group was 30. We, therefore, included 30 patients in 4 groups: treatment with Averrhoa carambola L., treatment with lonicera japonica Thunb, and treatment with both extracts and placebo.
Comparability of the patient groups
There was no difference in sex ratio between the 4 groups (p = 0.95), nor was there a difference in age, M= 38.1 years (p = 0.95).
The questionnaire at inclusion in the study showed that the duration since the onset of symptoms was identical in the 4 groups: between 6 and 12 months (p = 0.99) with a frequency of 1 attack per 1 to 2 weeks (p = 0.99).
Furthermore, the 5D itch scale showed no difference between the four groups in the recent evolution of pruritus: 12 to 18 hours per day, with moderate to severe intensity, with no real change during the last two weeks (p = 0.97 to 0.99).
Treatment effects
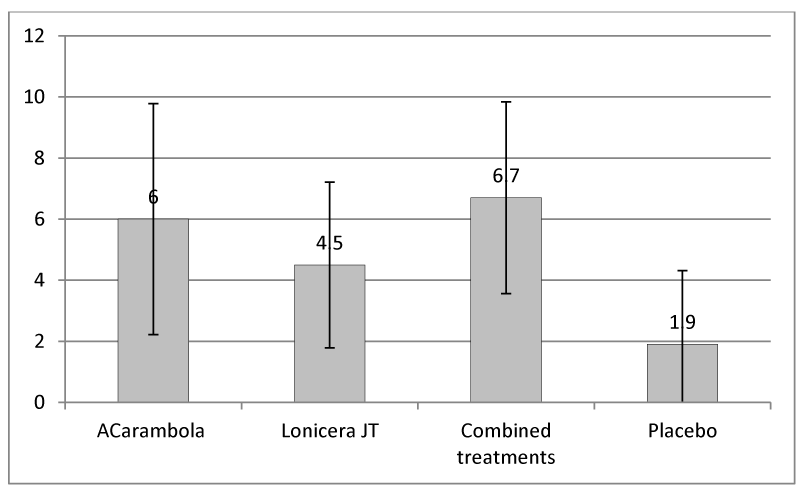
The visual analog scale of pruritus, graded from 0 (no itching) to 10 (worst itching imaginable) shows a significant variation between the assessment at inclusion and after 10 days of treatment for the treated groups, and much lower in the placebo group (Figure 1).
Representation of the variation evaluated by the patient before and after 10 days: average of 30 patients +/- S.D. in each of the four patient groups.
Statistical tests show a significant difference between the placebo group and the other three groups (p < 0.01). There was no significant difference between the three treatment groups (p = 0.19).
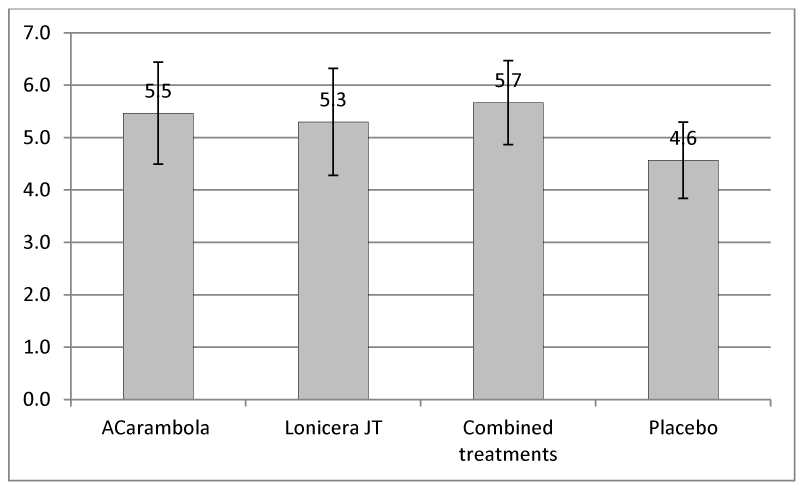
The evaluation of the impact of pruritus on the patient’s well-being, as assessed by the self-reported anxiety inventory, shows a significant variation between the evaluation at inclusion and after 10 days of treatment for the treated groups, and much lower in the placebo group (Figure 2).
Representation of the variation evaluated by the patient before and after 10 days: average over 30 patients +/- S.D. in each of the four patient groups.
Statistical tests show a significant difference between the placebo group and the other three groups (p < 0.01). There was a statistically significant difference between the three treatment groups (p = 0.02). Examination of these three groups of treated patients shows a significant difference between lonicera japonica Thunb and the group of the two combined treatments. On the other hand, despite an emerging trend, there was no significant difference between lonicera japonica Thunb and Averrhoa carambola L. or between Averrhoa carambola L. and the two treatments combined.
At the end of the 10 days of treatment, patients additionally completed the Patient’s Global Impression of Change (PGIC) questionnaire. Figure 3 shows the average responses to the questionnaire in each group.
The questionnaire was completed at the end of the 10-day protocol, represented by the mean +/- S.D. in each of the four patient groups.
The statistical study shows a statistically significant difference between the placebo group and the three treatment groups (p = 3. 10-5) but no difference between the three treatments.
We did not observe any statistical difference between the genders, nor according to the age of the patients (two-factor ANOVA, results not shown).
Discussion
The main purpose of this study was not to convince our colleagues of the efficacy of these two plants, Averrhoa carambola L. and lonicera japonica Thunb, on pruritus, especially in urticaria. Indeed, Vietnamese traditional medicine has been using these products for many decades with success. Our objective was to conduct a study capable of demonstrating with the tools of modern medical science that potential benefits exist in these treatments.
The idea of using a placebo could offend Vietnamese doctors who have been using these traditional medicine treatments for decades: why not give each patient a treatment whose effectiveness is recognized? On the other hand, for Western medicine, it is the superiority of the tested treatment compared to the placebo that proves its undeniable effectiveness. We wished, by the approach used in our study, to highlight the action of traditional treatments in an objective way, quantified by scales and scores that cannot be discussed, while proving a therapeutic effect superior to a simple placebo effect linked to the medical care of the patients.
We, therefore, asked the pharmacy specialists of the Department of Traditional Medicine of Haiphong Medical University to prepare the extracts of these two plants, as well as a placebo preparation, to be used in a traditional but double-blind manner.
After demonstrating that our four groups of patients did not differ in age and gender, but also in duration and recent course of pruritus, we compared the effect of the four treatments after 10 days.
The visual analog scale of pruritus shows a significant difference between the treated groups and the placebo group: the improvement of the sensation of pruritus after 10 days of treatment is less than 2 points in the placebo group, whereas it is very close to 5 in the two groups with treatment with one of the two plants, and close to 6 points in the group with a treatment combining the two plants. This undoubtedly attests to the therapeutic activity of the treatments on pruritus. In our study, there is no significant difference between the three treated groups.
The evaluation of the impact of pruritus on the patient’s well-being, assessed by the anxiety self-assessment inventory, shows a significant difference between the placebo group and the three groups of treated patients: the variation is less than 2 in the placebo group, whereas it is between 4.5 and 6.7 in the treatment groups. It is clearly demonstrated here that the improvement of pruritus by these traditional treatments allows a significant improvement in the quality of life felt by the patients. Moreover, our study shows a difference in this perception among the treated patients: lonicera japonica thusnb is statistically less effective than the other two treatments.
The assessment of the evolution of the sensation of pruritus and its impact on the quality of life of the patients (Figures 1,2) shows a tendency that does not appear statistically significant, according to which the combination of the two plants would be more effective than one or the other used alone, and that Averrhoa carambola would be more effective than Lonicera japonica Thunb. Further investigations on larger series of patients are necessary.
Finally, the subjective impression of efficacy questionnaire (SIZE) confirms a significant difference between the placebo and the treated groups (with no objective difference between these three groups of treated patients). In an area as highly subjective as pruritus (sensation subjectively felt by each patient), it is thus demonstrated that these traditional therapeutic preparations have an indisputable effect on the pruritus of chronic urticaria.
Reading the three figures of our study, it appears that the difference between the treatment and placebo groups is very significant for the visual analog scale of pruritus and the self-evaluation inventory of anxiety, and weaker, although significant when using the general satisfaction questionnaire on management. The first two measures were designed to objectively capture the sensations experienced by the patients, whereas the PGIC questionnaire reflects a purely subjective sensation. Medical care that recognizes the patient’s suffering and provides care, even without an active ingredient (as is the case with a placebo), is obviously capable of improving the patient’s feelings. It is the whole notion of placebo that is at stake here. It is therefore not surprising that the totally subjective part of the management in the placebo group is accompanied by an improvement in the feeling on the PGIC score. The much higher difference noted in the two other scores allows us to affirm that the therapeutic role of the plants studied is real and goes beyond a simple placebo effect.
Conclusion
The use of classical evaluation tools of pruritus and its repercussion on the quality of life of the patients allowed us to show that extracts of the two plants Averrhoa carambola L. and lonicera japonica Thunb used by the Vietnamese traditional medicine are active in this symptom.
The future objectives of our team will be to identify one or more indicators of modulation of the “pruritus effect” by Averrhoa carambola L. and lonicera japonica Thunb extracts on a cell co-culture system (keratinocytes and neurons) modeling certain aspects of pruritus [23] and then to test purified extracts in order to orientate towards the active principles responsible for the therapeutic effects observed in the present pilot study.
- Zhang T, Liu H, Bai X, Liu P, Yang Y, Huang J, Zhou L, Min X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int J Biol Macromol. 2020 May 15;151:1058-1066. doi: 10.1016/j.ijbiomac.2019.10.147. Epub 2019 Nov 15. PMID: 31739015.
- Su X, Zhu ZH, Zhang L, Wang Q, Xu MM, Lu C, Zhu Y, Zeng J, Duan JA, Zhao M. Anti-inflammatory property and functional substances of Lonicerae Japonicae Caulis. J Ethnopharmacol. 2021 Mar 1;267:113502. doi: 10.1016/j.jep.2020.113502. Epub 2020 Nov 12. PMID: 33189843.
- Yang C, Song C, Wang Y, Zhou W, Zheng W, Zhou H, Deng G, Li H, Xiao W, Yang Z, Kong L, Ge H, Song Y, Sun Y. Re-Du-Ning injection ameliorates radiation-induced pneumonitis and fibrosis by inhibiting AIM2 inflammasome and epithelial-mesenchymal transition. Phytomedicine. 2022 Jul 20;102:154184. doi: 10.1016/j.phymed.2022.154184. Epub 2022 May 21. PMID: 35665679.
- Shao T, Huang K. Network Pharmacology-Based Analysis on Lonicera japonica for Chronic Osteomyelitis Treatment. J Oncol. 2022 Jan 24;2022:1706716. doi: 10.1155/2022/1706716. PMID: 35111224; PMCID: PMC8803426.
- Xue H, Sun M, Zhao X, Wang Y, Yan J, Zhang W. Preparation and Characterization of Polysaccharide-Based Hydrogels for Cutaneous Wound Healing. Polymers (Basel). 2022 Apr 22;14(9):1716. doi: 10.3390/polym14091716. PMID: 35566885; PMCID: PMC9105569.
- Wu JY, Chen YJ, Bai L, Liu YX, Fu XQ, Zhu PL, Li JK, Chou JY, Yin CL, Wang YP, Bai JX, Wu Y, Wu ZZ, Yu ZL. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine. 2020 Mar;68:153173. doi: 10.1016/j.phymed.2020.153173. Epub 2020 Jan 19. PMID: 31999977.
- Ge L, Xie Q, Jiang Y, Xiao L, Wan H, Zhou B, Wu S, Tian J, Zeng X. Genus Lonicera: New drug discovery from traditional usage to modern chemical and pharmacological research. Phytomedicine. 2022 Feb;96:153889. doi: 10.1016/j.phymed.2021.153889. Epub 2021 Dec 20. PMID: 35026509.
- Sari Y, Indarto D, Wasita B. The Effects of Star Fruit (Averrhoa carambola Linn.) Extract on Body Mass Index, Fasting Blood Glucose, and Triglyceride Levels in Male Rats with Obesity and Type 2 Diabetes Mellitus. Macedonian Journal of Medical Sciences. 2022; Apr 18; 10(A):744-751.
- Kuntang W, Nuengchamnong N, Lamlertthon S. Metabolite Profile, Antioxidant Activity and Anti-Candida Activity of Fermented Star Fruit Bioextract (Averrhoa carambola L.). Trends in Sciences 2022; 19(10): 4183.
- Lakmal K, Yasawardene P, Jayarajah U, Seneviratne SL. Nutritional and medicinal properties of Star fruit (Averrhoa carambola): A review. Food Sci Nutr. 2021 Jan 23;9(3):1810-1823. doi: 10.1002/fsn3.2135. PMID: 33747490; PMCID: PMC7958541.
- Luan F, Peng L, Lei Z, Jia X, Zou J, Yang Y, He X, Zeng N. Traditional Uses, Phytochemical Constituents and Pharmacological Properties of Averrhoa carambola L.: A Review. Front Pharmacol. 2021 Aug 12;12:699899. doi: 10.3389/fphar.2021.699899. PMID: 34475822; PMCID: PMC8407000.
- Yasawardene P, Jayarajah U, De Zoysa I, Seneviratne SL. Mechanisms of star fruit (Averrhoa carambola) toxicity: A mini-review. Toxicon. 2020 Nov;187:198-202. doi: 10.1016/j.toxicon.2020.09.010. Epub 2020 Sep 20. PMID: 32966829.
- Chang CT, Chen YC, Fang JT, Huang CC. Star fruit (Averrhoa carambola) intoxication: an important cause of consciousness disturbance in patients with renal failure. Ren Fail. 2002 May;24(3):379-82. doi: 10.1081/jdi-120005373. PMID: 12166706.
- Oku H, Ogawa Y, Iwaoka E, Ishiguro K. Allergy-preventive effects of chlorogenic acid and iridoid derivatives from flower buds of Lonicera japonica. Biol Pharm Bull. 2011;34(8):1330-3. doi: 10.1248/bpb.34.1330. PMID: 21804227.
- Chan BC, Hon KL, Leung PC, Sam SW, Fung KP, Lee MY, Lau HY. Traditional Chinese medicine for atopic eczema: PentaHerbs formula suppresses inflammatory mediators release from mast cells. J Ethnopharmacol. 2008 Oct 30;120(1):85-91. doi: 10.1016/j.jep.2008.07.034. Epub 2008 Aug 3. PMID: 18725279.
- Hong SH, Kwon JT, Shin JY, Kim JE, Minai-Tehrani A, Yu KN, Lee S, Park SJ, Chang SH, Jiang HL, Vibin M, Han K, Son KH, Kwak WJ, Chae C, Bang SH, Cho MH. Therapeutic effect of Broussonetia papyrifera and Lonicera japonica in ovalbumin-induced murine asthma model. Nat Prod Commun. 2013 Nov;8(11):1609-14. PMID: 24427953.
- Numata T, Ito T, Egusa C, Kobayashi Y, Maeda T, Tsuboi R. A case of oral allergy syndrome due to star fruit sensitized from atopic hands. Allergol Int. 2015 Oct;64(4):393-5. doi: 10.1016/j.alit.2015.06.011. Epub 2015 Jul 26. PMID: 26433542.
- Reich A, Chatzigeorkidis E, Zeidler C, Osada N, Furue M, Takamori K, Ebata T, Augustin M, Szepietowski JC, Ständer S. Tailoring the Cut-off Values of the Visual Analogue Scale and Numeric Rating Scale in Itch Assessment. Acta Derm Venereol. 2017 Jun 9;97(6):759-760. doi: 10.2340/00015555-2642. PMID: 28224165.
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, Augustin M, Szepietowski JC, Ständer S. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Derm Venereol. 2012 Sep;92(5):502-7. doi: 10.2340/00015555-1246. PMID: 22170091.
- Elman S, Hynan LS, Gabriel V, Mayo MJ. The 5-D itch scale: a new measure of pruritus. Br J Dermatol. 2010 Mar;162(3):587-93. doi: 10.1111/j.1365-2133.2009.09586.x. Epub 2009 Dec 1. PMID: 19995367; PMCID: PMC2875190.
- Desai NS, Poindexter GB, Monthrope YM, Bendeck SE, Swerlick RA, Chen SC. A pilot quality-of-life instrument for pruritus. J Am Acad Dermatol. 2008 Aug;59(2):234-44. doi: 10.1016/j.jaad.2008.04.006. Epub 2008 Jun 11. PMID: 18550210.
- ECDEU Assessment Manual for Psychopharmacology. Guy W. Rockville, MD: US Department of Heath,Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, 1976.
- Bocciarelli C, Cordel N, Leschiera R, Talagas M, Marcorelles P, Misery L, Lebonvallet N. Development and validation of an in vitro co-culture model of differentiated keratinocytes and sensory neurons from stem cells to study the involvement of Zika virus in pruritus and cutaneous neurogenic inflammation. Annales de Dermatologie et de Vénéréologie. 2020; 147:A242-A243.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
