International Journal of Dermatology and Clinical Research
Low PD-1 expression and no prognostic impact in early-stage Mycosis Fungoides: 61 patients retrospective cohort analysis
Gustavo Moreira Amorim1,2,3*, Danielle Carvalho Quintella1, João Paulo Niemeyer Corbellini1, Luiz Claudio Ferreira4, Marcia Ramos-e-Silva1,2 and Tullia Cuzzi1,2,4
2Pathology Post-Graduation Program – Faculty of Medicine of Federal University of Rio de Janeiro (UFRJ), Brazil
3Faculty of Medicine of South Santa Catarina University (UNISUL), Brazil
4Evandro Chagas National Institute of Infectology – Oswaldo Cruz Foundation (FIOCRUZ), Brazil
Cite this as
Amorim GM, Quintella DC, Niemeyer Corbellini JP, Ferreira LC, Ramos-e-Silva M, et al. (2019) Low PD-1 expression and no prognostic impact in early-stage Mycosis Fungoides: 61 patients retrospective cohort analysis. Int J Dermatol Clin Res 5(1): 012-017. DOI: 10.17352/2455-8605.000032Background: Mycosis fungoides (MF) is an indolent behavior cutaneous T-cell lymphoma. Most patients present a slowly progressive course, over many years. However, some patients evolve early towards advanced stages of the disease, despite adequate treatment, having therefore, a worse prognosis. Increasing knowledge of risk factors that contribute to a better prognostic evaluation is considered of interest.
Aims: Evaluate positivity and prognostic importance of PD-1 marker in early-stage MF.
Methods: Retrospective cohort study with early-stage MF patients. Cases were defined by Pimpinelli criteria. PD-1 positivity was tested in complementary immunohistochemistry over the first skin biopsies done in our hospital. Disease progression, death and MF-related death was investigated 5 years after the date of the diagnostic definition according to the proposed criteria. The hazard ratio was calculated as association measurement. Adopted significance criterion was 5%.
Results: A total of 61 patients were included. PD-1 was positive in only 7 (11.5%) of them. Disease progression, death and MF-related death occurred, respectively, in 26.2%; 13.1% and 6.6% of patients. There was no significant association between PD-1 positivity and the studied outcomes. Conclusions: PD-1 positivity in our sample of early-stage MF was low and therefore, such positivity could not be correlated to the observed outcomes, not having prognostic importance. The retrospective character of the study and its small sample are limitations of our research.
Introduction
Immune checkpoint inhibitors are of great interest for several oncology fields given the success of immunobiological therapies that antagonize immunological signaling pathways in different cancer types. PD-1/PD-L1 and PD-L2 stand out among these pathways, which remain under constant study.
The programmed-cell-death-1 receptor (PD-1) is expressed during thyme maturation in immature lymphocytes, in mature T and B-lymphocytes, in natural killer T-cells, in monocytes and in some dendritic cells [1]. The PD-1 pathway plays an important role in the regulation of cellular immunity. The interaction between PD-1 and its ligands (PD-L1 and PD-L2) causes T lymphocyte proliferation blockades, decreased pro-inflammatory cytokines production, reduced cytolytic function of immune cell responses and shorter T-lymphocytes survival. These alterations lead to T-cell activity reduction in peripheral tissues presenting chronic inflammatory conditions, infections and even malignant neoplasms [1,2].
Mycosis fungoides (MF) is a low-grade malignant T-cell epidermotropic non-Hodgkin lymphoma that is limited to the skin at diagnosis time. It is the most prevalent form of primary cutaneous T-cell lymphoma (CTCL), since it accounts for 50% to 65% of cases. This lymphoma type can take chronic and indolent courses and allows 5-year global survival, on average, in 84.8% to 88% of the cases. This disease evolves slowly, although it has the potential to evolve to extracutaneous lymphoma in the long term [3-8].
In addition to hematological malignancies, PD-1 expression was investigated and correlated to worse prognosis in several solid-organ neoplasms [9]. PD-1 positivity values range from 13 to 60.2% in MF patients [9,10]. Ogurinade et al., suggested that PD-1 positivity could be of prognostic importance [11].
We investigated PD-1 positivity in immunohistochemistry assessments of skin fragments in order to study this topic. These skin fragments were collected from first biopsies of a retrospective cohort encompassing early-stage MF patients subjected to treatment and followed-up in the Photodermatology Sector of Clementino Fraga Filho University Hospital (HUCFF) of the Federal University of Rio de Janeiro (UFRJ).
Materials and Methods
It is an observational, longitudinal, retrospective cohort study with early-stage MF patients.
Inclusion criteria:
· Early-stage MF, defined by Pimpinelli et al., [12,13]. The aforementioned criteria were retrospectively applied based on the first biopsy examination performed at our institution. Four points at least were required (as proposed by the algorithm). We highlight that our institution doesn’t have access to clonality T-cell receptor gene rearrangements examination. In this way, clinical, histopathological and immunohistochemical criteria (CD2, CD3, CD5 and CD7) were used.
· Adult patients, 18 years-old or more;
· TNMB staging IA or IB (skin limited disease) [11,12];
· Five-year follow up or more.
Exclusion criteria:
· Insufficient data on medical charts;
· Unavailable histopathological examinations for revision or insufficient material on paraffin blocks to perform immunohistochemical analysis;
· Positive serology to HTLV 1 and 2.
The evaluated dependent variables were:
· Disease progression (staging) treated in a qualitative, dichotomous and nominal manner. Those who progressed to stage IIA or more were categorized in the “disease progression” group.
· Death rates were treated in a qualitative, dichotomous and nominal manner.
· MF-related death caused by the lymphoma or by complications resulting from systemic therapies. This variable was also treated in a qualitative, dichotomous and nominal manner.
The assessed independent variables were gender, age at diagnosis, the presence of plaques, disease extension, CLIPi score, TNMB, Pautrier’s microabscesses (PM), folliculotropism and lymphocytic atypia, as well as PD-1 expression.
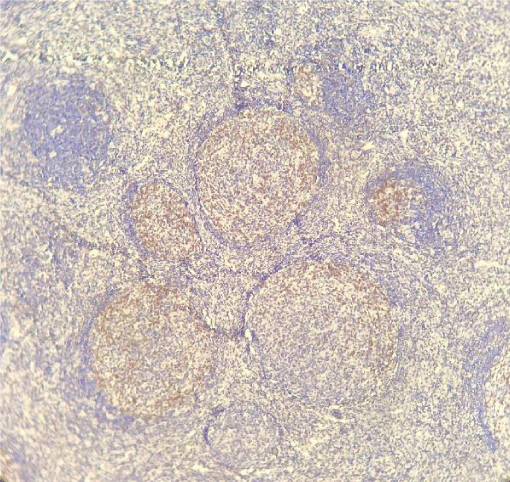
Immunohistochemistry was applied on paraffin blocks with skin fragments from the first biopsies performed in our hospital. We used PD-1 (NAT105) Mouse Monoclonal Antibody from Cell Marque, with a 1:100 dilution as proposed by the company (Cell Marque. Sierra College Boulevard, Rocklin, California. 95077. EUA). Two positive control tests for each sequence of reactions were done, using tonsil tissue, again, as suggested by Cell Marque. The percent of PD-1 neoplastic T cells were scored as minimal (<20%), moderate (20-49%) and high (>50%).
Data were gathered in printed and digitized Excel 2011 worksheets (Microsoft® Excel® for Mac 2011 / Version: 14.2.0) and analyzed in the SPSS statistical software, version 24.0. The chi-square test (X2) or Fischer’s exact test, were applied to investigate the association between qualitative independent variables.
Hazard ratios and their respective confidence intervals (CI: 95%) were calculated as association measurements. The significance criterion was 5%. Finally, Multivariate Poisson regression was performed to help identify independent predictors for the events.
The current study complies with the National Health Council Resolution 466/12; it is registered in Plataforma Brasil (Brazil Platform) and was approved by CEP-HUCFF/UFRJ - CAAE: 59235916.9.0000.5257.
Results
One hundred and thirty-five (135) patients were eligible for the study; however, 102 patients diagnosed with early-stage MF were included based on medical report’s analysis.
Among the 33 excluded patients, 17 were due to insufficient data, 10 had a different diagnosis after revision (lymphomatoid papulosis and granulomatous slack skin lymphoma) and 6 had positive HTLV serology.
Seventy-eight of the 102 patients selected had their first histopathological examination available for adequate revision. Sixty-seven of the 78 selected had Paraffin blocks containing the biopsied tissue for subsequent immunohistochemical analysis of CD2, CD3, CD5 and CD7.
Of thouse 67 patients, 61 scored 4 points after retrospective application of Pimpinelli’s criteria, therefore corresponding to the total sample [13].
Table 1 summarizes the clinical aspects of our sample.
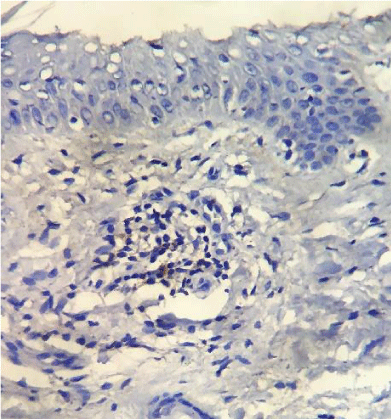
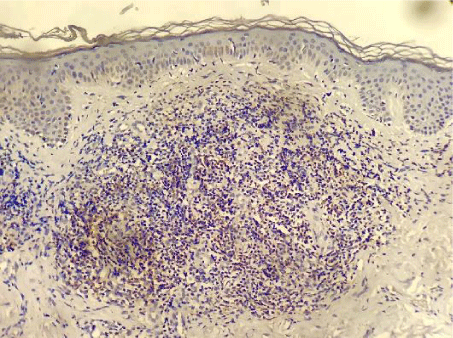
PD-1 was positive in 7 of the 61 investigated patients (Table 2). Positive PD-1 control on tonsil tissue and 3 examples of PD-1 positivy expression on figures 1-4.
Table 3 shows the frequency of the studied dependent variables.
Out of the 16 patients (16/61; 26.2%) who presented staging progress, 25.0% advanced to IIA (they developed to N1); 12.5%, to IIB (they presented T3); and 50%, to IIIA (they presented T4). In regard to the 2 remaining patients, 1 progressed to stage IIIB (T4 + B1); and 1, to stage IVA (T4 + B2) [12].
Eight (8/61; 13.1%) patients died; among them, 4 had a death associated with MF. These 4 patients presented staging progress and were subjected to systemic treatment. All four died despite chemotherapy.
Comparisons between independent and dependent variables are shown in table 4.
There was no correlation between PD-1 positivity and the observed outcomes. Also, no statistically significant association was found in the multivariate regression test when all variables were studied together.
Discussion
PD-1 markers were assessed in immunohistochemistry examinations from a cohort encompassing 61 early-stage MF patients, based on the Pimipinelli’s criteria. Positivity was evaluated and related to disease progression and death after a 5–year follow-up.
PD-1 positivity was low (11.5%). The percentage of marked lymphocytes was not expressive, even among positive cases.
Samimi et al., investigated PD-1 positivity in peripheral blood T lymphocytes of Sézary syndrome (SS) and MF patients. They recorded higher PD-1 expression percentage in SS patients when compared to MF patients [14].
Wada et al., had published a study on immunohistochemical investigations about PD-1 expression. They used a series of 26 MF and 11 SS biopsies from 37 patients and identified 40% positivity among MF patients with patches e plaques, and 60% positivity in patients with tumor stage lesions. Their cutoff for a positive expression was at least 30% marked lymphocytes [15].
Also, Cetinozman et al., had studied a sample with 27 SS and 60 MF cases to evaluate PD-1 positivity in immunohistochemistry tests applied to previous cutaneous-biopsy materials. The positive test cutoff percentage was determined in 50% of the total infiltrate lymphoid cells. Eight-nine percent of SS patients and 13% of MF patients showed PD-1 positivity in their samples. Tumor stage and with erythrodermic MF recorded higher PD-1 positivity when compared to early-stage MF [10].
Kantekura et al, and Park et al. (2014) found 80% (12/15) - cutoff positivity > 3% of marked lymphocytes; and 84% (21/25) cutoff: at least 11% of marked lymphocyte; PD-1 positivity in MF patients, respectively, after applying a similar methodology. However, their cutoff points for positivity determination were more sensitive [16,17].
Nguyen et al., published the results of a PD-1 immunopositivity research; they used immunohistochemical examinations from previous biopsies of cutaneous lymphoma patients in the study. In total, they assessed 103 specimens of MF patients. At least 50% PD-1 positivity in lymphoid cells was identified in 14/21 (66.7%), 23/34 (67.6%) and 11/17 (64.7%) MF patients with patches, plaques and tumor lesions, respectively [9].
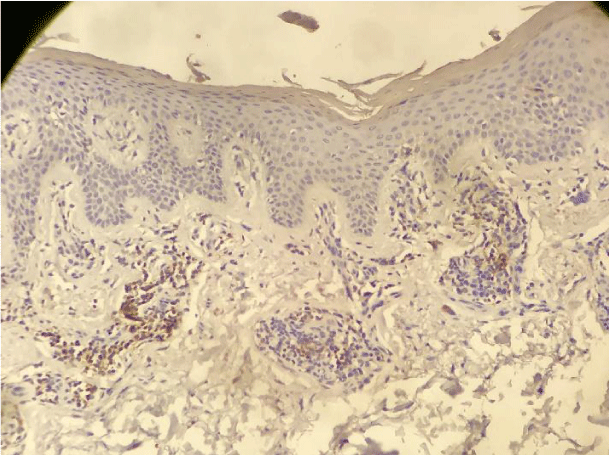
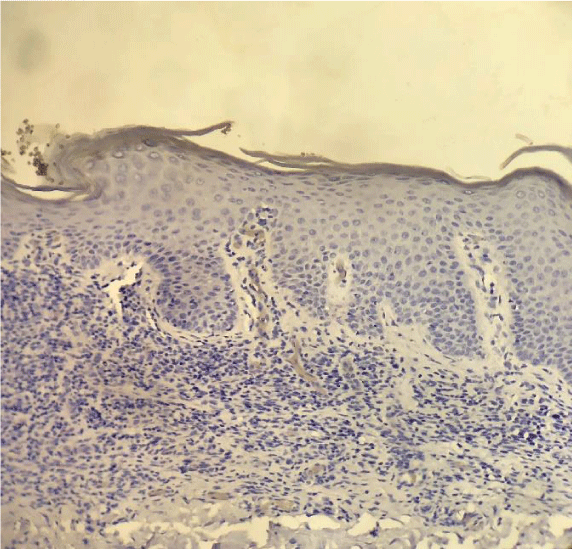
Our findings were similar to the ones published by Cetinozman et al., [10]. Low intensity superficial perivascular lymphoid infiltrate, characteristic of early-stage MF could, at least partly, justify the low PD-1 expression. On the other hand, we identified intense lymphoid infiltration cases, sometimes even with a lichenoid pattern, without PD-1 expression (Figure 5).
Percentages of disease progression, mortality and specific mortality found in our study are slightly higher when compared with other studies, especially when we take into account that those events were found only after 5 years, and not 10 [18-20]. Perhaps those findings are influenced by a majority of patients in our sample with plaques and stage IB.
There is growing interest in identifying relevant factors contributing to better prognostic analysis, besides the current staging model [21-25].
A case report published by Ogurinade et al (2014) caught our attention [11]. They had shown an MF patient who had a quick and aggressive disease, progressing from early-stage MF to tumor lesions, despite adequate management. Intense PD-1 positivity was documented in immunohistochemistry examinations. Also, they had hypothesized that PD-1 expression were related to rapidly progressive MF since cellular immunovigilance against tumor cells tends to diminish when it is activated with PD-L1 and PD-L2 ligands [11].
Another previous study by Nguyen et al. (2017), found 3 patients in the PD-1 positive with a longer follow-up and, apparently, they presented early progression to advanced stages of the disease. Therefore, they suggested that PD- 1 expression might have prognostic importance when analyzing MF patients [9].
We could not find a statistically significant association between PD-1 expression and the events studied. Therefore, in our sample, PD-1 could not be defended as a poor prognostic factor.
Important limitations of the present study are: retrospective nature, small sample, and relatively low incidence of events. With a quite a small sample size and only a relatively few events of progression and death, it is unlikely to find meaningful associations.
Conclusions
Based on the present results, PD-1 positivity in early-stage MF was low; therefore, such positivity could not be correlated to the studied events, which justified the statement that such marker didn’t have prognostic importance. The literature on the subject is still scarce and, as discussed, controversial. Conclusively, it is pertinent to state it that the retrospective character of the study and its small sample are limitations of our research. New studies are needed to get deeper insights on the subject.
- Francisco LM, Sage PT, Sharpe AH (2010) The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 236: 219-242. Link: https://bit.ly/2GlesP0
- Fife BT, Pauken KE (2011) The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci 1217: 45-59. Link: https://bit.ly/32D7UoH
- Wilcox RA (2017) Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am J Hematol 92: 1085–1102. Link: https://bit.ly/2M72AUq
- Pulitzer M (2017) Cutaneous T-cell Lymphoma. Clin Lab Med 37: 527-546. Link: https://bit.ly/2Y2zdoE
- Amorim GM, Niemeyer-Corbellini JP, Quintella DC, Cuzzi T, Ramos-e-Silva M (2018) Clinical and epidemiological profile of patients with early stage mycosis fungoides. An Bras Dermatol 93: 546-552. Link: https://bit.ly/2YcsUTK
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, et al. (2005) WHO-EORT classification for cutaneous lymphomas. Blood 105: 3768-3785. Link: https://bit.ly/2XRzwHH
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C (2014) Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome). Part II. Prognosis, management, and future directions. J Am Acad Dermatol 223: e1-e17. Link: https://bit.ly/2O0p9Ng
- Su C, Tang R, Bai HX, Girardi M, Karakousis G, et al. (2018) Disease site as a prognostic fator for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. Link: https://bit.ly/2Z0QKiu
- Nguyen GH, Olson LC, Magro CM (2017) Upregulation of inhibitory signaling receptor programmed death marker-1 (PD-1) in disease evolution from cutaneous lymphoid dyscrasias to mycosis fungoides and Sezary’s syndrome. Ann Diagn Pathol 28: 54-59. Link: https://bit.ly/2YfZcND
- Cetinozman F, Jansen PM, Vermeer MH, Willemze R (2012) Differential expression of programmed death-1 (PD-1) in Sézary syndrome and mycosis fungoides. Arch Dermatol 148: 1379-1385. Link: https://bit.ly/2XZNId9
- Ogurinade O, Ahn CS, Gergis U, Yassin AH, Magro C (2014) Cutaneous lymphocyte antigen expression loss and PD1 positivity in early cutaneous lesions of rapidly progressive mycosis fungoides. Clin Case Rep 2: 209-218. Link: https://bit.ly/2Z0FzWT
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim, Y, et al. (2007) Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the ICSL and the EORTC. Blood 110: 1713-1722. Link: https://bit.ly/2O2TSJz
- Pimpinelli N, Olsen EA, Santucci M, Vonderheid E, Haeffner AC, et al. (2005) Defining early mycosis fungoides. J Am Acad Dermatol 53: 1053-1063. Link: https://bit.ly/2Z2bpCG
- Samimi S, Benoit B, Evans K, Wherry EJ, Showe L, et al. (2010) Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma:implications for immune suppression. Arch Dermatol 146: 1382–1388. Link: https://bit.ly/2LqNdXL
- Wada DA, Wilcox RA, Harrington SM, Kwon ED, Ansell SM, Comfere NI et al. (2011) Programmed death 1 is expressed in cutaneous infiltrates of mycosis fungoides and Sézary syndrome. Am J Hematol 86: 325-327. Link: https://bit.ly/2GwxIcL
- Kantekura K, Yang Y, Raohunath P, Schaffer A, Woetmann A, et al. (2012) Expression patterns of the immunossupressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell Lymphoma/mycosis fungoides. Am J Dermatopathol 34: 126-128. Link: https://bit.ly/30Ft1EW
- Park JH, Han JH, Kang HY, Lee ES, Kim YC (2014) Expression of follicular helper T-cell markers in primary cutaneous T-cell lymphoma. Am J Dermatopathol 36: 465-470. Link: https://bit.ly/2Yco4FY
- Talpur R, Singh L, Daulat S, Liu P, Seyfer S, et al. (2012) Long-term outcomes of 1.263 patients with mycosis fungoides and Sézary syndrome from 1982 to 2009. Clin Cancer Res 18: 5051-5060. Link: https://bit.ly/32wg6XL
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, et al. (2010) Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organization for Research and Treatment of Cancer staging proposal. J Clin Oncol 28: 4730-4739. Link: https://bit.ly/2So70re
- Desai M, Shuling L, Sareeta P (2015) Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: A single-institution cohort. J Am Acad Dermatol 72: 276-285. Link: https://bit.ly/2LvoRMr
- Wernham AG, Shah F, Amel-Kashipaz R, Cobbold M, Scarisbrick J (2015) Stage I mycosis fungoides: frequent association with a favourable prognosis but disease progression and disease specific mortality may occur. Br J Dermatol 173: 1295-1295. Link: https://bit.ly/2O5Og1f
- Nikolaou V, Papavid E, Patsatsi A, Siakantaris M, Economidi A, et al. (2017) Prognostic indicators for mycosis fungoides in a Greek population. Br J Dermatol 176: 1321-1330. Link: https://bit.ly/2Splnvk
- Scarisbrick JJ, Kim YH, Whittaker SJ, Wood GS, Vermeer MH, et al. (2014) Prognostic factors, prognostic índices and staging in mycosis fungoides and Sézary syndrome: where are we now? Br J Dermatol 170: 1226-1236. Link: https://bit.ly/2LtEjso
- Benton EC, Crichton S, Talpur R, Agar NS, Fields PA, et al. (2013) A cutaneous lymphoma international prognostic index (CLIPi) for mycosis fungoides and Sezary syndrome. Eur J Cancer 49: 2859-2868. Link: https://bit.ly/2XTjvkH
- Scaribrisk JJ, Quaglino P, Prince HM, Papadavid E, Hodak E, et al. (2018) The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. Link: https://bit.ly/2JFYHEH
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
