International Journal of Dermatology and Clinical Research
Nε-(carboxymethyl) lysine represses hair follicle formation by inhibiting Sonic hedgehog expression in a NF-κB-independent manner
Kosuke Tanaka1, Kana Mizuno1, Chika Natsume1, Misaki Takanishi1, Yuki Shimada1, Ryo Saito2, Norihisa Fujita2 and Takashi Fujita1*
2Pharmacoinformatics Lab, Ritsumeikan University, Shiga, Japan
Cite this as
Tanaka K, Mizuno K, Natsume C, Takanishi M, Fujita T, et al. (2019) Nε-(carboxymethyl) lysine represses hair follicle formation by inhibiting Sonic hedgehog expression in a NF-κB-independent manner. Int J Dermatol Clin Res 5(1): 006-011. DOI: 10.17352/2455-8605.000031Nε-(carboxymethyl) lysine (CML), an advanced glycation end product (AGE), is an aging factor produced by glycation of protein. Higher levels of AGE in skin tissue are related to skin elasticity, but how CML that has accumulated in the skin affects hair follicle formation is unclear. This study constructed a simple model that mimics accumulated glycation from feeding by intradermally injecting Nε-(carboxymethyl) lysine (CML), and examined the effects on the morphogenesis of hair follicles (HF). The results showed weakening of the hair shaft and HF formation by CML. The in vitro inhibitory effect of CML on wound healing of dermal papilla cells (DPC) suggested that the mechanism influences the proliferation and migration of DPC, which are essential for HF morphogenesis. In addition, CML in DPC inhibited the expression of sonic hedgehog (Shh), a factor of tissue morphogenesis, in a NF-kB-independent manner. The findings suggest that the delay in HF formation was due to CML inhibiting proliferation and migration in DPC by inhibiting Shh expression.
Introduction
Advanced glycation end-products (AGEs) is a generic term for structures generated in the late stages of nonenzymatic glycosylation reaction between sugars, such as glucose, and proteins [1]. AGEs have been studied as being directly involved in the onset and progression of diabetic complications, diabetic retinopathy, diabetic nephropathy, diabetic neuropathy. Nε- (carboxymethyl) lysine (CML) is an abundant and well characterized non-crosslinking, non-fluorescence AGE that is commonly known as a biomarker of oxidative stress and long-term protein damage [2,3]. Accumulation of CML in the skin facilitates aging phenomena such as wrinkles and sagging [4]. CML stimulates receptor for advanced glycation endproducts (RAGE), an AGE receptor, leading to activation of a hallmark signaling axis; the NF-κB pathway [5].
Major signaling pathways, such as WNT, BMP, and Shh signaling, are involved in HF morphogenesis [6-10]. In mice, HF develops in several successive waves of induction that are dependent on canonical WNT activity and have different requirements with respect to ectodysplasin and BMP signals (10). Edar signaling has been implicated in refining Wnt/β-catenin pattern during primary placode induction, and it also acts as a suppressor of BMP signals, which has an inhibitory effect on placode formation [11]. Eda A1-Edar signaling axis stimulates Sonic hedgehog (Shh) expression in primary hair placodes by cell-to-cell communication [12]. Shh is the most well-known promoter of hair follicle formation, and hair follicle formation has been shown to be impaired in Shh-deficient mouse skin [13,14]. Shh binds to cell membrane receptors coded by two genes, Ptch1 and Ptch2, and information is transmitted to Gli family transcription factors (Gli1, 2, 3) that act downstream [15]. Eda A1 and EdaR are also able to activate NF-κB, suggesting a link to downstream signal cascades including Traf6 [6,10,16]. Activation of the NF-κB pathway therefore leads to Shh induction and HF formation, and activation of the NF-κB pathway downstream of RAGE, which is closely related to diabetes, leads to hair growth. Despite this, hair loss symptoms are often observed in diabetes.
In this study, to easily and quickly determine the effects of CML accumulation in HF morphogenesis, we prepared an in vivo model by intradermally injecting CML, and analyzed in detail the effects on HF morphogenesis. The results revealed that CML weakened the formation of HF, thereby inhibiting hair shaft growth through a possible mechanism of inhibiting Sonic hedgehog (Shh) expression mediated by a receptor for advanced glycation end-products (RAGE) in a nuclear factor (NF)-κB (NF-κB)-independent manner.
Materials and Methods
CML-BSA preparation
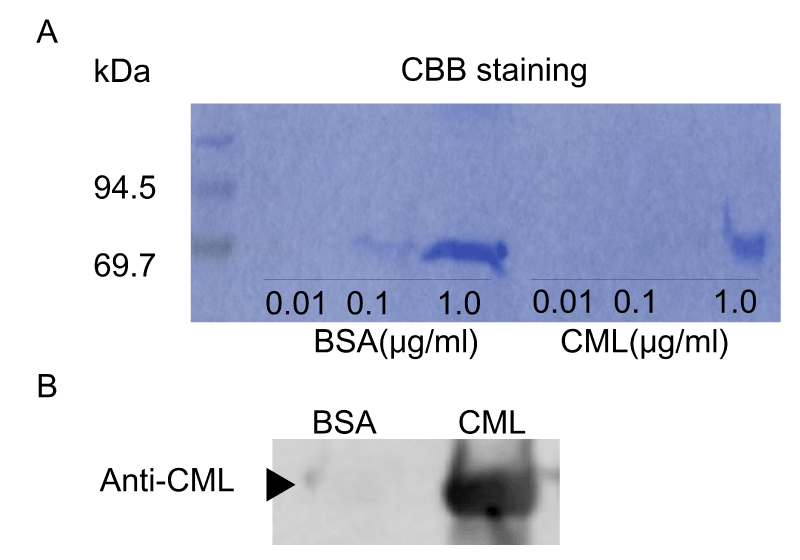
According to Takeuchi’s method, CML-BSA was prepared by incubating 50 mg/ml BSA (Sigma-Aldrich, St Louis, MO) with or without 50 mM glyoxylic acid and 150 mM sodium cyanoborohydride in 0.2 M phosphate buffer (pH 7.4) at 37°C for 24 h, before being subjected to PD-10 column chromatography (GE Healthcare UK, Buckinghamshire, UK) and dialysis using Slide-A-Lyzer™ (Thermo Fisher Scientific Inc., Tokyo, Japan) against PBS to remove low molecular weight reactants [17]. Then, proteins were concentrated by using a filtration device (VIVASPIN 500, 10,000 MWCO PES; Sartorius, Germany). Protein concentrations were determined with BCA protein assay reagent using BSA as a standard. Prepared CML-BSA was detected by Western blot using anti-CML antibody (Abcam Co., Ltd., Tokyo, Japan) (S.1A, B). Unless otherwise indicated, all other reagents were obtained from Wako Pure Chemicals (Osaka, Japan) or Nacalai Tesque (Kyoto, Japan).
Cell cultures
Mouse fibroblastic cells were prepared from E15.5 mouse embryonic skin and cultured in Dulbecco’s Modified Eagle Medium containing 10% fetal bovine serum (FBS) and antibiotics, as described previously [17]. Primary dermal papilla cells (DPCs) were prepared from the whiskers of 8-week-old female C57BL6N mice. Briefly, DPCs were surgically isolated as hair bulb tissues from excised whiskers under the microscope, and were then cultured in Papilla Cell Growth Medium (Toyobo Co., Ltd., Osaka, Japan). DPCs were cultured for up to three passages (within 1 month), and were then plated onto 24-well plates. After reaching confluency, DPC monolayers were scratched across the center of the wells with yellow tips. Twenty-four hours after scratching, the degree of wound recovery was evaluated in images captured using EVOS fl. For cell proliferation/cytotoxicity assay, Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan) was used. Unless otherwise indicated, all other reagents were obtained from Wako Pure Chemicals (Osaka, Japan) or Nacalai Tesque.
Forced hair depilation assay
First, hairs were forcibly removed from the backs of female BL6 mice (8 to 12 weeks old, n = 6) using silicon resin (Cemedine Co., Ltd., Tokyo, Japan). The next day, 200 μl of BSA (1 mg/ml) or CML-BSA (1 mg/ml) was intradermally injected into the depilated dorsal skin in 10 spots to the left (BSA) and right (CML) of the spinal column using 27G×1/2” injection needles (NIPRO, Osaka, Japan) with India ink for marking the injection site. Mice were euthanized 2 days later, and the dorsal skin, after the skin muscle layer had been removed, was used as a sample specimen and for quantitative real-time PCR (qPCR). All animals were obtained from a commercial vendor (SLC Japan, Shizuoka, Japan) and housed in a semi-barrier animal room (light/dark cycle 12:12 h, free-feeding). The protocol used meets the guidelines of the Japanese Society for Pharmacology and was approved by the Committee for Ethical Use of Experimental Animals at Ritsumeikan University.
Histological analysis
Mouse dorsal skin tissue samples were fixed in 10% formalin neutral buffer solution for 24 h, and were then embedded in paraffin. A series of specimens (5 μm in thickness) were prepared and subjected to hematoxylin & eosin (HE) staining or immunohistochemical (IHC) studies, as described previously [18]. For F-IHC, the following antibodies were used: mouse anti-Sonic Hedgehog antibody (Abcam Co., Ltd., Tokyo, Japan), and Alexa Fluor 488 goat anti-mouse IgG (Life Technologies) as the secondary antibody with Fluore-KEEPER with 4’,6-diamidino-2-phenylindole (DAPI). Hair loss activity was analyzed using Epilat hair removal tape (Kracie, Tokyo, Japan), and visualized using a Leica stereoscopic microscope system (MZ10F/DFC7000T; Leica Microsystems, Wetzlar, Germany). Hair cuticles were analyzed using an electron microscope (Keyence VE-8800; Keyence Co., Osaka, Japan). For the detection of apoptosis, an In situ Apoptosis Detection Kit (Takara Bio Inc., Shiga, Japan) was used.
Adenovirus transfer
Bicistronic adenoviruses expressing EGFP, dominant-negative-RAGE (DN-RAGE)-IRES-EGFP, or I-κB-SR was used as described previously [19]. The primary culture of E15.5 embryonic skin cells was infected with the EGFP-expressing adenoviruses at a multiplicity of infection (MOI) of 50 for 24 h. Expression levels of the introduced genes were monitored by qPCR or reporter assay.
qPCR
One microgram of total RNA was reverse-transcribed by ReverTra Ace® cDNA synthesis kit (TOYOBO, Osaka, Japan). Quantitative Real-time PCR (qPCR) was performed as described previously [20]. PCR products were normalized against housekeeping genes, and measurements between samples were compared by cycling threshold (Ct). Primer sequence used were as follows:
mGAPDH-F 5’-TGCACCACCAACTGCTTAG-3’
mGAPDH-R 5’-GGATGCAGGGATGATGTTC-3’
Shh-F 5’-GGAAAACACGGGAGCAGACC-3’
Shh-R 5’-CCACGGAGTTCTCTGCTTTC-3’
Gli-1-F 5’-GCTGTCGGAAGTCCTATT-3’
Gli-1-R 5’-ACTGGCATTGCTAAAGG-3’
Gli-2-F 5’-CTGACCCGCAACGCCTACT-3’
Gli-2-R 5’-CCGAATGCCGTCATCCAAG-3’
Gli-3-F 5’-AACCCTATTCTACCCTCCAAA-3’
Gli-3-R 5’-GCTGATAGTGCTGGTATTGCT-3’
Ptch-1-F 5’-AAAGAACTGCGGCAAGTTTTTG-3’
Ptch-1-R 5’-CTTCTCCTATCTTCTGACGGGT-3’
Ptch-2-F 5’-GGTCCTCCGCACCTCATATC-3’
Ptch2-R 5’-GCGCAGTCTGAATCAACATC-3’
The non-regulated housekeeping gene served as an internal control and was used to normalize for differences in RNA input. All measurements were performed in quadruplicate.
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). Significance was tested using the Student’s t-test or, where multiple comparisons were required, one-way analysis of variance (ANOVA). A P-value of less than 0.05 was considered to be significant.
Results
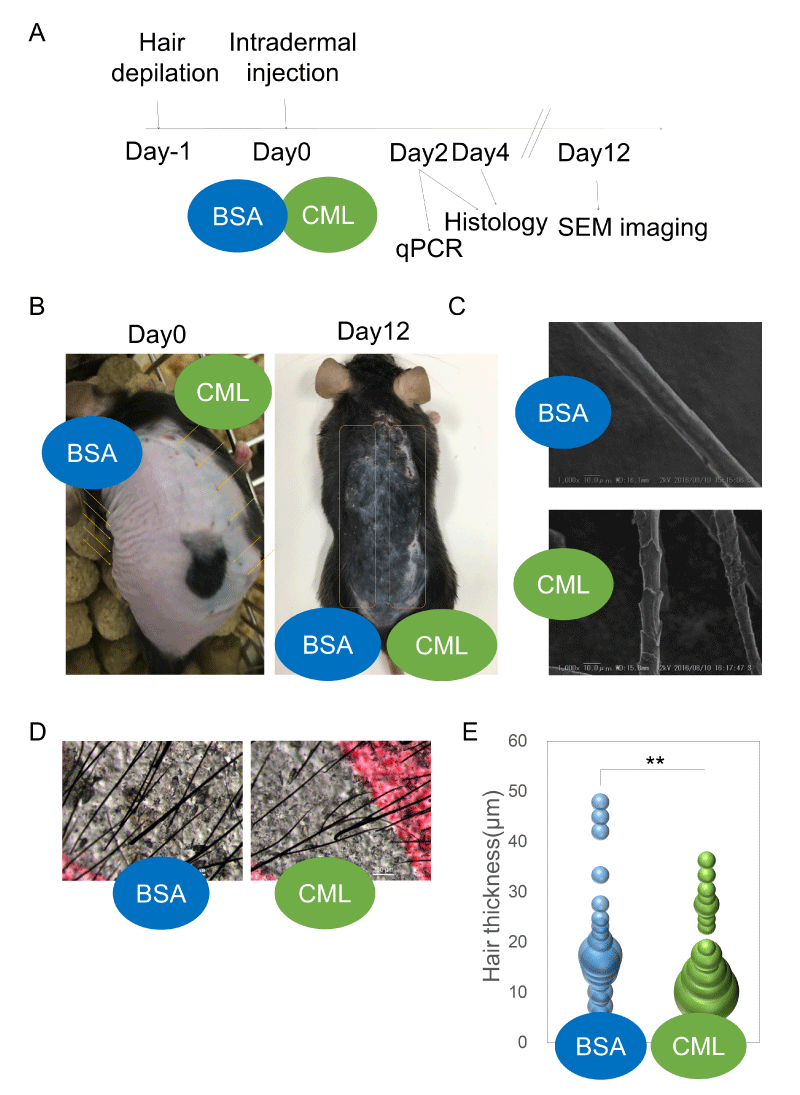
Effects of CML injection on hair shaft growth and HF formation First, an intradermal injection model of locally accumulated CML was created to examine the presence or absence of hair growth defects (Figure 1A). After forcibly removing hairs and synchronizing the hair cycle to the telogen phase [21], synthetic CML-BSA (Figure S1) was intradermally injected. Twelve days after intradermal injection, hair growth defects were observed growing concentrically around the injection site, showing the effects of CML (Figure 1B). Upon observation of the hair shafts growing in the area of hair growth defects, there was morphological weakening (Figure 1C,D) and clear thinning of the shaft diameter (Figure 1E).
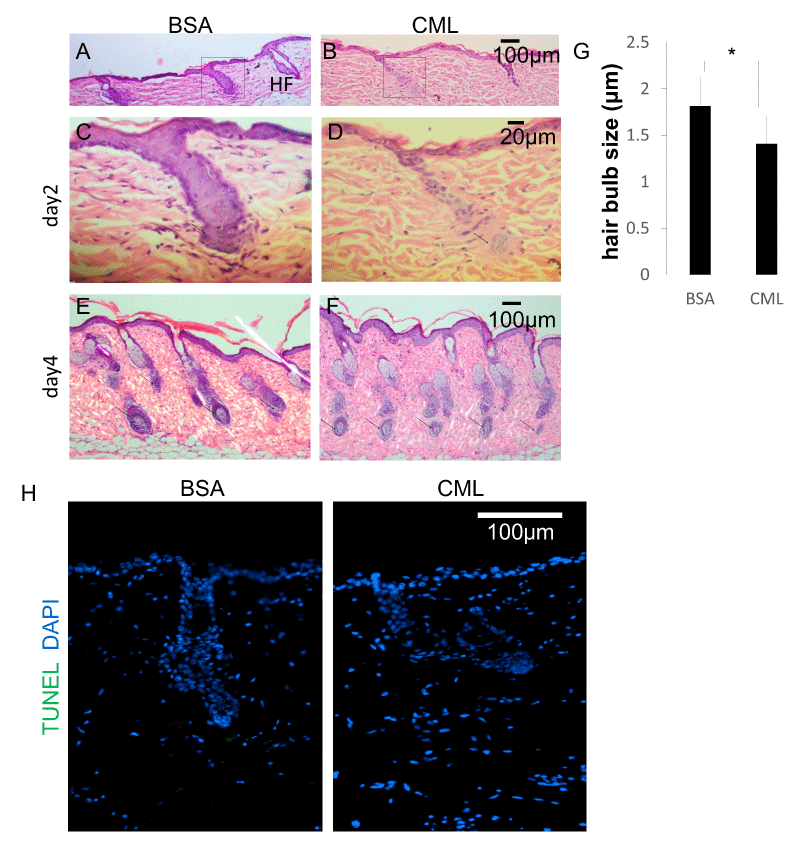
Next, the effects on HF morphology were examined. Studies have shown a close relationship between hair shaft diameter and HF diameter [22,23]. The degree of follicular regeneration during the anagen phase is known to influence follicle size. A time-course histological analysis was performed to investigate the causes of hair growth defects and morphological weakening of the hair shaft. After forcibly removing hairs and synchronizing the hair cycle to the telogen phase, skin tissue samples were collected on the second day and fourth day after intradermal injections of CML-BSA, and the changes in HF structure were observed after hematoxylin-eosin (HE) staining. On the second day after hairs were forcibly removed, defects in HF down-growth were observed at the site of CML-BSA injection (Figure 2A-D). On the fourth day after hairs were forcibly removed, a period of increased down-growth and aggregation at the hair bulb, the morphology of the hair bulb was weakened (Figure 2E,F) and miniaturization of the hair bulb diameter was observed (Figure 2G). This is thought to be an effect caused by delayed hair follicle formation. Meanwhile, TdT-mediated dUTP nick-end labeling (TUNEL) staining revealed that follicular miniaturization was not affected by apoptosis (Fig. 2H), suggesting that hair follicle growth is inhibited by the delay in hair follicle formation caused by CML.
Shh lowering effect of CML in RAGE-mediated NF-kB-independent pathway
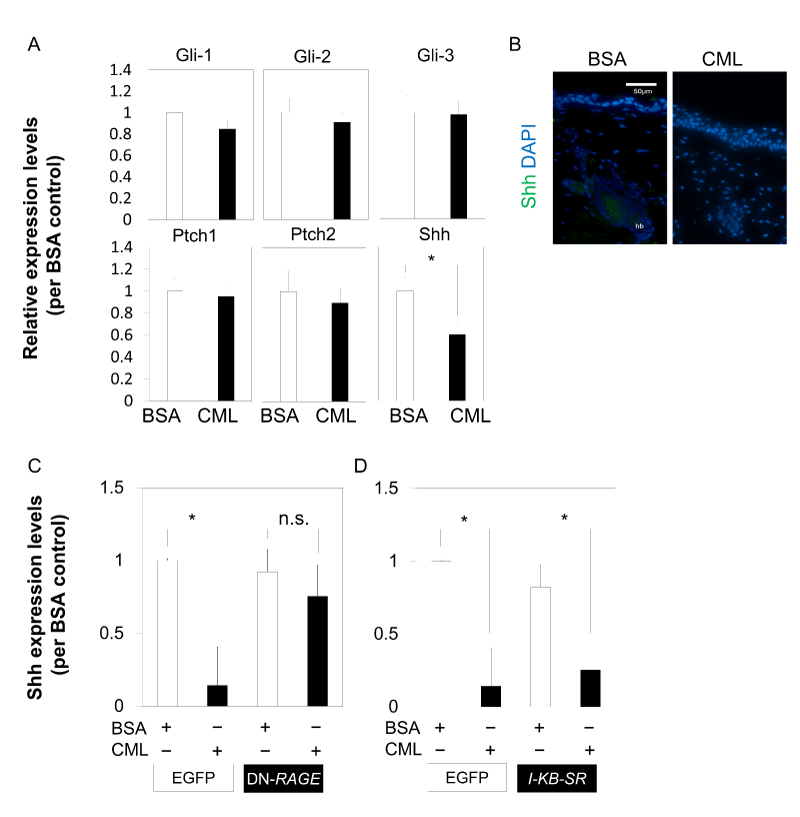
To determine the cause of inhibition of cell aggregation in the bulb region, the effect of CML-BSA injection on Shh was examined. In Shh-knockout mice, which are known for mesenchymal cell-specific expression during hair germ formation at the embryonic stage, placode formation of primordial HF was observed, but further development was inhibited. This demonstrated that Shh is essential for hair germ cell proliferation [24]. Skin tissue collected on the second day after hair loss was analyzed using qPCR, in an attempt to examine the effects on Shh to unravel the mechanism of CML affecting HF morphogenesis, which is continuously regenerated from the time of birth. The results showed that when CML-BSA was injected, Shh expression was significantly inhibited in the skin tissue collected during down-growth in the early anagen phase. On the other hand, inhibition of gene expression was not observed in Ptch1, Ptch2, Gli1, Gli2, and Gli3 (Figure 3A). In addition, there was no Shh expression in the CML-BSA injected immunostained tissue collected from the bulb region on the second day after hair loss (Figure 3B).
The direct effect of CML-BSA on Shh was then examined using Shh from a primary culture of embryonic skin-derived mixed epithelial-mesenchymal cells at E15.5, a stage corresponding to early HF formation. The results revealed that the addition of CML-BSA repressed Shh expression of E15.5 embryonic skin tissue-derived cells, and the inhibited expression was derepressed by dominant negative (DN)-RAGE, suggesting that CML-BSA inhibition of Shh expression is mediated by RAGE (Figure 3C). It is known that there is crosstalk between RAGE and Toll-like receptor (TLR) [25] and studies have shown that Shh expression is modulated through NF-kB [26]. Next, we investigated whether inhibition of NF-kB in IκB-SR stained cells derepressed the expression of Shh that had been repressed by the addition of CML-BSA. However, the expression was not derepressed (Figure 3D). These findings suggested that Shh inhibition by CML-BSA occurs through a NF-kB-independent pathway mediated by RAGE.
Direct inhibitory effect of CML-BSA on DPC
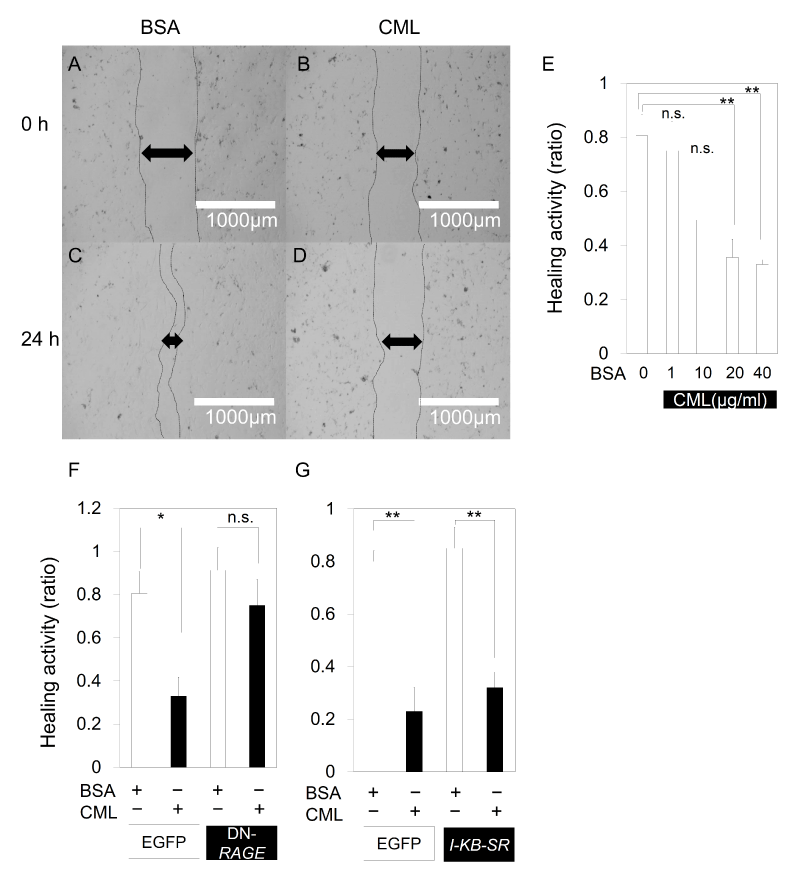
As there seems to be a close correlation between the number of DPC and the diameter of the hair shaft, the direct effect of CML on DPC was examined. The results of a scratch assay using primary cultured mouse DPC suggested that there is a direct inhibitory effect of CML-BSA on the migration and proliferation of DPC as the recovery of DPC from scratch decreased in a concentration-dependent manner (Figure 4A-E) with the addition of CML-BSA. Similarly, when scratch assay was performed on a primary culture of E15.5 mouse embryonic skin cells, recovery from scratch decreased after addition of CML-BSA. However, the declined recovery from scratch was derepressed with DN-RAGE (Figure 4F), demonstrating that the migratory and proliferative inhibition of CML-BSA of mesenchymal-like cells is mediated by RAGE. On the other hand, when NF-kB was repressed, the inhibited recovery from scratch was not derepressed (Figure 4G). CML-BSA may reduce the migration and proliferation of mesenchymal cells in a RAGE-mediated NF-kB-independent manner.
Discussion
Studies of forced expression of Dickkopf1 in the epidermis of transgenic mice have shown that Wnt signaling lies upstream and Shh, BMP4, Notch, FDF and the TNF family are downstream [27,28], in HF morphogenesis in the developmental process. These studies have demonstrated the important role of Shh signaling for complete HF formation after primordial HF formation. In the developmental process, the expression of Shh and Ptched receptors take place respectively in mesenchymal cells and primordial HF. As HF formation beyond the primordial HF is inhibited in Shh knock-out mouse, Shh must promote the proliferation of primordial HF in epithelial cells, serving an important function in HF formation beyond primordial HF [24]. The results of the present study revealed that CML injections caused thinning of the hair shaft and weakening of the HF, triggered by a decline in cell aggregation. Decreased Shh expression by CML triggered decreased proliferation of a group of follicular epithelial cells such as hair matrix cells involved in hair shaft formation and outer root sheath involved in follicular down-growth. In turn, this may have triggered HF weakening and thinning of the hair shaft by CML. On the other hand, when scratch tests were performed on DPC and primary cultures of E15.5 embryonic skin cells, a decrease in cell proliferation and migration was observed in mesenchymal cells in a RAGE-mediated NF-kB-independent manner. As described above, CML may be inhibiting HF morphogenesis by inhibiting the proliferation of epithelial and mesenchymal cells.
The mechanism for hair root regeneration may be different from that of the formation of HF in the embryonic stage and the regeneration cycle after birth. However, when fibroblasts, which are mesenchymal cells in the embryonic stage, begin to form dermal cell aggregates, genes different from the surrounding fibroblasts, Noggin and BMP4, are known to be expressed [29]. BMP4, in particular, induces epidermal cells to repress Shh expression. However, Shh is expressed in placode epidermal cells in contact with the dermal aggregates, as Noggin inhibits BMP4 expression [30]. In epidermal cells around the placode where Noggin from dermal aggregates is out of reach, Shh expression is inhibited by BMP4, and a cascade of organ-forming and organ-inhibiting reaction diffusion waves form regular HF on the skin [31]. In this study, CML directly repressed the proliferation and migration in DPC and embryonic skin cells in the scratch assay, and in vivo, CML injection repressed aggregation in the hair root region. Given that part of the HF expression process in the embryonic stage is known to be repeated after birth, we plan to examine which factors, such as Noggin, BMP4 and others that act in the developmental process, have an effect via decreased NF-kB-independent expression of Shh.
Although glycation typically accumulates over a long period of feeding, in this study, we proposed a simple model that mimics aging through daily living by easily and quickly reconstructing an intradermal injection model. In this model, it is possible to investigate the effect of anti-glycation compounds, and it will be possible to develop compounds that bring about a therapeutic effect to patients treated with aging or anti-diabetic drugs containing the phenotype of hair loss. Hair generated from weak HF was readily prone to hair loss, and data to support the signs of aging were obtained.
At the same time, the mutual interaction between epithelial and mesenchymal cells, which are involved in HF morphogenesis, is a common system [32-34], observed in many organs of the human body such as the kidneys, teeth, tear glands and salivary glands, and we believe that this is a very good model for studying organ morphogenesis. Use of the model shown in this study may lead to a detailed understanding of hair follicle formation and enable verification of the organ morphogenesis mechanisms that are affected by glycation factors.
IκB-SR was kindly provided by Dr. Jun-ichiro Ionue (Tokyo University, Japan).
- Gkogkolou P, Böhm M (2012) Advanced glycation end products: Key players in skin aging? Dermatoendocrinol 4: 259-270. Link: https://goo.gl/ZUtcfN
- Yamamoto M, Yamaguchi T, Yamauchi M, Sugimoto T (2009) Low serum level of the endogenous secretory receptor for advanced glycation end products (esRAGE) is a risk factor for prevalent vertebral fractures independent of bone mineral density in patients with type 2 diabetes. Diabetes Care 32: 2263-2268. Link: https://goo.gl/YTWRem
- Oren TW, Botolin S, Williams A, Bucknell A, King KB (2011) Arthroplasty in veterans: analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. J Rehabil Res Dev 48: 1195-1210. Link: https://goo.gl/ZBwt6g
- Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC (2012) Skin anti-aging strategies. Dermatoendocrinol 4: 308–319. Link: https://goo.gl/BiYMTi
- Haslbeck KM, Schleicher E, Bierhaus A, Nawroth P, Haslbeck M, et al. (2005) The AGE/RAGE/NF-(kappa)B pathway may contribute to the pathogenesis of polyneuropathy in impaired glucose tolerance (IGT). Exp. Clin. Endocrinol. Diabetes 113: 288-291. Link: https://goo.gl/GsJtWy
- Headon DJ, Overbeek PA (1999) Involvement of a novel Tnf receptor homologue in hair follicle induction. Nat. Genet 22: 370-374. Link: https://goo.gl/tkv5F8
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, et al. (1999) Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat. Cell Biol 1: 158-164. Link: https://goo.gl/4PThbs
- DasGupta R, Fuchs E (1999) Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557-4568. Link: https://goo.gl/AXC7wd
- Andl T, Reddy ST, Gaddapara T, Millar SE (2002) WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643-653. Link: https://goo.gl/C8Dv1M
- Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, et al. (2002) Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development 129: 2541-2553. Link: https://goo.gl/wvicwc
- Pummila M, Fliniaux I, Jaatinen R, James MJ, Laurikkala J, et al. (2007) Ectodysplasin has a dual role in ectodermal organogenesis: inhibition of Bmp activity and induction of Shh expression. Development 134: 117-125. Link: https://goo.gl/1uETRK
- Aggarwal BB (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol 3: 745-756. Link: https://goo.gl/TvTyhC
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, et al. (1998) Sonic Hedgehog signaling is essential for hair development. Curr Biol 8: 1058-1068. Link: https://goo.gl/dJVQ6d
- Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, et al. (1999) Essential role for Sonic Hedgehog during hair follicle morphogenesis. Dev Biol 205: 1-9. Link: https://goo.gl/vR2BWC
- Callahan CA, Oro AE (2001) Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic Hedgehog signaling. Current Opinion in Genetics & Development 11: 541-546. Link: https://goo.gl/P8NAfB
- Schmidt-Ullrich R, Tobin DJ, Lenhard D, Schneider P, Paus R, et al. (2006) NF-kappaB transmits Eda A1/EdaR signalling to activate Shh and cyclin D1 expression, and controls post-initiation hair placode down growth. Development 133: 1045-1057. Link: https://goo.gl/BkX3zc
- Takeuchi M, Takino J, Furuno S, Shirai H, Kawakami M, et al. (2015) Assessment of the Concentrations of Various Advanced Glycation End-Products in Beverages and Foods That Are Commonly Consumed in Japan. PLoS One 10: e0118652. Link: https://goo.gl/WxsV5V
- Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, et al. (2004) Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18: 952–963. Link: https://goo.gl/CYPpjd
- Kosaka T, Fukui R, Matsui M, Kurosaka Y, Nishimura H, Tanabe M, et al. (2014) RAGE, receptor of advanced glycation endoproducts, negatively regulates chondrocytes differentiation. PLoS One 9: e108819. Link: https://goo.gl/m2Fhn5
- Matsui M, Tanaka K, Higashiguchi N, Okawa H, Yamada Y, et al. (2016) Protective and therapeutic effects of fucoxanthin against sunburn caused by UV irradiation. J. Pharmacol. Sci 132: 55-64. Link: https://goo.gl/VpBbSx
- Hsu YC, Pasolli HA, Fuchs E (2011) Dynamics between Stem Cells, Niche, and Progeny in the Hair Follicle. Cell 144: 92-105. Link: https://goo.gl/1mRvuU
- Jahoda, CA (1992) Induction of follicle formation and hair growth by vibrissa pappilae implanted into rat ear wounds; vibrissa-type fibers are specified. Development 115: 1103-1109. Link: https://goo.gl/48p3Em
- Osada A, Kobayashi K, Masui S, Hamazaki TS, Yasuda K, et al. (2009) Cloned cells from the murine dermal papilla have hair-inducing ability. J Dermatol Sci 54: 129-131. Link: https://goo.gl/gcYrbq
- Chiang C, Swan RZ, Grachtchouk M, Bolinger M, Litingtung Y, et al. (1999) Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol 205: 1-9. Link: https://goo.gl/u37v3Z
- Lin L (2006) RAGE on the Toll Road? Cell Mol Immunol 3: 351–358. Link: https://goo.gl/H9e4ds
- Nakashima H, Nakamura M, Yamaguchi H, Yamanaka N, Akiyoshi T, et al. (2006) Nuclear factor-kappaB contributes to hedgehog signaling pathway activation through sonic hedgehog induction in pancreatic cancer. Cancer Res 66: 7041-7049. Link: https://goo.gl/puqfJj
- Andl T, Reddy ST, Gaddapara T, Millar SE (2002) WNT signals are required for the initiation of hair follicle development. Dev Cell 2: 643-653. Link: https://goo.gl/wJT79B
- Chan EF, Gat U, McNiff JM, Fuchs E (1999) A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 21: 410-413. Link: https://goo.gl/VxKV9D
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, et al. (1999) Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol 1: 158-164. Link: https://goo.gl/B7egmb
- Chuong CM, Noveen A (1999) Phenotypic Determination of Epithelial Appendages: Genes, Developmental Pathways, and Evolution. J Invest Dermatol Symp Proc 4: 307-311. Link: https://goo.gl/5rj52D
- Sick S, Reinker S, Timmer J, Schlake T (2006) WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314: 1447-1450. Link: https://goo.gl/y7dZXV
- Fuchs E (2007) Scratching the surface of skin development. Nature 445: 834-842. Link: https://goo.gl/nfxKA6
- Hardy MH (1992) The secret life of the hair follicle. Trends Genet 8: 55-61. Link: https://goo.gl/4NTexK
- Toyoshima KE, Asakawa K, Ishibashi N, Toki H, Ogawa M, et al. (2012) Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nat Commun 3: 784. Link: https://goo.gl/wkpCov
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
