International Journal of Dermatology and Clinical Research
A rare disease more common than perceived: Two case studies and brief review of IgA Vasculitis
Lydia Shedlofsky DO1* and Chelsea Crist2
2Southeastern Regional Medical Center, USA
Cite this as
Lydia Shedlofsky DO, Crist C (2019) A rare disease more common than perceived: Two case studies and brief review of IgA Vasculitis. Int J Dermatol Clin Res 5(1): 003-005. DOI: 10.17352/2455-8605.000030Immunoglobulin A (IgA) Vasculitis, more commonly known as Henoch-Schönlein Purpura (HSP), is a disorder which causes inflammation and bleeding in the small blood vessels of the skin, joints, intestines, and kidneys. We report 2 cases of IgA vasculitis found in a rural emergency department: 1) HSP in an 8-year-old male who was initially misdiagnosed with insect bites 2) HSP in an adult male patient. We present both cases and a literature review, indicating that low incidence may be secondary to under diagnosis, arguing for a need of further education on the subject.
Abbreviations
IgA: Immunoglobulin A; HSP: Henoch-Schönlein Purpura; EULAR/PReS: European League Against Rheumatism/Paediatric Rheumatology European Society
Introduction
Henoch-Schönlein Purpura (HSP) is a systemic vasculitis of unknown etiology, caused by IgA deposition in vessels. Patients of any age can be affected, but the mean age of diagnosis is 6.7 ± 2.41 years [1]. Cutaneous lesions are the most common presenting symptoms, comprised of elevated non-blanching palpable purpura typically on the lower extremities [2,3]. The classic triad for clinical diagnosis is the above rash, in addition to abdominal pain or renal involvement, and arthritis [4-6] and this is reflected in diagnostic criteria seen in table 1.
Criteria vary somewhat due to increased research into the subject, including the development that leukocytosis with IgA immune deposition in small vessels contributes to the etiology of HSP [10].
This is considered an extremely rare condition, with reported incidence estimated to be 15 cases/100,000 people per year in children, and one tenth of this incidence in adults [11]. The adult patients have a more severe condition requiring more aggressive therapy, though HSP is usually non-fatal and often self-limiting [12].
Due to this rarity, and typically benign course, HSP is often not taught in depth in medical schools, where few students are offered a dermatology course [13]. This persists into residency, where educational focus is on most commonly seen conditions [14]. Excluding dermatitis, internal medicine physicians spend less than 0.03% of their time on dermatologic diagnoses. This indicates that each internist could go their entire careers without seeing a case of HSP [15,16]. For the past 80 years medical interns have been berated with the now infamous adage “When you hear hoofbeats look for horses, not zebras [17].” Of course, this forces physicians to develop an internal bias where IgA Vasculitis is simply not included in differentials.
We argue that it is equally possible that the true incidence is higher than published data, due to under-reporting. In fact, there are multiple published cases of misdiagnosed HSP [18-22]. leading to significant morbidity for patients. This frequency of misdiagnosis contributes to the perception that HSP is so rare it can never be seen, in turn causing more misdiagnoses, lower incidence, and less teaching on the subject matter.
In one month at a rural emergency department two cases of HSP were initially misdiagnosed, supporting this theory.Clinical Case Reports
Case 1
An 8-year-old male was brought to the emergency department by his mother complaining of a non-pruritic eruption to his lower extremities. Physical examination revealed scattered purplish macules and papules with diffuse petechiae covering his ankles and distal lower extremities. There were no recent illnesses, or changes in any medications. Diascopy revealed non-blanching lesions. The condition was originally misdiagnosed as secondary to insect bites. However, further history taking revealed that the patient also had bilateral knee pain and abdominal pain made worse with food. Blood work and urinalysis where all within normal limits, including ASO titer. Clinical diagnosis was made based on classic triad, in addition to meeting both the criterion developed by Michel et al., and EULAR/PReS criterion.
Case 2
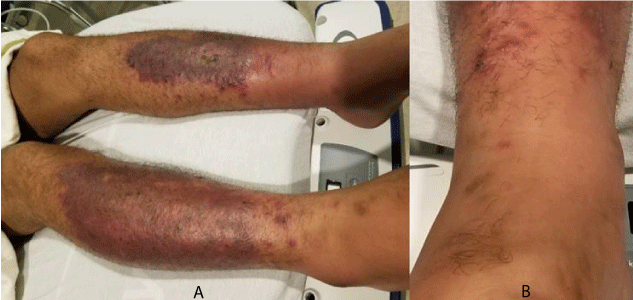
A 30-year-old male truck driver with well-controlled diabetes presented to the emergency department complaining of a worsening “rash”. He reported that he had first noticed discoloration to his bilateral shins several weeks ago, but earlier in the day he saw other “spots” appear as seen in figure 1. He also admitted to fatigue.
Clinical examination revealed bilateral 1+ pitting edema up to his knees, in addition to skin findings as seen above. Anterior tibias reveal violaceous patches with superficial erosions, brown hyperpigmented patches, and scattered erythematous macules. Initial blood work revealed white blood cell count of 15, normal platelet count, and CRP of 2.3. The differential was focused on venous stasis with overlying cellulitis. However, diascopy was performed revealing that the scattered macules were non-blanching, and palpable. Additional blood work revealed an ESR of 37 and IgA of 440. Urinalysis returned showing 3+ blood, and 3+ protein. Patient was admitted to the hospital, and subsequent skin biopsy revealed leukocytoclastic vasculitis.
Discussion
IgA vasculitis is one of the most common type of vasculitis found in children [23], but education on the subject during training focuses on the clinical trial, and the absolute rarity of the condition. Taught that the low incidence precludes making this diagnosis, it rarely enters possible differentials. However, in a single month at a rural emergency department two cases were discovered, and nearly misdiagnosed.
Case one was a textbook example of HSP, a young male with purpura, abdominal and joint pain presenting to the emergency department. However, it was nearly misdiagnosed because of the mother’s over-riding concerns about “bed bugs”. The adult in case two has a much more difficult presentation. He is one of approximately 115,000 cases worldwide each year [11], with long-standing lower extremity discoloration and co-morbidities. Differential was focused on venous stasis with overlying cellulitis, but fortunately diascopy was performed before empiric antibiotics were begun.
Additionally, the multitude of published cases of misdiagnosed IgA vasculitis, in addition to this subjective experience, indicates that there must be additional cases worldwide that are not discovered. Misdiagnosis of IgA vasculitis leads to significant morbidity and even possible mortality for patients [24-26].
Adult patients have much more severe renal histopathological changes compared to pediatric patients [27]. One in ten adults who has biopsy proven HSP die due to the disease course [28]. This is also considered a chronic disease of the mesangium [29], so of those adults who survive, many will continue to have renal abnormalities [30]. Only corticosteroid administration during the acute stage of the illness will prevent morbidity and mortality [31], which requires accurate and rapid diagnosis.
Overall, the misdiagnosis also contributes to under-reporting, indicating that the probable incidence is higher than published data indicates. This frequency of misdiagnosis contributes to the perception that HSP is so rare it can never be seen, in turn causing more misdiagnoses, lower incidence, and less teaching on the subject matter. We strongly recommend continuing medical education on the subject, but the ideal solution is additional education while still in training, either in medical school, or in residency.
With gratitude to Dr. Ahmad Jadaan, MD, FACP, FACC for his help and support in this endeavor.
- Zhang Q, Guo Q, Gui M, Ren Z, Hu B, et al. (2018) Henoch-Schonlein purpura with acute pancreatitis: analysis of 13 cases. BMC Pediatr 18: 159. Link: https://goo.gl/fFH8Ch
- Chen O, Zhu XB, Ren P, Wang YB, Sun RP, et al. (2013) Henoch Schonlein Purpura in children: clinical analysis of 120 cases. Afr Health Sci 13: 94-99. Link: https://goo.gl/ooLB8G
- Wang X, Zhu Y, Gao L, Wei S, Zhen Y, et al. (2016) Henoch-Schonlein purpura with joint involvement: Analysis of 71 cases. Pediatr Rheumatol Online J 14: 20. Link: https://goo.gl/53aLP5
- Kraft DM, Mckee D, Scott C (1998) Henoch-Schonlein purpura: a review. Am Fam Physician 58: 405-408. Link: https://goo.gl/aL26CC
- Saulsbury FT (2007) Clinical Update: Henoch-Schonlein Purpura. Lancet 369: 976-978. Link: https://goo.gl/LBt8GL
- Cojocariu C, Stanciu C, Ancuta C, Danciu M, Chiriac S, et al. (2016) Immunoglobulin A Vasculitis Complicated with Clostridium difficile Infection: a rare case report and brief review of the literature. J Gastrointestin Liver Dis 25: 235-238. Link: https://goo.gl/z571RC
- Michel BA, Hunder GG, Bloch DA, Calabrese LH (1992) Hypersensitivity vasculitis and Henoch-Schonlein purpura: a comparison between the 2 disorders. J Rheumatol 19: 721-728. Link: https://goo.gl/ZG8Ztp
- Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of henoch-schonlein purpura. Arthritis Rheum 33: 1114-1121. Link: https://goo.gl/WuxpFk
- Ozen S, Ruperto N, Dillon MJ, Bagga A, Barron K, et al. (2006). EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis 65: 936-941. Link: https://goo.gl/agkBeL
- Calvo-Río V, Loricera J, Mata C, Martín L, Ortiz-Sanjuán F, et al. (2014). Henoch-Schonlein Purpura in Northern Spain: clinical spectrum of the disease in 417 patients from a single center. Medicine (Baltimore) 93: 106-113. Link: https://goo.gl/jxmPEq
- Kang Y, Park JS, Ha YJ, Kang MI, Park HJ,et al. (2014) Differences in Clinical Manifestations and Outcomes between Adult and Child Patients with Henoch-Schonlein Purpura. J Korean Med Sci 29: 198-203. Link: https://goo.gl/LGnH2k
- Blanco R, Martínez-Taboada VM, Rodríguez-Valverde V, García-Fuentes M, González-Gay MA (1997) Henoch-Schonlein Purpura in adulthood and childhood. Arthritis Rheum 40: 859-864. Link: https://goo.gl/eyyiUq
- Murase JE (2015) Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept 5: 95-96. Link: https://goo.gl/wt9n6i
- Feldman SR, Fleischer AB Jr, McConnell RC (1998) Most Common Dermatologic Problems Identified by Internists, 1990-1994. Arch Intern Med 158: 726-730. Link: https://goo.gl/Wtas4B
- Fleischer AB Jr, Herbert CR, Feldman SR, O'Brien F (2000) Diagnosis of Skin Disease by Nondermatologists. Am J Manag Care 6: 1149-1156. Link: https://goo.gl/fJmFEb
- Steele K (1984) Primary dermatological care in general practice. J R Coll Gen Pract 34: 22-23. Link: https://goo.gl/8ubqUq
- Garson (2017) When You Hear Hoofbeats Look for Horses Not Zebras. Quote Investigator. Retrived Online: Link: https://goo.gl/ea89ut
- Caliskan B, Guven A, Atabek C, Gok F, Demirbag S, et al. (2009) Henoch-Schonlein purpura presenting with symptoms mimicking balanoposthitis. Pediatr Rep 1: e5. Link: https://goo.gl/H6ZHRf
- Lawee D (2008) Atypical Clinical Course of Henoch-Schonlein Purpura. Can Fam Physician 54: 1117-1120. Link: https://goo.gl/ztnkDi
- Rubin J, Moy J (2004) Atypical Presentation of Henoch-Schonlein Purpura. The Journal of Allergy and Clinical Immunology 113: 293. Link: https://goo.gl/3BceXA
- Morrison A, Cohen H, Sperling R, Adler L, Wood S (2016) An Atypical Presentation of Henoch-Schonlein Purpura with Intussusception. Consultant 56: Link: https://goo.gl/qHQYWw
- Carella S, Maruccia M, Fino P, Onesti MG (2013) An Atypical Case of Henoch-Schonlein Purpura in a Young Patient: Treatment of the Skin Lesions with Hyaluronic Acid-based Dressings. In Vivo 27: 147-151. Link: https://goo.gl/xcSSoe
- Palit A, Inamadar AC (2009) Childhood Cutaneous Vasculitis: A Comprehensive Appraisal. Indian J Dermatol 54: 110-117. Link: https://goo.gl/GzMCBZ
- Akbar DH (2000) Fatal Complication of Henoch-Schonlein Purpura: Case Report and Literature Review. Saudi J Gastroenterol 6: 165-168. Link: https://goo.gl/XwawDM
- Hodgekiss A (2012) The Tragic Story of the Nine-Year-Old Boy Who’d Never Been Ill – but died after developing a rash on his leg. Daily Mail. Retrieved Online. Link: https://goo.gl/kgs5uD
- Oshikata C, Tsurikisawa N, Takigawa M, Omori T, Sugano S, et al. (2013) An Adult Patient with Henoch-Schonlein Purpura and Non-Occlusive Mesenteric Ischemia. BMC Res Notes 6: 26. Link: https://goo.gl/Uwe55j
- Liu Z, Wei YD, Hou Y, Xu Y, Li XJ, et al. (2016) Differences in pathological characteristics and laboratory indicators in adult and pediatric patients with Henoch-Schonlein purpura nephrtitis. J Huazhong Univ Sci Technolog Med Sci 36: 659-666. Link: https://goo.gl/C39Rdu
- Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, et al. (2002) Henoch-Schonlein Purpura in Adults: Outcome and Prognostic Factors. J Am Soc Nephrol 13: 1271-1278. Link: https://goo.gl/6KHw2W
- Sinniah R, Feng PH, Chen BT (1978) Henoch-Schoenlein Syndrome: a clinical and morphological study of renal biopsies. Clin Nephrol 9: 219-228 Link: https://goo.gl/xzmfXq
- Faull RJ, Aarons I, Woodroffe AJ, Clarkson AR (1987) Adult Henoch-Schonlein Nephritis. Aust N Z J Med 17: 396-401. Link: https://goo.gl/hEpBhU
- Roth DA, Wilz DR, Theil GB (1985) Schonlein-Henoch Syndrome in Adults. QJM: Int J Med 55: 145-152. Link: https://goo.gl/eKxpN3
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
