Imaging Journal of Clinical and Medical Sciences
Endomyocardial fibrosis of the right ventricle
Diego Sarre-Alvarez1*, Cuitláhuac Arroyo-Rodríguez2, Juan Manuel Chino-Mendoza3, Yhaquelina Admeli Quispe-Villca4, and José Antonio Arias-Godínez2
2Department of Echocardiography, National Institute of Cardiology Ignacio Chavez, Mexico City, Mexico
3Fellow in Cardiovascular Magnetic Resonance and Angiotomography, National Institute of Cardiology Ignacio Chavez, Mexico City, Mexico
4Fellow in Coronary Care Unit, National Institute of Cardiology Ignacio Chavez, Mexico City, Mexico
Cite this as
Alvarez DS, Rodríguez CA, Chino-Mendoza JM, Quispe-Villca YA, Arias-Godínez JA (2019) Endomyocardial fibrosis of the right ventricle. Imaging J Clin Medical Sci 6(1): 094-096. DOI: 10.17352/2455-8702.000128Endomyocardial fibrosis is a rare cause of restrictive cardiomyopathy which predominates in low-income population living in tropical areas. It´s characterized by endomyocardial fibrous tissue deposition causing restrictive physiology with poor prognosis without proper management. We present a 40-year-old woman with right ventricular endomyocardial fibrosis complicated by pericardial effusion and a giant atrial thrombus. The use of multimodal imaging is very important for the diagnosis of this extremely rare pathology in our country.
Introduction
Endomyocardial Fibrosis (EMF) is a disease with unknown etiology characterized by endomyocardial fibrous tissue deposition causing restrictive physiology. It affects the poorest population in tropical areas conferring a poor prognosis without proper management. EMF is poorly recognized and frequently misdiagnosed, remaining a challenging diagnosis in non-endemic areas.
Case Report
A 40-year-old Mexican woman was referred to our hospital for a pre-surgical evaluation for hysterectomy because of cardiomegaly on a chest X-rays. She had a history of dyspnea in the last 25 years which worsened to small efforts dyspnea in the last year. She also had acid peptic disease and iron deficiency anemia secondary to abnormal uterine bleeding.
Physical examination revealed muscular hypotrophy, grade III jugular venous distention, regular heart sounds, the second sound was intense with fixed split; grade I/IV systolic murmur in the mitral position and a grade II/IV systolic murmur in the tricuspid position. Abdominal distension from ascites, + pedal edema. Peripheral pulses were regular and symmetric with adequate amplitude.
Electrocardiogram showed sinus rhythm with right atrial enlargement (qR pattern in V1). Besides iron deficiency anemia, her laboratories were normal. She never presented eosinophilia.
The initial echocardiogram reported a non-dilated left ventricle with normal systolic function, right atrial dilatation, moderate tricuspid regurgitation, Ebstein's anomaly with a 50% of tethering and a moderate pericardial effusion without hemodynamic compromise.
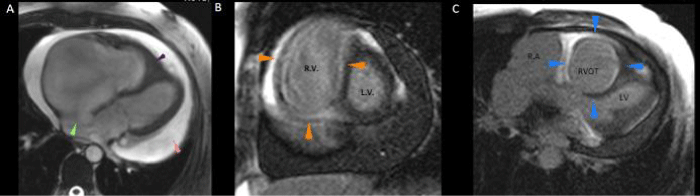
An MRI was performed to complement the pre-surgical anatomical features of Ebstein´s anomaly. It showed significant tricuspid regurgitation without leaflet adherence to the underlying myocardium and so, rejecting the diagnosis of Ebstein. Instead, typical features of right ventricular endomyocardial fibrosis were present (diffuse subendocardial right ventricular late gadolinium enhancement and right ventricular apical obliteration), severe pericardial effusion, dilatation of the right atrium, coronary sinus and both vena cava were also present (Figure 1).
Panel A. Four chambers 4c, Gradient Eco Sequence. GRE. Right ventricular apical obliteration (purple arrowhead). Left deviation of the interatrial septum (head green arrow). Pericardial effusion of 27mm. (pink arrow head). Right atrial dilatation. Panel B. Short axis (ventricle base) subendocardic diffuse late gadolinium enhancement of the right ventricle. (arrow head orange). Panel C. Short axis (right ventricular outflow tract) RVOT. Subendocardic diffuse late gadolinium enhancement in the pulmonary infundibulum. (blue arrow head). RA: Right atrium. RVOT: Right ventricular outflow tract, RV: Right ventricle. LV: Left ventricle. 4c: 4 chambers.
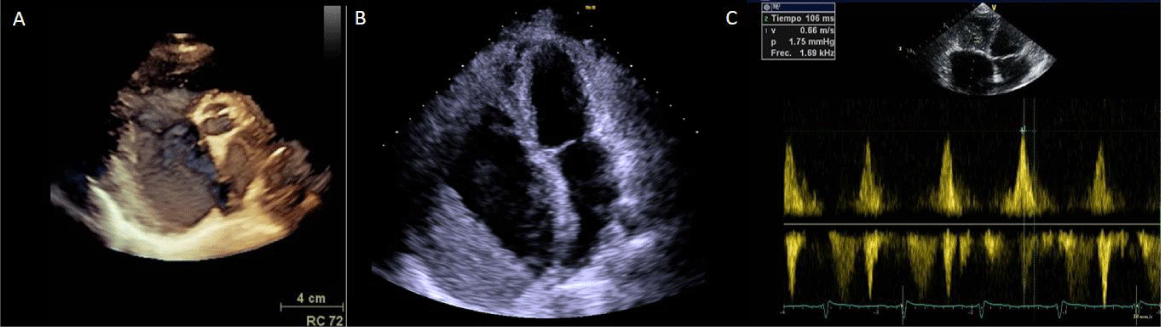
She was started on diuretics and with an angiotensin receptor blocker improving her functional status. After 2 years of follow up, she presented atrial fibrillation, syncope and increase on pericardial effusion. It was decided to perform a pericardial window, however, besides an initial improvement, she developed bilateral pleural effusion and recurrence of the pericardial effusion. This time, pericardiectomy and pleurodesis were done. The histopathological analysis concluded acute fibrinous and non-specific chronic pericarditis, without microorganisms or malignant cells. She was discharged for external follow up, continuing with medical therapy and oral anticoagulation. Two months later she required a pacemaker implantation due to a complete AV block. A new echocardiogram showed aneurismal right atrial dilatation with a giant thrombus, right ventricular restrictive cardiomyopathy and severe tricuspid regurgitation secondary to retraction and thickening of the valves and subvalvular apparatus (Figure 2).
Her clinical course has been torpid, with multiple hospitalizations due to heart failure, always associated with poor medical adherence. A surgical resection of the fibrotic tissue and tricuspid valve replacement was considered, however due to high surgical risk this possibility was ruled out. Economical and social limitations excluded the patient for a cardiac transplantation.
Discussion
Due to its low prevalence in non-endemic countries, endomyocardial fibrosis (EMF) is rarely suspected and not infrequently is confused with Ebstein´s anomaly, constrictive pericarditis and other cardiomyopathies. Echocardiography is the initial diagnostic study wherein atrial dilatation, ventricular apical obliteration with normal size ventricles increase the diagnostic suspicion. A restrictive filling pattern is usually seen only in advanced stages. Cardiac magnetic resonance (CMR) is the gold standard imaging technique for the diagnosis of EMF, it offers optimal visualization of the ventricular apex and possible apical thrombi. Late gadolinium enhancement (LGE-CMR) provides tissue characterization and identifies the distribution of fibrosis which aids in the differential diagnosis and gives useful information for the pre-surgical planning resection [1]. In addition, the degree of fibrous tissue deposition measured on LGE-CMR is related to NYHA functional class and mortality [2]. Endomyocardial biopsy provides the diagnosis and excludes other restrictive cardiomyopathies, particularly infiltrative and storage diseases. However, it´s an invasive procedure and allows the diagnosis in only about 50% of patients [3,4].
As seen in our patient, pericardial and/or pleural effusion are frequent, independently of the affected ventricle, as well as the involvement of the conduction tissue generating advanced blocks [5]. Atrial dilation favors atrial fibrillation and the formation of thrombus [6].
Endomyocardial fibrosis is a challenging diagnosis in in non-endemic areas, it can be misdiagnosed as Ebstein´s disease, other restrictive cardiomyopathies and constrictive pericarditis. A detailed analysis of the tricuspid valve apparatus and cardiac chambers with echocardiography along with LGE-CMR is of paramount importance for a precise diagnosis.
Hector González-Pacheco MD Coronary Care Unit, National Institute of Cardiology Mexico.
Francisco Azar-Manzur MD Coronary Care Unit, National Institute of Cardiology Mexico.
- Dato I (2015) How to recognize endomyocardial fibrosis? J Cardiovasc Med 16: 547-551. Link: http://bit.ly/2PC2aYg
- Salemi VMC, Rochitte CE, Shiozaki AA, Andrade JM, Parga JR, et al. (2011) Late Gadolinium Enhancement Magnetic Resonance Imaging in the Diagnosis and Prognosis of Endomyocardial Fibrosis Patients. Circ Cardiovasc Imaging 4: 304-311. Link: http://bit.ly/2Wvyy01
- Gutierrez PS, Campos FPF de (2017) Endomyocardial fibrosis. Autopsy Case Rep 7: 3-6. Link: http://bit.ly/2r2z2PA
- Barretto AC, Bellotti G, Higuchi M de L, Stolf NA, Dauar D, et al. (1986) Endomyocardial biopsy of the right ventricle in patients with endomyocardial fibrosis. Arq Bras Cardiol 46: 19-21. Link: http://bit.ly/2WyGYUw
- Grimaldi A, Mocumbi AO, Freers J, Lachaud M, Mirabel M, et al. (2016) Tropical Endomyocardial Fibrosis: Natural History, Challenges, and Perspectives. Circulation 133: 2503-2515. Link: http://bit.ly/338OnMs
- Pereira Barretto AC, Bellotti G Higuchi MLV, Stolf NAO, Dauar D, Mady C, et al. (1986) Biopsia endomiocárdica do ventrículo direito em portadores de endomiocardiofibrose. Arq Bras Cardiol 46: 19. Link: http://bit.ly/2WyGYUw
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
