Imaging Journal of Clinical and Medical Sciences
Stress and Glucose metabolism: A Review
Nirupama R1, Barathi Rajaraman2 and Yajurvedi HN3*
2Research Scholar, Sastra University, Thanjavur, Tamil Nadu, India
3Professor at DOS in Zoology, Mysore University, Manasagangotri, Mysore, Karnataka, India
Cite this as
Nirupama R, Rajaraman B, Yajurvedi HN (2018) Stress and Glucose metabolism: A Review. Imaging J Clin Medical Sci 5(1): 008-012. DOI: 10.17352/2455-8702.000037Stress is an inescapable fact of life. The perceived stress induces endocrine alterations characterized by the activation of hypothalamo-pituitary-adrenal axis and sympathetic adreno-medullary axis. The glucocorticoids and catecholamines which are secreted in response to stress induce variations in the physiology and behavior that help the individual to adapt to changing demands of the body. Glucocorticoids are known to play a central role in inducing the stress related pathophysiology. These hormones induce hypermetabolism in order to cope up with the increasing energy demands of the body. However when the stress is persistent the body adapts itself to continuous demands and starts regulating the metabolism at higher levels than the normal, termed as allostasis. This overwhelming load on the body will predispose the individual for the development of diseases. This mini-review focuses on long term chronic stress induced alterations in glucose metabolism and development of insulin resistance and glucose intolerance as a result of long term allostatic regulation.
Introduction
It is hard to dispute that most of us live at breakneck speed where numerous family, social and work obligations can easily overpower precious time and resources. This causes both physical and emotional stress that can take great toll on health [1]. Hence the field of biology of stress has been extensively studied by many researchers all over the world. However many of the aspects of stress remain obscure. Stress is a non-specific response of the body to any stimuli [2]. This review focuses on different pathways of glucose metabolism under long term chronic stress and its impact.
Stress is a state of threatened homeostasis which delineates the metabolic pathways in order to meet the increased demands of the host. The ability to cope with these changes is crucial for healthy life. The events that evoke stress response are called stressors which can be external or internal [3]. The external factors may be physical injury, extreme climatic conditions, etc. and the internal factors may be infections, hypoglycaemia, etc. or psychological factors (personal issues viz. job, health or finances, etc.) [4]. Stress may be acute i.e. exposure for a short duration or chronic i.e. long persistent stress. Acute stress is adaptive in nature and enables the organism face emergency situation precisely, flight or fight responses whereas the chronic stress is shown to have deteriorating effects on the health.
Stress, neuroendocrine axis and glucocorticoids
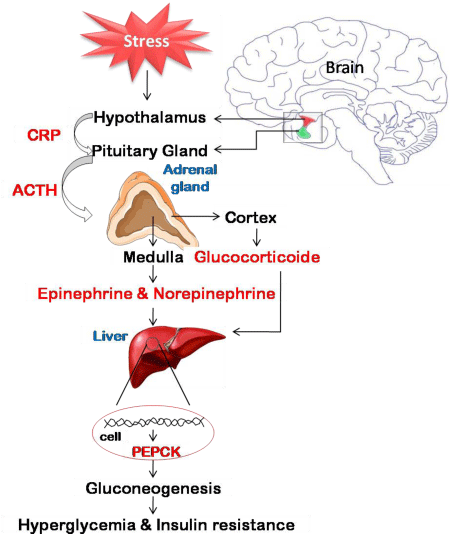
It is known that stress activates hypothalamo-pituitary- adrenal axis. The brain circuits that initiate and maintain stress response are located in the hypothalamus, which include paraventricular nuclei, locus ceruleus and the central sympathetic nervous system [5,6]. Stress activates the paraventricular nucleus (hypothalamus) in the brain and stimulates endocrine and neural mechanisms leading to an increase in the concentrations of stress hormones viz. glucocorticoids and catecholamines. Precisely, the activated paraventricular nucleus neurons secrete corticotropin releasing hormone (CRH) which regulates the adreno-corticotropic hormone (ACTH) release from the anterior pituitary. ACTH acts on the adrenal cortex to release glucocorticoids [7,8]. These neuroendocrine changes seem to lack habituation and possibly there was an abnormal hypothalamo- pituitary- adrenal axis (HPA axis) activation in response to repeated stress [9]. In addition, sympathetic nervous system is activated which releases norepinephrine direct to the circulation and epinephrine from the adrenal medulla. It is demonstrated that glucocorticoids and catecholamines act synergistically during stress [10] (Figure 1).
Catecholamines act transiently during the initial exposure to stress and at later stages would be accompanied by the secretion of glucocorticoids. Glucocorticoids are generally referred to as stress hormones [11] as their secretion will be high under stressful conditions. Glucocorticoids elicit their action by binding to glucocorticoid receptors (GRs) that regulate various physiological functions viz., inflammation, gluconeogenesis and adipocyte differentiation etc. [12]. Upon binding with ligand, it is dimerized and translocated into nucleus where it binds to glucocorticoids (GCs) response elements (GREs) and bring out the gene expression of the target genes.
The mechanisms of stress induced alterations in glucose metabolism leading to hyperglycaemia are also elucidated which involve the changes in different pathways. The chronic stress is reported to cause hypermetabolism characterized by enhanced glycolysis, gluconeogenesis, altered glucose uptake and reduced glycogenesis. Persistent stress predispose to the development of chronic illness accompanied by the metabolic dysregulation.
Glucose uptake and aerobic oxidation of glucose under stress
An important outcome of chronic stress is hyperglycemia. This may be due to either reduced uptake of glucose by cells or increased synthesis of glucose. Hyperglycemia is the immediate effect of stress as it serves energy to meet the energy requirements of the body to chronic stress. In an attempt to maintain the glucose homeostasis in response to elevated adrenocortical activity during stress, all the glucose pathways would be affected. Glucose uptake in the body is facilitated in 2 ways viz., facilitated diffusion and secondary active transport. The facilitated diffusion is against concentration gradient which may be insulin mediated or non-insulin mediated glucose uptake. The secondary active transport is seen predominantly in kidney involves the use of ATP [13].
GLUT receptors play a vital role in the uptake of glucose from the blood stream. There are 14 different types of glucose transporters, however the GLUT 1 -4 are significantly studied [14]. GLUT-1 and GLUT-3 are shown to have high affinity for glucose and GLUT-1 is the major receptor that acts in brain and GLUT-2 is responsible for the uptake of glucose in pancreas. The GLUT-4 is insulin sensitive and is predominantly involved in receptor mediated glucose uptake in muscle [15]. Counter regulatory hormones such as stress hormones (glucocorticoids and catecholamines) and glucagon are reported to inhibit insulin induced glucose uptake [16].
Glucose taken up by the cells enters glycolytic pathway. The end product of glycolytic pathway the pyruvate is metabolized either aerobically completely to carbon dioxide and water or anaerobically to lactate [13]. Under anaerobic conditions pyruvate is converted into lactate by the action of the enzyme lactate dehydrogenase. Under physiological conditions there will be equilibrium between the concentration of lactate and pyruvate. However under chronic stress conditions there will be increased concentrations of pyruvate and lactate [17-19] together with increased activity of lactate dehydrogenase (LDH) [20-22]. Under stressful conditions, the pyruvate produced by glycolysis may be channeled towards the production of glucose or it might end up in producing high lactate because of the reduced activity of pyruvate dehydrogenase (PDH). Reduced pyruvate dehydrogenase activity has been observed under chronic stress condition [23,24]. Stress is known to alter PDH activity [25] by increasing the concentration of pyruvate dehydrogenase kinase which inactivates PDH by phosphorylating it [26]. In spite of this, the activity of tricarboxolic acid (TCA) cycle will be high during stress because of the availability of substrates for TCA cycle by the oxidation of lipids (Nelson and Cox, 2004). Further the increased activity of TCA cycle provides substrates for gluconeogenesis. It is reported that stress induces lipolysis [27,28] and proteolysis which further elevate the concentration of the substrates for gluconeogenesis [29-31]. In addition, chronic stress caused hyperlactatemia an indication of hypermetabolism.
Glycogenesis and glycogenolysis during chronic stress
Chronic hyperglycemia during stress not only affects the glucose uptake and utilization but also enhances the synthesis of glucose endogenously. During normal conditions, the dietary glucose and endogenous glucose synthesized by the liver lead to the formation of glycogen in the liver. Stress is known to inhibit the glycogenesis in liver and skeletal muscles by inhibiting activity of glycogen synthase. The activity of glycogen synthase is inhibited by its phosphorylation by glycogen synthase kinase 3 (GSK-3) [32].
Glycogenolysis is the process of release of glucose from the glycogen. This usually occurs during starvation. Under stress, the glycogenolysis occurs to meet the increased energy demands by the body to withstand the perceived stress. A number of studies have shown the decreased liver glycogen content in response to chronic stress. For instance, our study [24] wherein rats were exposed for restraint and forced swimming every day for 2, 4 or 24 weeks, a reduction in hepatic glycogen content was observed. In addition, Kuznetsov and his coworkers [33] subjected rats to hypokinetic stress for 5, 15, 30, 45 and 60 days which resulted in decreased liver glycogen content.
Gluconeogenesis during chronic stress
Gluconeogenesis is synthesis of glucose from non-carbohydrate precursors [13]. Under normal conditions the gluconeogenesis occurs during starvation to supply glucose to the cells, especially the brain which is dependent on the glucose. Stress increases the hepatic glucose production by increasing the activities of key gluconeogenic enzymes viz. phosphoenol pyruvate carboxy kinase (PEPCK), pyruvate carboxylase, fructose 1,6 bisphosphatase (FBPase) and glucose-6-phosphatase (G6Pase). All these key regulatory enzymes are transcriptionally regulated by glucocorticoids. Stress increases the transcription of PEPCK genes [12,34]. CREB, C/EBP and FOXO1 are the transcription factors which induce PEPCK genes under stress conditions [35]. It is reported that 7 fold over expression of PEPCK causes hyperglycaemia and 2 fold over expression causes insulin resistance [35]. Glucocorticoids also stimulate the expression of pyruvate carboxylase [29] and glucose-6- phosphatase [36]. In addition stress increases the activities of aminotransferases, glutamic pyruvic transminase (GPT) and glutamic oxaloacetatic transaminase (GOT) [22,37] which further increase the concentration of substrates like pyruvate and oxaloacetate for gluconeogenesis. In addition to these, glucocorticoids are known to increase the blood glucose levels under stressful conditions by not only increasing gluconeogenesis but also by reducing insulin sensitivity. The glucocorticoids exert this action by antagonizing insulin stimulated translocation of glucose transporters from intracellular compartments to plasma membrane [12,38-40]. A similar mechanism is responsible for the glucocorticoid induced insulin resistance in the skeletal muscles [41].
Glucose metabolism, allostasis and allostatic load
The concept of allostasis was introduced by Sterling and Eyer in 1988 [42]. Allostasis is maintenance of physiological variables at altered level, different from the homeostatic set point in response to perceived or anticipated challenges or stressors. It regulates the metabolism by altering the biochemical pathways to achieve stability. Indeed the state of allostasis has been demonstrated by us in rats that were exposed to chronic stress [24]. In this study consistent hyperglycemia was observed in rats for 24 weeks, following exposure to restraint for 1 h followed by forced swimming for 15 minutes after a gap of 4 hours every day for 2, 4 or 24 weeks. The hyperglycemia was accompanied by altered pathways of glucose metabolism, predominantly increased activity of gluconeogenic enzymes. An exaggerated response of the body to persistent stress may lead to allostatic load i.e. altered physiological processes that may cause damage to the system [43]. For instance in our study [24] prolonged hyperglycemic condition due to stress was accompanied by insulin resistance and failure to tolerate glucose as shown by OGTT in rats. Thus when stress is persistent and it is prolonged for a long duration, the adaptive processes in the body become maladaptive resulting in pathophysiology. Brunner and coworkers has hypothesized [44] that neuroendocrine axis activated in response to stress stimuli plays a major role in the development of metabolic syndrome. In fact, many researchers have shown the development of insulin resistance and metabolic syndrome in response to chronic stress [12,45-49]. Therefore it is inferred that when the system is unable to cope up with the continuous demands, allostatic load might become overwhelming that predisposes the body for the development of the diseases.
Conclusion
It is evident from the above discussion that chronic stress has adverse effects on the glucose metabolism. The alterations that are observed during chronic stress appear to be due to allostatic regulation in response to demand on the body. However, long term allostatic regulation leads to allostatic load resulting in pathophysiological conditions such as metabolic syndrome. Since stress is an inescapable fact of life, one should aim at managing the stress. Non-pharmacological intervention and stress management would prove beneficial in controlling the deleterious effects of stress. Future studies should be aimed at developing a novel strategy to suppress the activation of HPA axis and sympathetic nervous system due to stress, so as to prevent deleterious effects of glucocorticoids on carbohydrate metabolism.
- Mitra A (2008) Diabetes and stress: A review. Ethno Med 2: 131-135. Link: https://goo.gl/DqV4T6
- Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138: 32. Link: https://goo.gl/VJRnQe
- Townsend J (1999) Feeling stress??? Stress and stress management. https://www.gaaymate .com/stressarticle.htm.feb1999.
- Meadows-Oliver M, Sadler LS, Swartz MK, Ryan-Krause P (2007) Sources of stress and support and maternal resources of homeless teenage mothers. J Child Adole Psych Nurs 20: 116-125. Link: https://goo.gl/YDJkcL
- Chrousos GP, Gold PW (1992) The concepts of stress system disorders. Overview of physical and behavioural homeostasis. JAMA 267: 1244-1252. Link: https://goo.gl/BEyWGF
- Tsigos C, Chrousos GP (1994) Physiology of the hypothalamic– pituitary–adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinol Metab Clin North Am 23: 451– 466. Link: https://goo.gl/azdb5R
- Antoni FA (1986) Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr Rev 7: 351–378. Link: https://goo.gl/cA44ZS
- Whitnall MH (1993) Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol 40: 573–629. Link: https://goo.gl/vY4xti
- Li HY, Sawchenko PE (1988) Hypothalamic effector neurons and extended circuitries activated in `neurogenic' stress: a comparison of foot shock effects exerted acutely, chronically, and in animals with controlled glucocorticoid levels. J Comp Neurol 393: 244-266. Link: https://goo.gl/1zg11U
- Ganong WF (1998) The stress response: A dynamic overview. Hosp Prac 23: 155-171. Link: https://goo.gl/TTXbPY
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM (2009) The Stresses CNS: When glucocorticoids aggravate inflammation. Neuron 64: 33-39. Link: https://goo.gl/sZnPZX
- Wang M (2005) The role of glucocorticoids action in the pathophysiology of metabolic syndrome. Nutr. Metab 2: 3. Link: https://goo.gl/m8vCBh
- Nelson DL, Cox MM (2000) Principles of biochemistry. 4th edition
- Thorens B, Mueckler M (2010) Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab 298: 141–145. Link: https://goo.gl/CkgtM7
- Huang S, Czech MP (2007) The GLUT-4 glucose transporter. Cell metabol 5: 237-252. Link: https://goo.gl/xRPBxv
- Kuo T, McQueen A, Chen TC, Wang JC (2015) Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol 872: 99-126. Link: https://goo.gl/cmqm6j
- Podvigina TT, Troshikhian GV, Bogdanova TS (1978) Characteristics of the intensity of glycolytic and oxidative processes in rat brain tissue under hypoxia and hyperoxia. Ukr Biokhhim Zh50: 155-159. Link: https://goo.gl/zCvLB9
- Gartner K, Buttoner D, Dohler K, Friedel R, Lindena J, et al. (1980) Stress response of rats to handling and experimental procedures. Lab Anim 14: 267-274. Link: https://goo.gl/16Y5Da
- Teagu CR, Dhabhar FS, Barton H, et al. (2007) Metabonomic studies on the physiological effects of acute and chronic psychological stress in Sprague- Dawley rats. J Proteome Res 6: 2080-2093. Link: https://goo.gl/X4xot1
- Arakawa H, Kodama H, Matsuoka N, Yamaguchi I (1997) Stress increases plasma enzyme activity in rats: Differential effects of adrenergic and cholinergic blockades. J Pharmacol Exp Ther 280: 1296-1303. Link: https://goo.gl/7VkaZJ
- Chang Ah Chu, Sindelar DK, Neal DW, Allen EJ, Donahue EP, et al. (1997) Comparison of the direct and indirect effects of epinephrine on hepatic glucose production. J Clin Invest 99: 1044-1056. Link: https://goo.gl/JvVWPA
- Nayanatara AK, Nagaraja HS, Ramaswamy C, et al. (2009) Effect of chronic unpredictable stressors on some selected lipid parameters and biochemical parameters in Wistar rats. J Chin Clin Med 4: 92-97. Link: https://goo.gl/kX2beJ
- Nirupama R, Devaki M, Yajurvedi HN. (2010) Repeated acute stress induced alterations in the carbohydrate metabolism in rat. J Stress PhysiolBiochem 6: 44-55. Link: https://goo.gl/bNbHAv
- Nirupama R, Devaki M, Yajurvedi HN (2012) Chronic stress and Carbohydrate metabolism: Persistent changes and slow return to normalcy in male albino rats. Stress 15: 262-271. Link: https://goo.gl/M4jtjV
- Miles PDG, Yamatani K, Lickley HLA, Vranic M (1991) Mechanism of glucoregulatory responses to stress and their deficiency in diabetes. Proc Natl Acad Sci 88: 1296-1300. Link: https://goo.gl/QG7ASq
- Pengfei WU, Juichi Sato, Yu Zhao, et al. (1998) Starvation and diabetes increase the amount of pyruvate dehydrogenase kinase isoenzyme 4 in rat heart. Biochem J 329: 197-201. Link: https://goo.gl/mpuJY7
- Brindley DN (1995) Role of glucocorticoids and fatty acids in the impairment of lipid metabolism observed in the metabolic syndrome. Int J Obes Relat Metab Disord 19: 69-75. Link: https://goo.gl/kLzNfi
- Sato T, Yamamoto H, Sawada N, et al. (2006) Restraint stress alters the duodenal expression of genes important for lipid metabolism in rat. J Bone Min Metab 24: 291-299. Link: https://goo.gl/6HK5E2
- Eisenstein A (1973) Effect of adrenal cortical hormones on carbohydrate, protein and fat metabolism. J ClinNutr 26: 113-120. Link: https://goo.gl/NU8Bt8
- Kayali AG, Young VR, Goodman MN (1987) Sensitivity of myofibrillar proteins to glucocorticoid- induced muscle proteolysis. Am J Physiol Endocrinol Metab252: E621-E626. Link: https://goo.gl/EjtkP9
- Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, et al. (1996) Energy- ubiquitin- dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest 92: 339-348. Link: https://goo.gl/q5wdnU
- Dokken BB, Saengsirisuwan V, Kim JS, Teachy MK, Henriksen EJ (2008) Oxidative stress- induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. Am J Physiol 294: E615-E621. Link: https://goo.gl/42eq84
- Kuznetsov VI, Saraev Lu V, Chirkin AA (1989) Glycolysis activation, a decrease in the glycogen reserve and the absence of glucocorticoids control over the enzymes of carbohydrate metabolism in the liver of rats under hypokinetic stress. Patol Fiziol Eksp Ter 4: 56-59. Link: https://goo.gl/w8Bmyc
- Andrew RC, Walker BR (1999) Glucocorticoids and insulin resistance; Old hormone, new targets. Clin Sci 96: 513-523. Link: https://goo.gl/U9q9QR
- Burgess CS, He TT, Yan Z, Lindner J (2007) Cytosolic phosphoenol pyruvate carboxy kinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 5: 313-320. Link: https://goo.gl/Ta4bGq
- Rooney DP, Neely RDG, Cullen C, Ehnis CN, Sheridan B (2008) The effect of cortisol on glucose/ glucose-6-phosphate cycle activity and insulin action. J Clin Endocrinol Metab 77: 1180-1183. Link: https://goo.gl/ogUhEJ
- Nagaraja HS, Anupama BK, Jaganathan PS (2006) Stress response in albino rats. Thai J Physiol Sci 19: 8-15. Link: https://goo.gl/1NnkSH
- Carter-Su C, Okamato K (1987) Effect of insulin and glucocorticoid on glucose transporters in rat adipocytes. Am J Physiol 252: E441-E453. Link: https://goo.gl/5be6d2
- Horner HG, Munck A, Lienhard GE (1987) Dexamethasone causes translocation of glucose transporters from the plasma membrane to an intracellular site in human fibroblasts. J Biol Chem 262: 17696-17702. Link: https://goo.gl/Mes75b
- Oda N, Nakai A, Mokuno T, Sawai Y, Nishida Y (1995) Dexamethasone induced changes in glucose transporter 4 in rats heart muscle, skeletal muscle and adipocytes. Eur J Endocrinol 133: 121-126. Link: https://goo.gl/Pnfaop
- Dimitriadis G, Leighton B, Parry-Billings M, Sasson S, Young M, et al. (1987) Effect of glucocorticoid excess on the sensitivity of glucose transport and metabolism to insulin in skeletal muscle. Biochem J 321: 707-712. Link: https://goo.gl/Uy22uB
- Sterling P, Eyer J (1988) Allostasis: A new paradigm to explain arousal pathology. Fisher S and Reason J, editors. Handbook of life stress, cognition and health 629-649. Link: https://goo.gl/pcrxDp
- Maloney E, Gurbasani B, Jones J, Coelho L, Pennachin C, et al. (2006) Chronic fatigue syndrome and high allostatic load. Pharmacogenomics 7: 467-473. Link: https://goo.gl/rs792n
- Brunner EJ, Hemingway H, Walker BR, et al. (2002) Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation 106: 2659–2665. Link: https://goo.gl/jdbyuz
- Rossetti L, Shulman GI, Zawalich W, DeFronzo RS (1987) Effect of hyperglycaemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 80: 1037-1044. Link: https://goo.gl/9RdUXM
- Kurowski TG, Lin Y, Tsichillo PN, Buse MG, Heydrick SJ, et al. (1999) Hyperglycemia inhibits insulin activation of Akt/ protein kinase B not phosphatidylinositol-3- kinase in rat skeletal muscle. Diabetes 48: 1-6. Link: https://goo.gl/9xZcnC
- Haber CA, Lan TKT, Yu Z, et al. (2003) N-acetylcysteine and taurine prevent hyperglycaemia- induced insulin resistance in vivo: possible role of oxidative stress. Am J Physiol Endocrinol Metab 285: E733-E753. Link: https://goo.gl/w5EUYS
- Robinson A, Lindsay E (2004) Insulin resistance and hyperglycemia in critical illness: Role of insulin in glycemic control. Adv Pract Acute crit Care 15: 45-62. Link: https://goo.gl/XeBLgK
- Rezvanfar MR, Dalvandy M, Emami AR, Rafiee M, Eshratee B (2009) Hyperglycemia and mortality in critically ill patients. Pak J Med Sci 25: 232-237. Link: https://goo.gl/ZDs3aj
- Bamberger CM, Schulte HM, Chrousos GP (1996) Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev 17: 245-261. Link: https://goo.gl/jnL1uX
- Schacke H, Rehwinkel H (2004) Dissociated glucocorticoid receptor ligands. Curr Opin Investig Drugs 5: 524-528. Link: https://goo.gl/9Gdg5G
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
