Imaging Journal of Clinical and Medical Sciences
GG Polymorphism of Platelet ITGA2B Gene Increases the Magnitude of Interleukin-6 Release after Cardiopulmonary Bypass
Koray Ak1,2*, Samet Ergun2,3, Hilal Altınoz2,4, Z Oya Uyguner5 and Sermin Tetik2,6
2Health Sciences Institute Department of Biochemistry, Turkey
3Taksim Education and Research Hospital Pathology Division, Turkey
4Istanbul Süreyyapasa Thoracic Disease and Thoracic Surgery Education and Research Hospital, Turkey
5Department of Medical Genetics, Istanbul Medical Faculty, Istanbul University, Turkey
6Cyprus International University, Lefkosa, Cyprus
Cite this as
Koray Ak, Ergun S, Altınoz H, Uyguner ZO, Tetik S (2016) GG Polymorphism of Platelet ITGA2B Gene Increases the Magnitude of Interleukin-6 Release after Cardiopulmonary Bypass. Imaging J Clin Medical Sci 3(1): 024-029. DOI: 10.17352/2455-8702.000032Objective: Cardiopulmonary bypass (CPB) induces a systemic inflammatory response which is thought to be a significant cause of postoperative organ dysfunction and mortality. In this study we aimed to investigate the effect of ITGA2B (integrin alpha 2b, platelet glycoprotein IIb of IIb/IIIa complex) gene polymorphism on the magnitude of inflammatory response after CPB.
Methods: Twenty patients undergoing coronary artery bypass grafting were included. Blood samples were taken at the three different times for analyses of Interleukin-6, Interleukin-10 and Nuclear Factor Kappa B by ELISA (t1: before operation, t2:10 minutes after removal of aortic cross clamping and t3: 24 hours after operation. ITGA2B gene polymorphisim, c.2621T>G, resulting missense alteration, p.Ile874Ser (rs5911) was studied in patients and 27 healthy volunteers by targeted polymerase chain reaction (PCR) and restriction enzyme digestion. Perioperative organ dysfunction was evaluated by cardiac surgery scorring (CASUS) system.
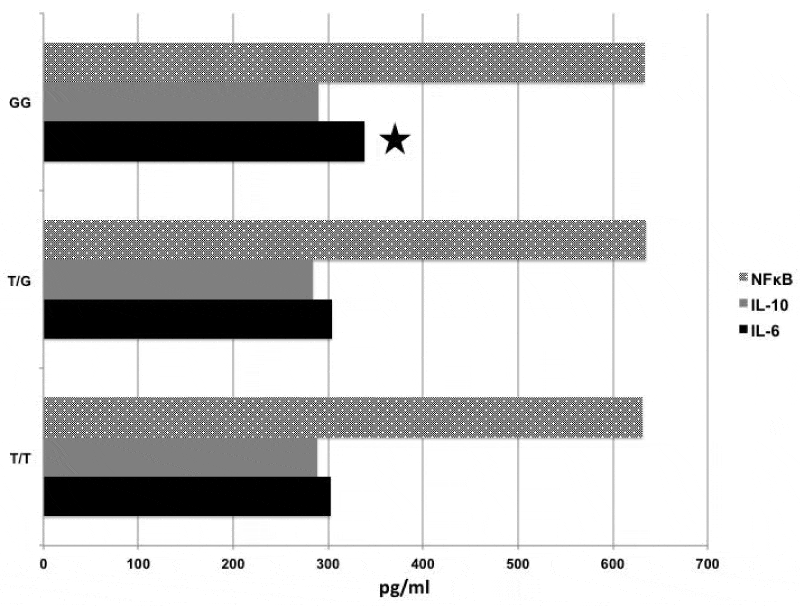
Results: There was no perioperative mortality. The mean ages of the patients and the controls were 67.45±12.30 and 51,38±7,03 years, respectively (p=0.001). Thirty-five percent (n=7) of the patients revealed TT, 45% (n=9) TG and 20% (n=4) GG polymorphism. The allele frequencies of the study group were similar to the controls (33,3%, n=9 revealed TT, 55,5 %, n=15 TG and 11,3%, n=3 GG). There was no significant difference in the frequency of genetic polymorphism between the patients and the controls. In the study group, patients with GG allele had significantly higher interleukin 6 levels 24 hours after operation than the others (GG (338,14±22.20pg/ml) versus TT (306,14±22, 10 pg/ml), p=0.025, and GG versus TG (308, 12±14,50pg/ml), p=0.039).
Conclusion: Our results reveal that GG allele of ITGA2B gene might have a significant role in the magnitude of the inflammatory response after CPB.
Introduction
Cardiac surgery with cardiopulmonary bypass (CPB) is associated with a systemic inflammatory response syndrome (SIRS) that is known to be one of the main causes of postoperative organ dysfunction (1). Several factors such as contact of blood with foreign surfaces, surgical trauma, ischemia reperfusion to the organs, and release of endotoxin have been widely documented to cause SIRS after CPB. Recently it has been shown that individual genetic polymorphisms in the genes encoding inflammatory cytokines, apolipoprotein E4 and lipoprotein lipase and the like have a major impact on the magnitude of SIRS in patients undergoing CPB (2-4).
ITGA2B is a receptor for fibronectin, fibrinogen, plasminogen, prothrombin, thrombospondin and vitronectin and functions in platelet activation (5). Current dogma suggest that several genetic polymorphisms of ITGA2B have been associated with a wide variety of clinical events including myocardial infarction (MI) at a young age, stroke and resistance to antiplatelet agents (6-8). Furthermore, it has been stated that different mutations of this gene results in a loss in the ability of aggregate and the production of an immune response (9). Recently, a growing body of evidence suggests that platelets have the pivotal role in the pathogenesis of several poor prognostic mediacal conditions like inflammation and cancer (10). We hypothesized that different polymorphism of the ITGA2B gene may influence the magnitute of inflammatory response after coronary artery bypass grafting (CABG) since the critical role of ITGA2B receptor in inflammation”. We investigated the relationship between the ITGA2B polymorphism and the magnitude of perioperative inflammatory cytokines and organ dysfunction in patients undergoing CABG with CPB.
Materials and Methods
After obtaining approval to conduct the study from the local ethics committee and written informed consent from each patient, we enrolled 20 consecutive patients undergoing elective first time coronary artery bypass grafting (CABG) procedure into the study. Exclusion criteria were previous CABG history, acute coronary syndrome, known infection, steroid or nonsteroidal anti-inflammatory therapy within the last three months, an autoimmune condition, a known pathology of platelets, bone marrow disease, preoperative intra-aortic balloon pump insertion, emergency revascularization, chronic renal disease requiring dialysis, and hepatic failure. Twenty-seven healthy control individuals (aged between 35 and 60 years) were used to investigate genotype distribution in comparison with patients. According to the results of medical history, physical examination, and laboratory data, they were judged healthy.
Medical history, demographic data, and the postoperative course for each patient were collected prospectively. Preoperative evaluation included routine blood biochemistry, complete blood count, pulmonary function test, transthoracic echocardiography, and coronary angiography. In the postoperative period, patients were followed up and treated according to the same institutional protocol.
Postoperative daily assessment of organ function in the intensive care unit (ICU) was performed by the cardiac surgery scoring (CASUS) system (11). In brief, CASUS contains 10 variables that were graded according to the severity for daily risk stratification in the ICU. Operative day CASUS score was calculated when the patient was admitted to the ICU. Then, subsequent calculations were performed in the morning of each ICU day. Perioperative myocardial infarction was defined by new electrocardiographic changes and an increase of 3.5-fold in creatine kinase (CK) isoenzyme MB level. Need for an inotropic agent was judged by systolic blood pressure lower than 90 mm Hg or more than 40 mm Hg below usual systolic pressure under appropriate fluid management. Postoperative acute renal dysfunction was defined as a postoperative serum creatinine level greater than 200 μmol/L or need for dialysis therapy or hemofiltration before hospital discharge. The surgical team and the clinicians who were responsible for the postoperative care of the patient were blinded to the study.
Anesthesia and operative technique
Midazolam was used for premedication, and the anesthetic agent consisted of a combination of fentanyl, midazolam, and pancuronium. Anesthesia was maintained with midazolam and vecuronium infusion and with inhaled sevoflurane. Median sternotomy was carried out in all patients. Standard aortocaval cannulation was done to establish CPB. CPB was performed with a roller pump system (Jostra AG, Hirrlingen, Germany) and a hollow-fiber membrane oxygenator. Also, a 40-μm arterial blood filter was used in all patients. Mild–to–moderate (28°C–32°C) hypothermia and pulsatile flow of 2.2 to 2.4 L/m2 were maintained throughout CPB in all patients. Myocardial protection was provided by antegrade tepid blood cardioplegic solution and topical cooling during aortic crossclamping. Repeated infusions of cardioplegic solutions were given every 20 minutes or earlier if electrical activity occurred. Rewarming to a normal nasopharyngeal temperature was achieved with a heat-exchange oxygenator and warming blanket. Left internal thoracic artery and saphenous vein were preferred as bypass conduits in all patients. The rest of the operation was completed in a standard fashion. Perioperative anticoagulation with heparin was reversed after CPB with protamine sulfate. Aprotinin and steroids were not used. All patients were followed up in a standard fashion during postoperative period.
Biochemical measurements
Venous blood sampling was used for routine biochemical measurements and cytokine analyses. Leukocyte count of the blood samples was determined by the use of the Coulter system (Coulter HMX-AL system hematology analyzer; Beckman Coulter Corporation, Miami, Fla) before induction, immediately after CPB, and 24 hours after the operation. Lactate levels were analyzed (GEM Premier 3000 blood gas/electrolyte analyzer model 5700; Instrumentation Laboratory, Lexington, Mass) from radial artery blood samples taken at preoperatively, immediately after CPB, and 6, 12, and 24 hours postoperatively. CK (Cobas Integra 700; Roche Diagnostics, Basel, Switzerland) and CK-MB levels (Elecsys 2010; Roche Diagnostics) were measured preoperatively and 6 and 24 hours after the operation.
Analyses of Interleukin (IL)-6, IL-10 and Nuclear factor kappa-light-chain-enhancer of ctivated B cells (NfκB)
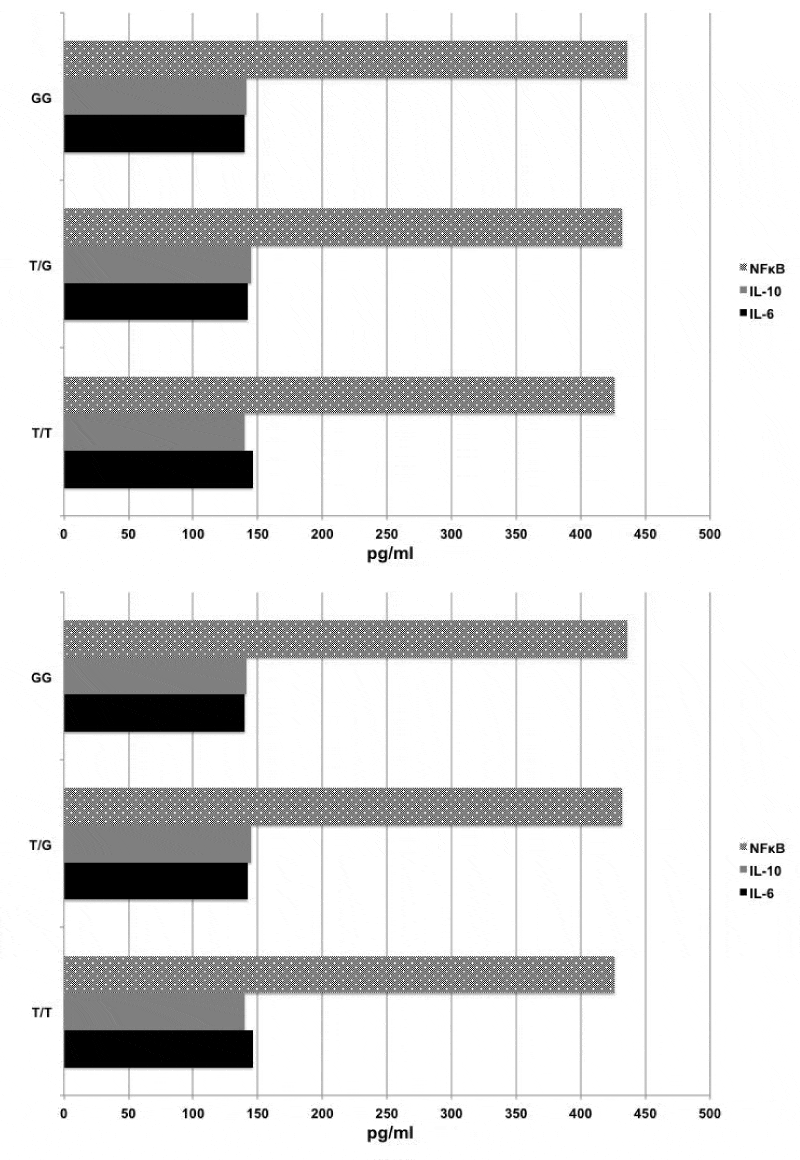
Levels of IL-6, IL-10 and NFκB were measured by enzyme-linked immunosorbent assay (Invitrogen, BioSource Division, and Carlsbad, Calif) according to the manufacturer’s recommendations. The blood samples were collected into sterile ethylenediaminetetraacetic acid tubes. After centrifugation, the plasma was collected and then stored at −20°C until biochemical analysis. Levels of IL-6, IL-10 and NFκB were measured at 3 different time points: t1, before induction of anesthesia; t2, 10 minutes after removal of aortic cross clamping, and t3, 24 hours after operation (Figures 1-3).
Isolation of DNA and genotyping
Whole blood samples for genotyping were drawn preoperatively from both patients and healthy control individuals. Isolation of DNA is performed by solution based kit (Mammalian Blood, Roche), pair of primers flanking the polymorphic site is designed (5’- GCT GGG TGG AAG AAA GAC CT-3’ for forward and 5’- CTG CTC ACT ACG AGA ACT GGA-3’ for reverse) (Invitrogen). The rs5911 is a single nucleotide polymorphism (SNP) located at the exon 26 of the ITGA2B gene. PCR amplification generates 244 bp product which is digested into three fragments 31, 78 and 135 bps for TT homozygotes, into two fragments 78 and 166 bps, for GG homozygotes and four fragments 31, 78, 135 and 166 bps for T/G heterozygotes when digested with BccI enzyme (New England Biolabs). Fragments were separated by electrophoresis on a 2.75 % horizontal agarose gels.
Statistical analysis
Statistical analysis was performed with the SPSS for Windows 16.0 version (SPSS, Inc, Chicago, Ill). The χ2 test was used to analyze relationships between categorical data. Nonparametric Mann–Whitney U test was used to compare IL-6, IL-10 and NFκB levels and different outcomes among groups. Nonparametric testing was chosen because the study population was relatively small in size and the data did not follow a normal distribution. Association between two continuous variables was determined by Spearman rank correlation. Continuous variables were given as mean ± standard error of the mean. A χ2 test was used to compare the observed numbers of each genotype with those expected for a population to establish whether they were in the Hardy–Weinberg equilibrium. Regression analyses were used to adjust for confounding factors. All tests were 2-sided. All measurements between and within the groups were checked for different time points by repeated-measures analysis of variance with Bonferroni corrections for multiple testing.
Results
The resuluts of genetic analyses related to the study group and the healthy controls were summarized in Table 1. Of the 20 patients enrolled in the study, 9 patients (45%) revealed polymorphic heterozygote pattern (T/G) of the ITGA2B gene which was found to be statistically nonsignificant to the control population (55.5%). The frequencies of the normal (G/G) and polymorphic homozygote (T/T) patterns did not differ between the study and the control group (for GG; 20% (n=4) versus 11,3% (n=3) and for TT, 35% (n=7) versus 33,3% (n=9), respectively). The mean age was 67.45±12.30 years in the study group and 51,38±7,03 years in the controls (p=0.001). The 70% of the patients (n=14) and 62.9% of the controls (n=17) were male (p=0.614). Comparison of the demographics and the perioperative profile of the patients were given in the Table 2. All patients had significant triple vessel CAD and revealed normal renal function preoperatively. Aortic cross clamp time, CPB time and the number of performed distal anastomosis did not differ amongh the diffrent polymorphisms. Moreover, ionotrophic drug requirement, 24 hour blood loss, ICU and hospital stay did not create a difference.
There was no perioperative mortality in both groups. Postoperative daily assessment of organ dysfunction (CASUS scorring) in ICU revealed no difference in postoperative day 0 (operative day) and day 1 CASUS scores among three pomymorhisms (Table 3).
Preoperative levels of the IL-6, 10 and NFκB were detectable in almost all patients. In all patients, the levels of IL-6, 10 and NFκB revealed an upward trend at the t2 but this was not prominent compared to those at the t1. CPB induced a very significant increase in the levels of IL-6, IL-10 and NFκB 24 hour after operation (tı versus t3; for IL-6: 161,25±28,31 pg/ml versus 322,34±42±23 pg/ml, p=0.000), for IL-10: 134,64±14,30 pg/ml versus 289,20±32±30 pg/ml (p=0.001) and for NFκB: 461,17±51,40 pg/ml versus 634,34±13,23 pg/ml, p=0.001). Among three alleles, we could not detect in any statistically significant differences in the levels of IL-10 and NFκB at three time points. However, IL-6 levels at t3 were significantly higher in patients with G/G polymorphism compared to those with T/G and T/T (G/G (338,14±22.20 pg/ml) versus T/T (306,14±22,10 pg/ml), p=0.025, and G/G versus T/G (308,12±14,50 pg/ml), p=0.039).
Discussion
In the present study, our results showed a similar frequency of the rs5911 SNP of the ITGA2B gene located on chromosome 17 in patients undergoing CABG compared to the healthy subjects. The study group was older than the healthy controls. In patients undergoing CABG, a significant correlation between postoperative 24-hour IL-6 levels and the presence of the G/G polymorphism of the ITGA2B gene was detected. Levels of IL-10 and NFκB did not reveal a difference according to the genotype. We could not detect any correlation between early clinical outcome and the ITGA2B genotype. Cardiopulmonary bypass is still a prerequisite for many cardiac surgical procedures. Cardiac surgery with CPB launches an acute phase reaction, namely SIRS. Postoperative SIRS remains as one of the most important determinants of postoperative organ dysfunction and mortality. Pathophysiology of SIRS includes complement activation, release of free oxygen radicals and activation of the humoral and cellular immune system, which leads to increased inflammatory cytokine release like IL-6, IL-8, IL-10 and TNF-α) (1). Additionally, NF-κB is a ubiquitously expressed transcription factor that regulates expression of genes involved in SIRS and cellular apoptosis related to CPB (12).
A growing body of evidence suggests that certain polymorphisms of several genes coding lipoprotein lipase, inflammatory cytokines, endothelial nitric oxide synthatase (eNOS), and apolipoprotein E have a particular impact on the magnitude of inflammatory reaction and, therefore, on the severity of clinical consequences of SIRS related to CPB. In our previous study, we demonstrated that patients with lipoprotein lipase S447X stop codon mutation is associated with higher levels of IL-8 and less favorable early clicinal outcome after CABG with CPB (4). Presence of IL-6-174 G/C genotype revealed higher post-CPB IL-6 levels, longer stays in the hospital and in the ICU and higher degree of renal and pulmonary complications after CABG (13). Jouan et al., revealed that presence of the IL6-572G>C and IL10-592C>A single nucleotide polymorphisms could be an important tool for identifying patients at the highest risk of poor tolerance to the inflammatory response to cardiopulmonary bypass (14).
The subunits of the GpIIb/IIIa complex are coded by seperate genes located on the long arm of chromosome 17. It has been documented that platelet surface glycoproteins are highly polymorphic and can be presented as autoantigens. Of which, p.Ile843Ser is associated to platelet-specific alloantigen BAK. The p.Leu33Pro polymorphism (rs5918) of the platelet Gp IIIa (ITGB3) gene is the one of the most frequently implicated in syndromes of immune-mediated platelet destruction, particularly neonatal alloimmune thrombocytopenia and posttransfusion (15). Genetic polymorphisms of several platelet Gp receptors have been shown to contribute to the pathophysiology of important clinical events like antiplatelet drug resistance, myocardial infarction, stroke and venous thrombosis. In recent study, Santiago-German et al demonstrated a significant correlation between GpIIIa PIAA2 and plasminogen activator inhibitor 4G alleles with ST elevation acute myocardial infarction (STEAMI) in young Mexican subjects (16). Lekakis et al., claimed that presence of GpIIb homozygous human platelet antigen (HPA)-3b polymorphism in patients with acute coronary syndrome increases the risk of having transmural MI (17). Esen et al revealed a probable link between GP1BA (platelet glycoprotein Ib alpha) Kozak T/C polymorphism and the risk of ischemic stroke, especially in those with undetermined etiology (18). Pourgheysari et al speculated that the presence of higher prevalence of PLA2 polymorphism of GPIIa/IIIb in patiets with venous thromboembolism (19). In our collective, we could not detect a difference in the distribution of the ITGA2B genetic variants (rs5911) between the patients who had severe triple vessels coronary artery disease and underwent CABG and the healthy control subjects. In the literature, the rs5911 polmorphism has been studied in a very limited number of studies. Knowles et al studied the role of 49 SNPs (including rs5911) in patients with coronary artery disease. They concluded that platelet rs5911 SNP had not any effect on the risk of acute MI (20). Altinoz et al studied the impact of rs5911 SNP on inflammation in patients with COPD and they claimed a probable correlation between the G/G genotype and the magnitude of IL-10 levels in these patients (21).
Out of their role in thrombosis, platelets have also a pivotal role in inflammation. Following activation, platelets express P-selectin and bind rapidly to monocytes and neutrophiles or endothelial cells via P-selectin Gp ligand-1 receptor (22). This binding triggers the tissue factor pathway and leads to leukocyte actication and production of inflammatory mediators (23). Moreover recent evidence suggests that activated platelets also contribute to chronic inflammatory milieu in the process of atherosclerosis (24). In our study, the magnitude of IL-6 level 24 hour after operation was higher in patients with the G/G allele than the other alleles. The levels of IL-10 and NF-κB remained similar among the alleles. IL-6 has both proinflammatory and antiinflammatory properties. It stimulates the release of hepatic proteins and is involved in neutrophil-mediated ischemia/reperfusion injury. Nevertheless, IL-10 is a potent antiinflammatory cytokine that reduces neutrophil adhesion to activated endothelial cells (1-3). As the post-CPB increase in IL-10 produces heart and lung protection, increased levels of IL-6 and TNF-α have been associated with cardiac dysfunction after CPB (1). In our study we could not find any correlation between the levels of the cytokines and perioperative organ dysfunction. In our study, there were no major differences in terms of morbidity and mortality among the three alleles. This could be attributed to the small number of the patients in the study group. Also, the patient population in this cohort was relatively young and had only a few comorbidities. The effect of the genotype related organ dysfunction would be expected to be more pronounced in patients with more comorbidities and risk factors.
It is very difficult to explain why patients with the G/G allele had a higher magnitude of IL-6 release 24 after operation with respect to the others in our study. In the literature, the impact of platelet Gp receptor polymorphisms on CPB-related inflammatory response has not been questioned at all. Kucarska-Newton et al demonstrated that individuals with the Leu33Pro polymorphism of the GPIIIa glycoprotein may be predisposed to increased risk of atherosclerotic plaque rupture due to both a thinning fibrous cap and as a result of a sustained pro-inflammatory state (25). With this study, they stressed the significance of platelet polymorphisms on chronic inflammatory process of atherosclerosis. Faraday et al revealed that presence of ITGB3 of p.Leu33Pro (rs5918) and GP1BA of p.Thr145Met polymorphisms increases the risk of postoperative MI after major vascular surgery like infrainguinal, abdominal or thoracoabdominal aortic surgery (26). This increased risk of perioperatrive MI would be secondary to more pronounced inflammatory response related to major surgical procedure. Our study has some limitations. First, the number of the patients included into the study is small. Increasing the number would increase the statistical power of the study. Second, it would be beneficial to measure other inflammatory cytokines of the SIRS like IL-1, IL-2, IL-8 and TNF-α. Third, it would be beneficial to include patients with more pronounced co-morbidities to test the impact of different ITGA2B gene alleles on perioperative clinical outcome.
In conclusion, our results show that GG polymorphism of platelet ITGA2B gene attenuates the severity of SIRS by decreasing the levels of IL-6 24 hours after operation. Preoperative genotyping for platelet ITGA2B gene might be beneficial in detecting patients with a genetically determined risk for an exaggerated cytokine response.
- Levy JH, Tanaka KA (2003) Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 75: S715–S717.Link: https://goo.gl/R12ggZ
- Drabe N, Zünd G, Grünenfelder J, Sprenger M, Hoerstrup SP, et al. (2001) Genetic predisposition in patients ungergoing cardiopulmonary bypass surgery is associated with an increase of inflammatory cytokines. Eur J Cardiothrac Surg 20: 609–613. Link: https://goo.gl/MkCeKV
- Grünenfelder J, Umbehr M, Plass A, Bestmann L, Maly FE, et al. (2004) Genetic polymorphisms of apolipoprotein E4 and tumor necrosis factor beta as predisposing factors for increased inflammatory cytokines after cardiopulmonary bypass. J Thorac Cardiovasc Surg 128: 92–97. Link: https://goo.gl/mzLUHk
- Ak K, Isbir S, Tekeli A, Ergen A, Atalan N, et al. (20037 Presence of lipoprotein lipase S447X stop codon affects the magnitude of interleukin 8 release after cardiac surgery with cardiopulmonary bypass. J Thorac Cardiovasc Surg 134: 477-483. Link: https://goo.gl/EPmJ1V
- Tetik S, Kaya K, Demir M, Eksioglu-Demiralp E, Yardimci T (2010) Oxidative modification of fibrinogen affects its binding activity to glycoprotein (GP) IIb/IIIa. Clin Appl Thromb Hemost 16: 51-59. Link: https://goo.gl/4CK9lb
- Addad F, Elalamy I, Chakroun T, Abderrazek F, Dridi Z, et al. (2010) Platelet glycoprotein IIIa (platelet antigen 1/platelet antigen 2) polymorphism and 1-year outcome in patients with stable coronary artery disease. Blood Coagul Fibrinolysis 21: 674-678. Link: https://goo.gl/a6D8qG
- Santiago-Germán D, Leaños-Miranda A, García-Latorre E, Borrayo-Sánchez G, Majluf-Cruz A, et al. (2012) Platelet glycoprotein IIIA PIA2 polymorphism is associated with ST elevation acute myocardial infarction in young Mexican population. J Thromb Thrombolysis 33: 389-396. Link: https://goo.gl/ypnLNu
- Shanker J, Gasparyan AY, Kitas GD, Kakkar VV (2011) Platelet function and antiplatelet therapy in cardiovascular disease: implications of genetic polymorphisms. Curr Vasc Pharmacol 9: 479-489. Link: https://goo.gl/kSS9IZ
- Nurden AT (1995) Polymorphisms of human platelet membrane glycoproteins: structure and clinical significance. Thromb Haemost 74: 345-351. Link: https://goo.gl/MiqJnq
- Xu XR, Zhang D, Oswald B, Carrim N, Wang X, et al. (2016) Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci 53: 409-430. Link: https://goo.gl/gUE7Gl
- Hekmat K, Kroener A, Stuetzer H, Schwinger RH, Kampe S, et al. (2005) Daily assessment of organ dysfunction and survival in intensive care unit cardiac surgical patients. Ann Thorac Surg 79: 1555-1562. Link: https://goo.gl/sZO6WY
- Shao H, Shen Y, Liu H, Dong G, Qiang J, et al. (2007) Simvastatin suppresses lung inflammatory response in a rat cardiopulmonary bypass model. Ann Thorac Surg 84: 2011-2018. Link: https://goo.gl/jEDbVU
- Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, et al. (2003) The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation: is atrial fibrillation an inflammatory complication? Circulation 108: II195-199. Link: https://goo.gl/Y58vko
- Jouan J, Golmard L, Benhamouda N, Durrleman N, Golmard JL, et al. (2012) Gene polymorphisms and cytokine plasma levels as predictive factors of complications after cardiopulmonary bypass. J Thorac Cardiovasc Surg 144: 467-473. Link: https://goo.gl/FfPbj7
- Newman PJ, Derbes RS, Aster RH (1989) The human platelet alloantigens, PlA1 and PlA2, are associated with a leucine33/proline33 amino acid polymorphism in membrane glycoprotein IIIa, and are distinguishable by DNA typing. J Clin Invest 83: 1778-1781. Link: https://goo.gl/SPPgo7
- Santiago-Germán D, Leaños-Miranda A, García-Latorre E, Borrayo-Sánchez G, Majluf-Cruz A, et al. (2012) Platelet glycoprotein IIIA PIA2 polymorphism is associated with ST elevation acute myocardial infarction in young Mexican population. J Thromb Thrombolysis 33: 389-396. Link: https://goo.gl/4MR8Bn
- Lekakis J, Bisti S, Tsougos E, Papathanassiou A, Dagres N, et al. (2008) Platelet glycoprotein IIb HPA-3 polymorphism and acute coronary syndromes. Int J Cardiol 127: 46-50. Link: https://goo.gl/U9fbw2
- Esen FI, Hancer VS, Küçükkaya RD, Yeşilot N, Coban O, et al. (2012) Glycoprotein Ib-alpha Kozak polymorphism in ischemic stroke. Neurol Res 34: 68-71. Link: https://goo.gl/EQ25RM
- Pourgheysari B, Boroujeni HR, Hasheminia AM, Drees F (2013) PLA2 polymorphism of platelet glycoprotein IIb/IIIa but not Factor V Leiden and prothrombin G20210A polymorphisms is associated with venous thromboembolism and more recurrent events in central Iran. Blood Coagul Fibrinolysis 24: 471-476. Link: https://goo.gl/H6WSXs
- Knowles JW, Wang H, Itakura H, Southwick A, Myers RM, et al. (2007) Association of polymorphisms in platelet and hemostasis system genes with acute myocardial infarction. Am Heart J 154: 1052-1058. Link: https://goo.gl/hWo7od
- Altinoz H, Ergun S, Ak K, Uyguner ZO, Yardimci T, et al. (2012) C0166 The relationship between platelet glycoprotein IIb RS5911 polymorphism and inflammation in COPD patients. Thrombosis Research 130: S102. Link: https://goo.gl/gwG5qu
- Zimmerman GA (2001) Two by two: the pairings of P-selectin and P-selectin glycoprotein ligand 1. Proc Natl Acad Sci U S A 28: 98: 10023-10024. Link: https://goo.gl/J3i5Qa
- Weyrich AS, Lindemann S, Zimmerman GA (2003) The evolving role of platelets in inflammation. J Thromb Haemost 1: 1897-1905. Link: https://goo.gl/aiXjJZ
- Linden MD, Jackson DE (2010) Platelets: pleiotropic roles in atherogenesis and atherothrombosis. Int J Biochem Cell Biol 42: 1762-1766. Link: https://goo.gl/qPBrJ8
- Kucharska-Newton AM, Monda KL, Campbell S, Bradshaw PT, Wagenknecht LE, et al. (2011) Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 216: 151-156. Link: https://goo.gl/2od3Go
- Faraday N, Martinez EA, Scharpf RB, Kasch-Semenza L, Dorman T, et al. (2004) Platelet gene polymorphisms and cardiac risk assessment in vascular surgical patients. Anesthesiology 101:1291-1297. Link: https://goo.gl/jvPpp5
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
