International Journal of Clinical Endocrinology and Metabolism
Omega-3/omega-6 fatty acids: The effects on the psychophysical well-being of adolescents and adults
Giulio Perrotta*
Cite this as
Perrotta G (2023) Omega-3/omega-6 fatty acids: The effects on the psychophysical well-being of adolescents and adults. Int J Clin Endocrinol Metab 9(1): 008-018. DOI: 10.17352/ijcem.000057Copyright License
© 2023 Perrotta G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Fatty acids of the omega-3/omega-6 groups are used especially in cases of pregnancy, lactation, and malnutrition. In recent decades, pediatrics has been trying to find out whether the use of omega-3/omega-6 has effects on human growth and neurodevelopment.
Aims: Check the state of the art on the use of omega-3/omega-6 type fatty acids in the diet, in adolescent and adult populations.
Materials and methods: A total of 72 original articles on the topic of human growth and nutrition in pediatrics have been selected on PubMed through September 2022.
Results: In literature, the use of omega-3/omega-6, with greater prevalence in the former group than the latter group, appears to be most effective in hypertension hypothesis, dyslipidemia, and high C-reactive protein values, cardiovascular risk, and neuropathic pain, while appearing to have less impact on neurodegenerative (except in multiple sclerosis) and mental disorders (except in depression). Interesting benefits can be detected when combining omega-3/omega-6 with spirulina algae, chitosan, probiotics, vitamin D, fiber and plant extracts.
Conclusion: Significant evidence emerges on the importance of omega-3 and omega-6 fatty acid supplementation, but important structural shortcomings of research designs still emerge from the published studies; moreover, many studies assume that fatty acid supplementation can have a curative effect on already active diseases, when in fact such prescriptions should be considered as adjuvant therapies to prevent or promote symptomatic regression, precisely because of their anti-inflammatory, antioxidant and immunomodulating virtues. However, there is no concrete and robust evidence of the positive impact on psychological well-being. Future research that can resolve the critical issues highlighted is hoped to promote a better approach to the topic of omega-3/omega-6 supplementation in human health.
Background and aims
Omega-3/Omega-6
Fatty acids are aliphatic monocarboxylic acids derived from or contained in esterified form in a vegetable or animal fat, oil, or wax, and are divided into Short-Chain (SCFA), Medium-Chain (MCFA), Long-Chain (LCFA), or Very Long Chain (VLCFA), depending on the number of carbon atoms present [1]. α-linoleic acid (ALA), cervonic or docosahexaenoic acid (DHA), and thymnodonic or Eicosapentaenoic Acid (EPA) are among the major fatty acids of the omega-3 group, which, together with arachidonic acid (AA) of the omega-6 group, are generally considered to be potent anti-inflammatory antioxidants and immunomodulators [2].
Introductory sources can be both animal (animal oils and fats, fish oil, especially cod liver, herring and oily fish, salmon, and in lesser amounts in cod, trout, and human milk) [3] and plant sources (corn seed oil, sunflower oil, nuts, transgenic Camelina sativa seed oil, CSOs [4,5], the blueberries [6] and microalgae [7]), both in natural and synthetic forms; in particular, n-3 PUFAs of marine and plant origin have different effects on erythrocyte fatty acid composition and regulation of glycolipid metabolism [8].
However, the exact dose to be administered has not been determined, although there are studies that emphasize both personalizations of therapy (as is the case with obese people, who may be affected by different assimilation/absorption due to their clinical condition [9]) and use at night, in that in the absence of dietary intake of EPA and DHA, circulating levels of these fatty acids decrease during the nighttime period and reach their lowest point in the morning, and therefore, overnight consumption of n-3 PUFAs, which counteracts this pattern, may have functional significance [10]. One study went on to focus on the assumption that Omega-3 (n-3) Fatty Acid (FA) supplements increase blood concentrations of EPA and DHA and that most supplements on the market are esterified to Triglycerides (TG) or Ethyl Esters (EE), which limits their absorption and may cause gastrointestinal side effects. With this in mind, and intending to compare the 24-hour plasma concentrations of EPA, DHA, and EPA+DHA when provided esterified in Monoglycerides (MAG), this study showed that the plasma concentration of n-3 FA in adults is higher after acute supplementation with n-3 FA esterified in MAG than in EE or TG, suggesting that with a lower dose of n-3 FA MAG, the plasma concentrations of n-3 FA achieved are similar to those achieved after higher doses of n-3 FA esterified in EE or TG [11].
Omega-3/omega-6 in adolescent and adult populations
In the literature, most studies published on the adolescent population focus on allergic disease and metabolic disorders such as diabetes (and its consequences), unlike the adult population, which also broadens the audience to include autoimmune diseases, neurodegenerative disorders, inflammatory and allergic disorders, and many others.
Specifically, in the obese adolescent (as well as in the adult) it has been shown that LCPUFA-ω3 supplementation does not affect body weight [12], although it results in improved muscle tone [13,14] (while reducing linoleylcarnitine [15]) and significant platelet aggregation [16]; on insulin values, studies are conflicting [12,17], however, if omega-3 is combined with acetylsalicylic acid there is an improvement in parameters in diabetes mellitus [18], even in the presence of Duchenne dystrophy [19].
The use of omega-3/omega-6 would also appear to be effective in regulating the effects of metabolic changes that lead to obesity [20], high blood pressure, and dyslipidemia (with little evidence regarding the impact on liver fat [21] (with a preference for DHA + EPA over ALA) [22], unless it is non-alcoholic fatty liver disease [23,24] (in which case it is suggested to add vitamin d3 to the omega-3 formulation [25]).
It has also been shown that the consumption of seed oils high in omega-6 polyunsaturated fat (PUFA), and linoleic acid (LA), contributes to low-grade inflammation, oxidative stress, endothelial dysfunction, and atherosclerosis [26] and not surprisingly, a low serum level of arachidonic acid (AA) was instead associated with an unfavorable functional outcome in patients with acute intracerebral hemorrhage [27].
Recent studies have shown that the combined dietary supplement of DHA / EPA improves the status of triglycerides and HDL, but can increase LDL levels compared to acid α-lipoic acid (ALA) if a low n-6 / n-3 ratio is not maintained [28]. Specifically, PUFAs with a low n-6 / n-3 ratio have been shown to significantly reduce triglyceride concentrations and increase HDL-cholesterol concentrations, while plant-derived n-3 PUFAs significantly reduce total cholesterol and LDL-cholesterol concentrations, and EPA and DHA n-3 PUFAs significantly reduce triglyceride concentrations and increase HDL-C concentrations [29].
Cardiac and vascular diseases have been studied for decades concerning the use of omega-3/omega-6, showing efficacy in cardiovascular risk, atherosclerosis, cardiac arrhythmic disorders, and cardiac ischemic forms, with greater preference for ALA over DHA and EPA and the latter over DHA, although there is a risk of increased fibrous plugin coronary atherosclerotic plaques, but only if supplemental omega-3 intake is < 3.5%, discouraging the combination of DHA + EPA + Acetylsalicylic Acid (although there is discordance) as capable of affecting cyclooxygenase activity in platelets [18,30-40].
Inflammatory diseases, which have high C-reactive protein values, also find benefits when treated with omega-3/omega-6 supplementation, demonstrating greater efficacy of EPA over DHA [41,42].
The therapeutic utility of omega-3/omega-6 use has also been demonstrated concerning the improvement of allergic symptoms of a muscular-tensive, neuropathic [43,44] or autoimmune nature from rheumatoid arthritis [16], and in cystic fibrosis [45], periodontitis [46], sperm motility [47], of male hypotestosteronism in overweight or obese individuals (but only when supplementing with DHA) [48] and the risk of preeclampsia [49,50] and placental disorders, but only in low-risk pregnancies and if supplementation occurs early in pregnancy and not late [51], as well as slowing the outcomes of maculopathy [52] and the disabling symptoms of dry eye syndrome [53].
Concerning about cognitive performance and psychological stability, there are encouraging results in the literature with respect to cognitive function [54] (although it would appear that DHA supplementation has efficacy on attention in ADHD [55-57], EPA supplementation has efficacy on long-term memory, working memory, and problem solving function [58,59], while DHA+EPA has efficacy on executive functions in Alzheimer-type dementia [60]), especially if DHA/EPA supplementation is combined with curcumin [61] and piperine [62]; on the other hand, the positive effect on psychiatric symptoms (anxiety, obsessive, depressive, psychotic and nonpsychotic in bipolar and eating disorders, with a higher positive EPA>DHA ratio for depressive symptoms) [63-80] and neurodegenerative disorders (such as Alzheimer’s and Multiple Sclerosis) [81-88], which might be affected by the placebo effect or a slight improvement brought about by the anti-inflammatory and oxidative effects of the administered fatty acids, appears less encouraging.
Materials and methods
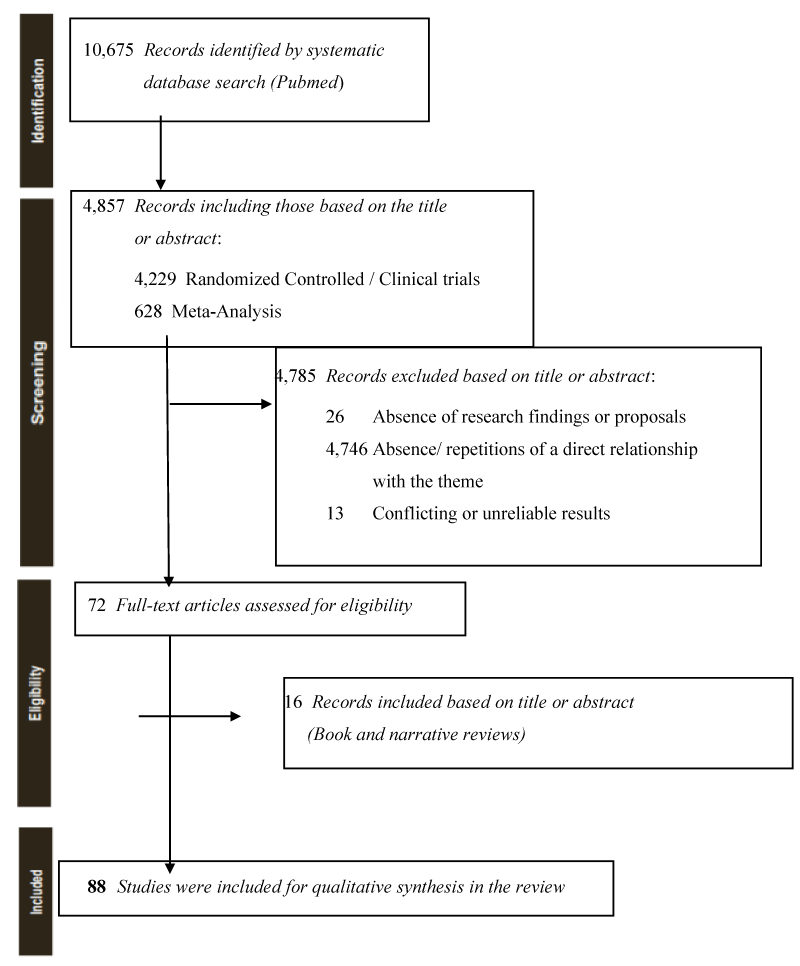
We searched in PubMed Central, Internet Archive Scholar, CORE, CiteSeerX, Semantic Scholar, and Europe PMC, until September 30, 2022, for meta-analyses, clinical trials, and randomized controlled trials, using the keyword “omega-3/omega-6 fatty acids”, “DHA/EPA/ALA/AA”, “adolescent” and “adult”, content on the abstract and title, have been selected 10,675 useful results, of which 72 original articles were used for the present review as they focused on the topics of growth and neurodevelopment. A single reference (book) related to the analyzed topic from sources outside PubMed was added in the first note. Simple reviews, opinion contributions, or publications in popular volumes were excluded because they were irrelevant or redundant for this paper, and publications that did not present results or statistical samples but only research protocols and proposals, those that did not specifically address the topic of investigation, those with contradictory data, unreliable data, or otherwise with a deficient research design. The search was limited to English-language articles. No limit was placed on the year of publication, covering the time window from 1979 to the present period Figure 1.
Results, discussion and limitations
In the medical literature, omega-3/omega-6 supplementation is advisable, with proper precautions and specific purposes, although to date, the reference dietary intakes have not yet been established with certainty, and published studies have important structural shortcomings, such as frequent small sample sizes for each category evaluated, questionable quality of included studies, and technical error, as well as the noncomparability of blood levels of omega-3 long-chain polyunsaturated fatty acids and the possible influence of genetic factors (such as in the case of the presence of the APOEɛ4 -APOE4-allele that accelerates the oxidation of omega-3 polyunsaturated fatty acids -PUFAs- or in the hypothesis of the minor allele of rs3834458 in FADS2 that results in lower delta-6 desaturase activity leading to increased ALA and decreased EPA, DPA and DHA in the blood) and environmental. These limitations could qualitatively affect the conclusive results.
The use of omega-3/omega-6, with greater prevalence in the former group than the latter group, appears to be most effective in hypertension hypothesis, dyslipidemia, and high C-reactive protein values, although there is still debate about efficacy on inflammatory factors such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α.
The impact on cardiovascular risk brought about by the addition of omega-3 to statin therapy also appears to be nonsignificant, although studies often consider only high-risk patients and not all other subjects as well, according to a rationale opposite to prevention, in fact leading to a potential bias that would lead one to believe on their no efficacy, when in fact they could have a preventive efficacy (even reduced or potential) on cardiovascular risk. In particular, studies show differentiation by type of morbid condition, modulating the prescription according to the specific clinical profile of the patient, since fatty acids of animal origin do not have the same function and effectiveness as those of marine and plant origin; in fact: if the former are more effective in reducing systolic blood pressure and dyslipidemia, the latter are better suited to intervene in erythrocyte fatty acid composition and regulation of glycolipid metabolism; if DHA has a greater anti-inflammatory effect, on the metabolic and nervous system, EPA has greater impact in the cardiovascular system and depressive and mood symptoms, but their DHA + EPA combination may not be suitable in the presence of coronary atherosclerotic plaques (as long as a low n-6/n-3 ratio is maintained), hypercholesterolemia with high LDL, coronary ischemic heart disease, and the presence of cardiac arrhythmias, and in such cases ALA supplementation is better; DHA + EPA + aspirin is always discouraged, because they might affect the activity of cyclooxygenase in platelets, and promote thrombotic episodes, in more predisposed individuals (smoking, obesity, dyslipidemia, genetic thrombophilia, and taking birth control pills).
In obesity then, the clinical picture becomes more complicated, as the factors at play are multiple, complex, and interlinked, although one study has nonetheless demonstrated a clinical benefit in the administration of DHA+EPA, as well as the use of spirulina algae, chitosan, probiotics, vitamin D, fiber, and plant extracts, to promote moderate weight loss, under controlled dietary intake.
The same discourse can also be applied to psychiatric symptomatology, which is affected as much by exact drug therapy as by the exact daily intake of the omega-3 supplement taken (as there is high subjective variability in the response to the supplement in erythrocytes plasma, and whole blood depending on different doses), but also to neurodegenerative diseases such as Alzheimer’s, which appear to be unaffected by DHA/EPA supplementation as much as glucose-insulin ratio, malnutrition, and hypovitaminosis; the exception appears to be Multiple Sclerosis, in which omega-3 and fish oil supplementation have beneficial effects on reducing relapse rates, inflammatory markers, and improving quality of life. Evidence on psychological well-being is not robust, although there are studies already cited that point to secondary effects in certain neurodegenerative pathologies and in anxiety and mood states Table 1.
Conclusion
Significant evidence emerges regarding the importance of omega-3 and omega-6 fatty acid supplementation in pregnancy and lactation, malnutrition states, inflammatory diseases, cardiac and vascular risk, neurodegenerative disorders, and mental disorders. However, while several findings are promising, important structural shortcomings of the research designs still emerge from the published studies; moreover, many studies assume that fatty acid supplementation can have a curative effect on already active diseases, when in fact such prescriptions should be considered as adjuvant therapies to prevent or promote symptomatic regression, precisely because of their anti-inflammatory, antioxidant and immunomodulatory virtues. Such findings could be capable of undermining the research results. However, there is no concrete and robust evidence of the positive impact on psychological well-being. Future research that can resolve the critical issues noted is hoped, to promote a better approach to the topic of omega-3/omega-6 supplementation, in human health.
- Arienti G. Le basi molecolari della nutrizione. Piccin. 2003.
- Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002 Oct;56(8):365-79. doi: 10.1016/s0753-3322(02)00253-6. PMID: 12442909.
- Yang S, Lin R, Si L, Li Z, Jian W, Yu Q, Jia Y. Cod-Liver Oil Improves Metabolic Indices and hs-CRP Levels in Gestational Diabetes Mellitus Patients: A Double-Blind Randomized Controlled Trial. J Diabetes Res. 2019 Dec 28;2019:7074042. doi: 10.1155/2019/7074042. PMID: 31956660; PMCID: PMC6949680.
- West AL, Miles EA, Lillycrop KA, Han L, Sayanova O, Napier JA, Calder PC, Burdge GC. Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br J Nutr. 2019 Jun;121(11):1235-1246. doi: 10.1017/S0007114519000825. Epub 2019 Apr 12. PMID: 30975228; PMCID: PMC6658215.
- Schwab US, Lankinen MA, de Mello VD, Manninen SM, Kurl S, Pulkki KJ, Laaksonen DE, Erkkilä AT. Camelina Sativa Oil, but not Fatty Fish or Lean Fish, Improves Serum Lipid Profile in Subjects with Impaired Glucose Metabolism-A Randomized Controlled Trial. Mol Nutr Food Res. 2018 Feb;62(4). doi: 10.1002/mnfr.201700503. Epub 2018 Jan 31. PMID: 29272068.
- McNamara RK, Kalt W, Shidler MD, McDonald J, Summer SS, Stein AL, Stover AN, Krikorian R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol Aging. 2018 Apr;64:147-156. doi: 10.1016/j.neurobiolaging.2017.12.003. Epub 2017 Dec 12. PMID: 29458842; PMCID: PMC5822748.
- Dawczynski C, Dittrich M, Neumann T, Goetze K, Welzel A, Oelzner P, Völker S, Schaible AM, Troisi F, Thomas L, Pace S, Koeberle A, Werz O, Schlattmann P, Lorkowski S, Jahreis G. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin Nutr. 2018 Apr;37(2):494-504. doi: 10.1016/j.clnu.2017.02.021. Epub 2017 Mar 6. PMID: 28302406.
- Liu H, Wang F, Liu X, Xie Y, Xia H, Wang S, Sun G. Effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acids on erythrocyte fatty acid composition in type 2 diabetic patients. Lipids Health Dis. 2022 Feb 10;21(1):20. doi: 10.1186/s12944-022-01630-0. PMID: 35144649; PMCID: PMC8832668.
- Fisk HL, Childs CE, Miles EA, Ayres R, Noakes PS, Paras-Chavez C, Kuda O, Kopecký J, Antoun E, Lillycrop KA, Calder PC. Modification of subcutaneous white adipose tissue inflammation by omega-3 fatty acids is limited in human obesity-a double blind, randomised clinical trial. EBioMedicine. 2022 Mar;77:103909. doi: 10.1016/j.ebiom.2022.103909. Epub 2022 Mar 2. PMID: 35247847; PMCID: PMC8894262.
- Jackson PA, Husberg C, Hustvedt SO, Calder PC, Khan J, Avery H, Forster J, Kennedy DO. Diurnal rhythm of plasma EPA and DHA in healthy adults. Prostaglandins Leukot Essent Fatty Acids. 2020 Mar;154:102054. doi: 10.1016/j.plefa.2020.102054. Epub 2020 Jan 15. PMID: 32018166.
- Chevalier L, Vachon A, Plourde M. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J Nutr. 2021 May 11;151(5):1111-1118. doi: 10.1093/jn/nxaa458. PMID: 33564872; PMCID: PMC8112767.
- Chevalier L, Vachon A, Plourde M. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J Nutr. 2021 May 11;151(5):1111-1118. doi: 10.1093/jn/nxaa458. PMID: 33564872; PMCID: PMC8112767.
- Rondanelli M, Perna S, Riva A, Petrangolini G, Di Paolo E, Gasparri C. Effects of n-3 EPA and DHA supplementation on fat free mass and physical performance in elderly. A systematic review and meta-analysis of randomized clinical trial. Mech Ageing Dev. 2021 Jun;196:111476. doi: 10.1016/j.mad.2021.111476. Epub 2021 Mar 26. PMID: 33781784.
- Rondanelli M, Perna S, Riva A, Petrangolini G, Di Paolo E, Gasparri C. Effects of n-3 EPA and DHA supplementation on fat free mass and physical performance in elderly. A systematic review and meta-analysis of randomized clinical trial. Mech Ageing Dev. 2021 Jun;196:111476. doi: 10.1016/j.mad.2021.111476. Epub 2021 Mar 26. PMID: 33781784.
- Guo XF, Tong WF, Ruan Y, Sinclair AJ, Li D. Different metabolism of EPA, DPA and DHA in humans: A double-blind cross-over study. Prostaglandins Leukot Essent Fatty Acids. 2020 Jul;158:102033. doi: 10.1016/j.plefa.2019.102033. Epub 2019 Nov 12. PMID: 31740197.
- Tomic-Smiljanic M, Vasiljevic D, Lucic-Tomic A, Andjelkovic N, Jakovljevic V, Bolovich S, Veselinovic M. Influence of different supplementation on platelet aggregation in patients with rheumatoid arthritis. Clin Rheumatol. 2019 Sep;38(9):2443-2450. doi: 10.1007/s10067-019-04569-3. Epub 2019 May 10. PMID: 31076942.
- Abbott KA, Burrows TL, Acharya S, Thota RN, Garg ML. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot Essent Fatty Acids. 2020 Aug;159:102154. doi: 10.1016/j.plefa.2020.102154. Epub 2020 Jun 13. PMID: 32563863.
- Holub A, Mousa S, Abdolahi A, Godugu K, Tu XM, Brenna JT, Block RC. The effects of aspirin and N-3 fatty acids on telomerase activity in adults with diabetes mellitus. Nutr Metab Cardiovasc Dis. 2020 Sep 24;30(10):1795-1799. doi: 10.1016/j.numecd.2020.06.014. Epub 2020 Jun 25. PMID: 32723580; PMCID: PMC7494550.
- Holub A, Mousa S, Abdolahi A, Godugu K, Tu XM, Brenna JT, Block RC. The effects of aspirin and N-3 fatty acids on telomerase activity in adults with diabetes mellitus. Nutr Metab Cardiovasc Dis. 2020 Sep 24;30(10):1795-1799. doi: 10.1016/j.numecd.2020.06.014. Epub 2020 Jun 25. PMID: 32723580; PMCID: PMC7494550.
- González-Becerra K, Ramos-Lopez O, Barrón-Cabrera E, Riezu-Boj JI, Milagro FI, Martínez-López E, Martínez JA. Fatty acids, epigenetic mechanisms and chronic diseases: a systematic review. Lipids Health Dis. 2019 Oct 15;18(1):178. doi: 10.1186/s12944-019-1120-6. PMID: 31615571; PMCID: PMC6792183.
- Parker HM, Cohn JS, O'Connor HT, Garg ML, Caterson ID, George J, Johnson NA. Effect of Fish Oil Supplementation on Hepatic and Visceral Fat in Overweight Men: A Randomized Controlled Trial. Nutrients. 2019 Feb 23;11(2):475. doi: 10.3390/nu11020475. PMID: 30813440; PMCID: PMC6413081.
- Zhou Q, Zhang Z, Wang P, Zhang B, Chen C, Zhang C, Su Y. EPA+DHA, but not ALA, Improved Lipids and Inflammation Status in Hypercholesterolemic Adults: A Randomized, Double-Blind, Placebo-Controlled Trial. Mol Nutr Food Res. 2019 May;63(10):e1801157. doi: 10.1002/mnfr.201801157. Epub 2019 Apr 23. Erratum in: Mol Nutr Food Res. 2020 Apr;64(7):e2070012. PMID: 30900815.
- Song L, Zhao XG, Ouyang PL, Guan Q, Yang L, Peng F, Du H, Yin F, Yan W, Yu WJ, Yan H. Combined effect of n-3 fatty acids and phytosterol esters on alleviating hepatic steatosis in non-alcoholic fatty liver disease subjects: a double-blind placebo-controlled clinical trial. Br J Nutr. 2020 May 28;123(10):1148-1158. doi: 10.1017/S0007114520000495. Epub 2020 Feb 14. PMID: 32054543.
- Guo XF, Wang C, Yang T, Ma WJ, Zhai J, Zhao T, Xu TC, Li J, Liu H, Sinclair AJ, Li D. The effects of fish oil plus vitamin D3 intervention on non-alcoholic fatty liver disease: a randomized controlled trial. Eur J Nutr. 2022 Jun;61(4):1931-1942. doi: 10.1007/s00394-021-02772-0. Epub 2022 Jan 24. PMID: 35067753.
- Yang J, Fernández-Galilea M, Martínez-Fernández L, González-Muniesa P, Pérez-Chávez A, Martínez JA, Moreno-Aliaga MJ. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients. 2019 Apr 18;11(4):872. doi: 10.3390/nu11040872. PMID: 31003450; PMCID: PMC6521137.
- Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: implications for atherosclerosis. J Am Coll Nutr. 2001 Apr;20(2 Suppl):97-105. doi: 10.1080/07315724.2001.10719021. PMID: 11349944.
- Takahashi J, Sakai K, Sato T, Takatsu H, Komatsu T, Mitsumura H, Murakami H, Iguchi Y. Serum arachidonic acid levels is a predictor of poor functional outcome in acute intracerebral hemorrhage. Clin Biochem. 2021 Dec;98:42-47. doi: 10.1016/j.clinbiochem.2021.09.012. Epub 2021 Oct 6. PMID: 34624254.
- Chen H, Deng G, Zhou Q, Chu X, Su M, Wei Y, Li L, Zhang Z. Effects of eicosapentaenoic acid and docosahexaenoic acid versus α-linolenic acid supplementation on cardiometabolic risk factors: a meta-analysis of randomized controlled trials. Food Funct. 2020 Mar 26;11(3):1919-1932. doi: 10.1039/c9fo03052b. PMID: 32175534.
- Li N, Jia M, Deng Q, Wang Z, Huang F, Hou H, Xu T. Effect of low-ratio n-6/n-3 PUFA on blood lipid level: a meta-analysis. Hormones (Athens). 2021 Dec;20(4):697-706. doi: 10.1007/s42000-020-00248-0. Epub 2020 Oct 29. PMID: 33123975.
- Du Y, Taylor CG, Zahradka P. Modulation of endothelial cell responses and vascular function by dietary fatty acids. Nutr Rev. 2019 Jun 22:nuz026. doi: 10.1093/nutrit/nuz026. Epub ahead of print. PMID: 31228246.
- Chen H, Deng G, Zhou Q, Chu X, Su M, Wei Y, Li L, Zhang Z. Effects of eicosapentaenoic acid and docosahexaenoic acid versus α-linolenic acid supplementation on cardiometabolic risk factors: a meta-analysis of randomized controlled trials. Food Funct. 2020 Mar 26;11(3):1919-1932. doi: 10.1039/c9fo03052b. PMID: 32175534.
- Guo XF, Li KL, Li JM, Li D. Effects of EPA and DHA on blood pressure and inflammatory factors: a meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2019;59(20):3380-3393. doi: 10.1080/10408398.2018.1492901. Epub 2019 Feb 4. PMID: 29993265.
- Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K, Nissen SE. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA. 2020 Dec 8;324(22):2268-2280. doi: 10.1001/jama.2020.22258. PMID: 33190147; PMCID: PMC7667577.
- Khan SU, Lone AN, Khan MS, Virani SS, Blumenthal RS, Nasir K, Miller M, Michos ED, Ballantyne CM, Boden WE, Bhatt DL. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine. 2021 Jul 8;38:100997. doi: 10.1016/j.eclinm.2021.100997. PMID: 34505026; PMCID: PMC8413259.
- Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KH, Summerbell CD, Worthington HV, Song F, Hooper L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2020 Feb 29;3(3):CD003177. doi: 10.1002/14651858.CD003177.pub5. PMID: 32114706; PMCID: PMC7049091.
- Kita Y, Watanabe M, Kamon D, Ueda T, Soeda T, Okayama S, Ishigami K, Kawata H, Horii M, Inoue F, Doi N, Okura H, Uemura S, Saito Y. Effects of Fatty Acid Therapy in Addition to Strong Statin on Coronary Plaques in Acute Coronary Syndrome: An Optical Coherence Tomography Study. J Am Heart Assoc. 2020 Aug 18;9(16):e015593. doi: 10.1161/JAHA.119.015593. Epub 2020 Aug 1. PMID: 32805184; PMCID: PMC7660823.
- Alfaddagh A, Elajami TK, Saleh M, Mohebali D, Bistrian BR, Welty FK. An omega-3 fatty acid plasma index ≥4% prevents progression of coronary artery plaque in patients with coronary artery disease on statin treatment. Atherosclerosis. 2019 Jun;285:153-162. doi: 10.1016/j.atherosclerosis.2019.04.213. Epub 2019 Apr 13. PMID: 31055222; PMCID: PMC7963401.
- Xu B, Xu Z, Xu D, Tan X. Effect of n-3 polyunsaturated fatty acids on ischemic heart disease and cardiometabolic risk factors: a two-sample Mendelian randomization study. BMC Cardiovasc Disord. 2021 Nov 8;21(1):532. doi: 10.1186/s12872-021-02342-6. PMID: 34749668; PMCID: PMC8576934.
- Block RC, Shearer GC, Holub A, Tu XM, Mousa S, Brenna JT, Harris WS, Tintle N. Aspirin and omega-3 fatty acid status interact in the prevention of cardiovascular diseases in Framingham Heart Study. Prostaglandins Leukot Essent Fatty Acids. 2021 Jun;169:102283. doi: 10.1016/j.plefa.2021.102283. Epub 2021 Apr 24. PMID: 33964664; PMCID: PMC8159885.
- Gallini A, Yrondi A, Cantet C, Poncet M, Vellas B, Schmitt L, Andrieu S. Red Blood Cell Omega-3 Fatty Acid Composition and Psychotropic Drug Use in Older Adults: Results from the MAPT Study. J Nutr Health Aging. 2019;23(9):805-812. doi: 10.1007/s12603-019-1252-4. PMID: 31641729..
- MacIntosh BA, Ramsden CE, Honvoh G, Faurot KR, Palsson OS, Johnston AD, Lynch C, Anderson P, Igudesman D, Zamora D, Horowitz M, Gaylord S, Mann JD. Methodology for altering omega-3 EPA+DHA and omega-6 linoleic acid as controlled variables in a dietary trial. Clin Nutr. 2021 Jun;40(6):3859-3867. doi: 10.1016/j.clnu.2021.04.050. Epub 2021 May 12. PMID: 34130033; PMCID: PMC8293619.
- Ramsden CE, Zamora D, Faurot KR, MacIntosh B, Horowitz M, Keyes GS, Yuan ZX, Miller V, Lynch C, Honvoh G, Park J, Levy R, Domenichiello AF, Johnston A, Majchrzak-Hong S, Hibbeln JR, Barrow DA, Loewke J, Davis JM, Mannes A, Palsson OS, Suchindran CM, Gaylord SA, Mann JD. Dietary alteration of n-3 and n-6 fatty acids for headache reduction in adults with migraine: randomized controlled trial. BMJ. 2021 Jun 30;374:n1448. doi: 10.1136/bmj.n1448. PMID: 34526307; PMCID: PMC8244542.
- Galán-Arriero I, Serrano-Muñoz D, Gómez-Soriano J, Goicoechea C, Taylor J, Velasco A, Ávila-Martín G. The role of Omega-3 and Omega-9 fatty acids for the treatment of neuropathic pain after neurotrauma. Biochim Biophys Acta Biomembr. 2017 Sep;1859(9 Pt B):1629-1635. doi: 10.1016/j.bbamem.2017.05.003. Epub 2017 May 8. PMID: 28495596.
- Pham TL, Bazan HEP. Docosanoid signaling modulates corneal nerve regeneration: effect on tear secretion, wound healing, and neuropathic pain. J Lipid Res. 2021;62:100033. doi: 10.1194/jlr.TR120000954. Epub 2021 Feb 6. PMID: 32788291; PMCID: PMC7933495.
- Watson H, Stackhouse C. Omega-3 fatty acid supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2020 Apr 10;4(4):CD002201. doi: 10.1002/14651858.CD002201.pub6. PMID: 32275788; PMCID: PMC7147930.
- Stańdo M, Piatek P, Namiecinska M, Lewkowicz P, Lewkowicz N. Omega-3 Polyunsaturated Fatty Acids EPA and DHA as an Adjunct to Non-Surgical Treatment of Periodontitis: A Randomized Clinical Trial. Nutrients. 2020 Aug 27;12(9):2614. doi: 10.3390/nu12092614. PMID: 32867199; PMCID: PMC7551834.
- Hosseini B, Nourmohamadi M, Hajipour S, Taghizadeh M, Asemi Z, Keshavarz SA, Jafarnejad S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-analysis. J Diet Suppl. 2019;16(2):245-256. doi: 10.1080/19390211.2018.1431753. Epub 2018 Feb 16. PMID: 29451828.
- Hosseini B, Nourmohamadi M, Hajipour S, Taghizadeh M, Asemi Z, Keshavarz SA, Jafarnejad S. The Effect of Omega-3 Fatty Acids, EPA, and/or DHA on Male Infertility: A Systematic Review and Meta-analysis. J Diet Suppl. 2019;16(2):245-256. doi: 10.1080/19390211.2018.1431753. Epub 2018 Feb 16. PMID: 29451828.
- Zhou SJ, Yelland L, McPhee AJ, Quinlivan J, Gibson RA, Makrides M. Fish-oil supplementation in pregnancy does not reduce the risk of gestational diabetes or preeclampsia. Am J Clin Nutr. 2012 Jun;95(6):1378-84. doi: 10.3945/ajcn.111.033217. Epub 2012 May 2. PMID: 22552037.
- Bakouei F, Delavar MA, Mashayekh-Amiri S, Esmailzadeh S, Taheri Z. Efficacy of n-3 fatty acids supplementation on the prevention of pregnancy induced-hypertension or preeclampsia: A systematic review and meta-analysis. Taiwan J Obstet Gynecol. 2020 Jan;59(1):8-15. doi: 10.1016/j.tjog.2019.11.002. PMID: 32039806.
- Carvajal JA. Docosahexaenoic acid supplementation early in pregnancy may prevent deep placentation disorders. Biomed Res Int. 2014;2014:526895. doi: 10.1155/2014/526895. Epub 2014 Jun 12. PMID: 25019084; PMCID: PMC4082939.
- Zhong Y, Wang K, Jiang L, Wang J, Zhang X, Xu J, Yao K. Dietary fatty acid intake, plasma fatty acid levels, and the risk of age-related macular degeneration (AMD): a dose-response meta-analysis of prospective cohort studies. Eur J Nutr. 2021 Sep;60(6):3013-3027. doi: 10.1007/s00394-020-02445-4. Epub 2021 Jan 19. PMID: 33469697.
- Downie LE, Ng SM, Lindsley KB, Akpek EK. Omega-3 and omega-6 polyunsaturated fatty acids for dry eye disease. Cochrane Database Syst Rev. 2019 Dec 18;12(12):CD011016. doi: 10.1002/14651858.CD011016.pub2. PMID: 31847055; PMCID: PMC6917524.
- van der Wurff ISM, Meyer BJ, de Groot RHM. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (n-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients. 2020 Oct 12;12(10):3115. doi: 10.3390/nu12103115. PMID: 33053843; PMCID: PMC7599612.
- van der Wurff ISM, Meyer BJ, de Groot RHM. Effect of Omega-3 Long Chain Polyunsaturated Fatty Acids (n-3 LCPUFA) Supplementation on Cognition in Children and Adolescents: A Systematic Literature Review with a Focus on n-3 LCPUFA Blood Values and Dose of DHA and EPA. Nutrients. 2020 Oct 12;12(10):3115. doi: 10.3390/nu12103115. PMID: 33053843; PMCID: PMC7599612.
- Checa-Ros A, Haro-García A, Seiquer I, Molina-Carballo A, Uberos-Fernández J, Muñoz-Hoyos A. Early monitoring of fatty acid profile in children with attention deficit and/or hyperactivity disorder under treatment with omega-3 polyunsaturated fatty acids. Minerva Pediatr. 2019 Aug;71(4):313-325. doi: 10.23736/S0026-4946.18.04975-7. Epub 2018 Nov 7. PMID: 30419741.
- Perrotta G. Attention Deficit Hyperactivity Disorder: definition, contexts, neural correlates and clinical strategies. J Addi Adol Beh. 2019; 2(1). doi: 10.31579-007/2688-7517/08.
- Emery S, Häberling I, Berger G, Walitza S, Schmeck K, Albert T, Baumgartner N, Strumberger M, Albermann M, Drechsler R. Omega-3 and its domain-specific effects on cognitive test performance in youths: A meta-analysis. Neurosci Biobehav Rev. 2020 May;112:420-436. doi: 10.1016/j.neubiorev.2020.02.016. Epub 2020 Feb 15. PMID: 32070694.
- Perrotta G. Executive functions: definition, contexts and neuropsychological profiles. J. Neuroscience and Neurological. 2019; 4(3). doi: 10.31579/2578-8868/077.
- Kosti RI, Kasdagli MI, Kyrozis A, Orsini N, Lagiou P, Taiganidou F, Naska A. Fish intake, n-3 fatty acid body status, and risk of cognitive decline: a systematic review and a dose-response meta-analysis of observational and experimental studies. Nutr Rev. 2022 May 9;80(6):1445-1458. doi: 10.1093/nutrit/nuab078. PMID: 34605891.
- Kuszewski JC, Howe PRC, Wong RHX. Evaluation of Cognitive Performance following Fish-Oil and Curcumin Supplementation in Middle-Aged and Older Adults with Overweight or Obesity. J Nutr. 2020 Dec 10;150(12):3190-3199. doi: 10.1093/jn/nxaa299. PMID: 33097947.
- Khairani S, Fauziah N, Wiraswati HL, Panigoro R, Setyowati EY, Berbudi A. The Potential use of a Curcumin-Piperine Combination as an Antimalarial Agent: A Systematic Review. J Trop Med. 2021 Oct 11;2021:9135617. doi: 10.1155/2021/9135617. PMID: 34671402; PMCID: PMC8523290.
- Liao Y, Xie B, Zhang H, He Q, Guo L, Subramanieapillai M, Fan B, Lu C, McIntyre RS. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl Psychiatry. 2019 Aug 5;9(1):190. doi: 10.1038/s41398-019-0515-5. Erratum in: Transl Psychiatry. 2021 Sep 7;11(1):465. PMID: 31383846; PMCID: PMC6683166.
- van der Burg KP, Cribb L, Firth J, Karmacoska D, Mischoulon D, Byrne GJ, Bousman C, Stough C, Murphy J, Oliver G, Berk M, Ng CH, Sarris J. EPA and DHA as markers of nutraceutical treatment response in major depressive disorder. Eur J Nutr. 2020 Sep;59(6):2439-2447. doi: 10.1007/s00394-019-02090-6. Epub 2019 Sep 25. PMID: 31555976.
- Guu TW, Mischoulon D, Sarris J, Hibbeln J, McNamara RK, Hamazaki K, Freeman MP, Maes M, Matsuoka YJ, Belmaker RH, Jacka F, Pariante C, Berk M, Marx W, Su KP. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother Psychosom. 2019;88(5):263-273. doi: 10.1159/000502652. Epub 2019 Sep 3. PMID: 31480057.
- Robinson DG, Gallego JA, John M, Hanna LA, Zhang JP, Birnbaum ML, Greenberg J, Naraine M, Peters BD, McNamara RK, Malhotra AK, Szeszko PR. A potential role for adjunctive omega-3 polyunsaturated fatty acids for depression and anxiety symptoms in recent onset psychosis: Results from a 16 week randomized placebo-controlled trial for participants concurrently treated with risperidone. Schizophr Res. 2019 Feb;204:295-303. doi: 10.1016/j.schres.2018.09.006. Epub 2018 Sep 19. PMID: 30241990; PMCID: PMC6402999.
- Li W, Lei D, Tallman MJ, Patino LR, Gong Q, Strawn JR, DelBello MP, McNamara RK. Emotion-Related Network Reorganization Following Fish Oil Supplementation in Depressed Bipolar Offspring: An fMRI Graph-Based Connectome Analysis. J Affect Disord. 2021 Sep 1;292:319-327. doi: 10.1016/j.jad.2021.05.086. Epub 2021 Jun 5. PMID: 34139404; PMCID: PMC8282765.
- McPhilemy G, Byrne F, Waldron M, Hibbeln JR, Davis J, McDonald C, Hallahan B. A 52-week prophylactic randomised control trial of omega-3 polyunsaturated fatty acids in bipolar disorder. Bipolar Disord. 2021 Nov;23(7):697-706. doi: 10.1111/bdi.13037. Epub 2021 Jan 10. PMID: 33340432.
- Zhang MM, Zou Y, Li SM, Wang L, Sun YH, Shi L, Lu L, Bao YP, Li SX. The efficacy and safety of omega-3 fatty acids on depressive symptoms in perinatal women: a meta-analysis of randomized placebo-controlled trials. Transl Psychiatry. 2020 Jun 17;10(1):193. doi: 10.1038/s41398-020-00886-3. PMID: 32555188; PMCID: PMC7299975.
- Satogami K, Tseng PT, Su KP, Takahashi S, Ukai S, Li DJ, Chen TY, Lin PY, Chen YW, Matsuoka YJ. Relationship between polyunsaturated fatty acid and eating disorders: Systematic review and meta-analysis. Prostaglandins Leukot Essent Fatty Acids. 2019 Mar;142:11-19. doi: 10.1016/j.plefa.2019.01.001. Epub 2019 Jan 11. PMID: 30773209..
- Perrotta G. Anxiety disorders: definitions, contexts, neural correlates and strategic therapy. J Neurol Neurosci. 2019; 6(1):046.
- Perrotta G. Neural correlates in eating disorders: Definition, contexts and clinical strategies. J Pub Health Catalog. 2019; 2(2):137-148.
- Perrotta G. Post-traumatic stress disorder: Definition, contexts, neural correlations and cognitive-behavioural therapy. J Pub Health Catalog. 2019; 2(2):40-7.
- Perrotta G. Sleep-wake disorders: Definition, contexts and neural correlations. J Neurol Psychol. 2019; 7(1):09.
- Perrotta G. Depressive disorders: Definitions, contexts, differential diagnosis, neural correlates and clinical strategies. Arch Depress Anxiety. 2019; 5(2):009-033, DOI: 10.17352/2455-5460.000038.
- Perrotta G. Panic disorder: definitions, contexts, neural correlates and clinical strategies. Curr Tr Clin & Med Sci. 2019; 1(2).CTCMS.MS.ID.000508.
- Perrotta G. Obsessive-Compulsive Disorder: definition, contexts, neural correlates and clinical strategies. Journal of Neurology. 2019; 08-16.
- Perrotta G. Behavioral addiction disorder: definition, classifications, clinical contexts, neural correlates and clinical strategies. J Addi Adol Beh. 2019; 2(1). doi: 10.31579/ JARAB.19/007.
- Perrotta G. Bipolar disorder: definition, differential diagnosis, clinical contexts and therapeutic approaches. J. Neuroscience and Neurological Surgery. 2019; 5(1), DOI: 10.31579/2578-8868/097.
- Perrotta G. Suicidal risk: definition, contexts, differential diagnosis, neural correlates and clinical strategies. J. Neuroscience and Neurological Surgery. 2020; 6(2):114. DOI: 10.31579/2688-7517/114.
- Bianchi VE, Herrera PF, Laura R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr Neurosci. 2021 Oct;24(10):810-834. doi: 10.1080/1028415X.2019.1681088. Epub 2019 Nov 4. PMID: 31684843.
- AlAmmar WA, Albeesh FH, Ibrahim LM, Algindan YY, Yamani LZ, Khattab RY. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: a systematic review. Nutr Neurosci. 2021 Jul;24(7):569-579. doi: 10.1080/1028415X.2019.1659560. Epub 2019 Aug 28. PMID: 31462182.
- Tomaszewski N, He X, Solomon V, Lee M, Mack WJ, Quinn JF, Braskie MN, Yassine HN. Effect of APOE Genotype on Plasma Docosahexaenoic Acid (DHA), Eicosapentaenoic Acid, Arachidonic Acid, and Hippocampal Volume in the Alzheimer's Disease Cooperative Study-Sponsored DHA Clinical Trial. J Alzheimers Dis. 2020;74(3):975-990. doi: 10.3233/JAD-191017. PMID: 32116250; PMCID: PMC7156328.
- Perrotta G. Alzheimer's disease: definition, contexts, neural correlates, strategies and clinical approaches. J Aging Stud Ther. 2019; 104:1(1). doi: 10.16966/ jast.
- Perrotta G. Parkinson's disorder: definition, contexts, neural correlates, strategies and clinical approaches. J Neurosci Neurol Surg. 2019; 4(5). doi: 10.31579/2578-8868/079.
- Perrotta G. General overview of “human dementia diseases”: definitions, classifications, neurobiological profiles and clinical treatments. Gerontol & Geriatric stud. 2020; 6(1). GGS.000626. doi: 10.31031/GGS.2020.06.000626.
- Chen X, Wu Y, Zhang Z, Zheng X, Wang Y, Yu M, Liu G. Effects of the rs3834458 Single Nucleotide Polymorphism in FADS2 on Levels of n-3 Long-chain Polyunsaturated Fatty Acids: A Meta-analysis. Prostaglandins Leukot Essent Fatty Acids. 2019 Nov;150:1-6. doi: 10.1016/j.plefa.2019.08.005. Epub 2019 Aug 24. PMID: 31487670.
- Scholtz SA, Kerling EH, Shaddy DJ, Li S, Thodosoff JM, Colombo J, Carlson SE. Docosahexaenoic acid (DHA) supplementation in pregnancy differentially modulates arachidonic acid and DHA status across FADS genotypes in pregnancy. Prostaglandins Leukot Essent Fatty Acids. 2015 Mar;94:29-33. doi: 10.1016/j.plefa.2014.10.008. Epub 2014 Nov 7. PMID: 25500337; PMCID: PMC4339528.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
