International Journal of Clinical Endocrinology and Metabolism
Teriparatide in a patient with severe osteoporosis, hypoparathyroidism and thalassemia major
Graziani A1, Cannito M1, Putti MC2 and Camozzi V1*
2Clinic of Pediatric Hematology-Oncology, Department of Women’s and Children’s Health, University of Padova, Padova, Italy
Cite this as
Graziani A, Cannito M, Putti MC, Camozzi V (2022) Teriparatide in a patient with severe osteoporosis, hypoparathyroidism and thalassemia major. Int J Clin Endocrinol Metab 8(1): 009-012. DOI: 10.17352/ijcem.000055Copyright License
© 2022 Graziani A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.β-Thalassemia Major (TM) is a form of beta-thalassemias. TM complications include, among others, osteoporosis, whose frequency, among TM patients, varies from 13.6% to 50%. The overall etiological mechanisms of TM-related osteoporosis remain unclarified. The primary approach to osteoporosis in patients with TM is the management of TM and its complications and the use of antiresorptive agents, such as Bisphosphonates (BPs), as the first line-drug of treatment. In this article, we present the case of 45 years old-woman with TM and severe osteoporosis, with multiple fractures, albeit the assumption of BPs for many years.
The anti-fracture efficacy and safety of BPs are not well-established in TM patients. Data suggest that etidronate and zoledronic acid should be considered as first-line agents in the management of TM- associated osteoporosis. Regarding Teriparatide (TP), there are only a few case reports published about its use in TM patients. It is also noticed that, at the dismission of TP therapy, its benefits are rapidly lost. Finally, regarding romosozumab, our patient presents a significant cardiovascular risk due to the presence of insulin-treated Diabetes Mellitus (DM) and TM-related cardiomyopathy, suggesting we avoid this drug.
This case report shows that the therapy of osteoporosis in patients with TM remains an open problem. TM patients often present multiple comorbidities which create limitations to osteoporosis’s treatment. Moreover, these comoboridites are often unavoidable risk factors for osteoporosis.
Introduction
β-Thalassemia Major (TM) is one of the three main forms of beta-thalassemias [1]. These are a group of hereditary blood diseases characterized by alterations in the synthesis of the beta-chains of hemoglobin that further result in several consequences and phenotypes, ranging from patients with severe anemia to asymptomatic ones. The overall annual incidence of symptomatic TM patients is estimated to be 1 in 100.000 throughout the world and 1 in 10.000 in the European Union [2]. Individuals with TM begin to be treated between ages 6 and 24 months; they subsequently Require Red Blood Cells (RBC) transfusions to survive [1]. RBC transfusions lead to iron overload-related complications, such as endocrine disorders (growth retardation, failure of sexual maturation, diabetes mellitus, insufficiency of the parathyroid, thyroid, pituitary, and adrenal glands), liver failure and dilated myocardiopathy [2]. Other complications include HIV infection, viral hepatitis, and osteoporosis [3].
Therefore, the removal of iron is one of the most important management for individuals who undergo RBC transfusions. Iron chelators are represented by deferasirox, deferiprone, and deferoxamine [3]. Regarding osteoporosis, the reported frequency of this complication, even in well-treated patients with TM, varies from 13.6% to 50%, with an additional 45% of patients who are affected by osteopenia [4].
Chronic anemia, bone marrow expansion due to ineffective erythropoiesis, iron toxicity, calcium and zinc deficiencies, low vitamin D levels and endocrine complications seem to contribute to the etiology of bone diseases in TM. Nevertheless, the complex etiological mechanisms of this heterogeneous osteopathy remain unclarified [4].
The primary approach to osteoporosis in patients with TM is the management of TM and its complications. Despite RBC transfusions, chelation therapy, and adequate hormonal replacement therapy, TM patients tend to lose bone mass. The observation of increased bone resorption in TM- associated osteoporosis has led to the use of antiresorptive agents, such as bisphosphonates (BPs), as the first-line drugs to treat osteoporosis in these patients [5].
A final and effective approach to TM patients with osteoporosis is not available at the moment. Here we present the case of patients with TM and severe osteoporosis which required different lines of therapy.
Case presentation
In 2016 a 45 years old-woman was referred to our Unit because of severe osteoporosis. The patient was affected by TM, known since childhood, follow-up by hematologists and treated with RBC transfusion, splenectomy and chelating therapy. Her medical history was also characterized by the presence of insulin-dependent diabetes mellitus, a past cerebral emorragiae with a consequent paresis to left emisoma, past HCV infection, kidney stones, hypocitraturia and early menopause ad 26 yrs old. She also suffered from past Papillary Thyroid Cancer (PTC), staging pT1N1a, treated with surgery (total thyroidectomy and limphadenectomy) and successive hormonal therapy (suppressive and then semi-suppressive).
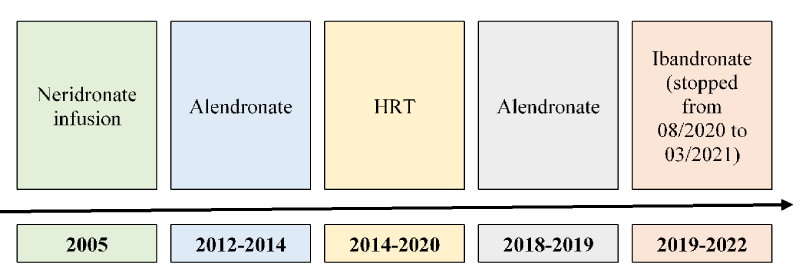
She had suffered a previous femoral fracture in 2012. In order to treat the known osteoporosis she had previously been treated with nedridonate infusion in 2005, alendronate from 2012 to 2014 and hormonal replacement therapy (HRT) with estro-progestinic from 2014 to half of 2020. She had already begun supplementation of vitamin D, previously insufficient.
After a few years of follow-up, in 2018 the patients re-began therapy with alendronate and since 2019 with ibandronate. Ibandronate has been stopped from August 2020 to march 2021 because of an episode of mandibular osteonecrosis with multiple teeth abscesses.
In November 2021, while the patient was still taking ibandronate, she suffered a new tibial fracture and then, in February 2022, a new femoral fracture.
Furthermore, during her clinical history, the patient developed a mild form of asymptomatic hypoparathyroidism, with a slow reduction of calcium and PTH levels to the lower range of normality. Her hypoparathyroidism may be a consequence of both the surgical operation at the neck and the TM- related infiltration of the parathyroid glands.
We report here available biochemical exams acquired during our follow-up period: Hb (NV 123-153 g/L):
99 g/L (03/2016) → 101 g/L (12/2018) → 104 g/L (12/2022) → 111 g/L (12/2022)
Creatinine (n.v. 45 - 84 umol/L):
54 umol/L (03/2016) → 55 umol/L (12/2018) → 43 umol/L (12/2020) → 51 umol/L (12/2022) Calcium (n.v.2.10 -2.55 mmol/L):
2.31 mmol/L (03/2016) → 2.14 mmol/L (02/2019) → 2.25 mmol/L (03/2021) → 2.38 mmol/L (10/2022)
Phosporus (n.v. 0.87 → 1.45 mmol/L):
0.72 mmol/L (03/2016) → 0.94 mmol/L (02/2019) → 0.96 mmol/L (03/2021) → 0.98 mmol/L (10/2022)
PTH (n.v. 6.5 - 36.8 ng/L):
13.5 ng/L (11/2016) → 10.7 ng/L (02/2019) → 8.6 ng/L (03/2021) → 7.2 ng/L (05/2022) → 4.8 ng/L (10/2022)
Vitamin D3 (n.v. > 75 nmol/L):
82 nmol/L (02/2019) → 58 nmol/L (03/2021) → 61 nmol/L (10/2022).
Furthermore, here below we report the Dual-energy X-Ray asborptiometries (DXAs) and bone turn- over markers (CTX, C-terminal telopeptide of type I collagen) of the patient during her medical history (Tables 1-3) and her therapy for osteoporosis (Figure 1).
Discussion
Fracture prevalence in TM patients ranges from 12.1% to 35.1%. Moreover, bone pain is often reported especially at the lower back site, although it is not always due to osteoporosis and bone fractures [5]. Even if the number of studies is limited, BP was shown to prevent bone loss, improve bone mineral density (BMD) and to be well tolerated in patients with TM. However, BPs’ anti-fracture efficacy and safety are not well established, due to the short follow-up and small sample size of trials conducted [5].
Data suggest that etidronate and zoledronic acid should be considered as first-line agents in the management of TM-associated osteoporosis, due to their relevant beneficial effects on BMD, bone turnover and pain and to the relatively greater number of patients enrolled in the studies [6].
Our patient had already been treated with neridronate, alendronate and ibandronate for a few years, apparently without significant efficacy on DXA parameters and fracture risk.
Regarding second-line therapies for osteoporosis, Teriparatide (TP) is the only anabolic therapy approved in Italy for the management of osteoporosis. In TM patients, TP might address the anabolic bone impairment by inducing osteoblast lining cells differentiation, stimulating osteoblast activity and limiting osteoblast and osteocyte apoptosis [5].
To our knowledge, there are only a few case reports published about TP treatment in TM patients. In reports, TP treatment improved BMD was well-tolerated and safe. However, the clinical use of TP in TM-associated osteoporosis has been very limited so far [5].
The most concerning adverse effect of teriparatide therapy is the risk of skeletal carcinogenicity, most notably osteosarcoma [7]. Our patient suffered from past PTC, which was treated with surgery. This does not represent an absolute contraindication, but it is generally considered, in clinical practice, a relative controindication that limited the use of TP. Moreover, TP could enhance the risk of hypercalciuria and/or urolithiasis [7]. Our patient has also suffered from kidney stones. Finally, is it to consider the theoric risk of leading to the development of extramedullary hematopoiesis when treating TM patients with TP [5].
It is also noticed that, according to most recent literature, after therapy con, TP is dismissed, and its benefits are rapidly lost, therefore this therapy should be followed by therapy with anti-resorptive agents such as BPs [8].
Another anabolic drug is romosobumab, which recently become available in Italy. It is a monoclonal antibody that selectively binds to sclerostin and inhibits its action [9]. Romosozumab should not be used in patients with a recent cardiovascular event and should be used cautiously in patients with high cardiovascular risk [10].
Our patient presents a significant cardiovascular risk due to the presence of insulin-treated DM and TM-related cardiomyopathy. Thus, romosozumab should be avoided as a therapy for her osteoporosis.
The patient presented multiple risk factors for osteoporosis, most of them due to TM. In particular, we underlined the role of insulin-treated DM, past vitamin D deficiency, past femoral fractures and past suppressive therapy after total thyroidectomy and early menopause. She presented partial immobility due to left emisoma paresis too.
Furthermore, the patient present hypoparathyroidism, which is probably caused both by the previous neck surgery and by the TM. In fact, hypoparathyroidism may be present in 4.4% - 6.8% of patients with TM, therefore requiring per sé adequate vitamin D supplementation [11].
We hence suggested the patient begin a cycle of therapy with TP (20 ug/die sc), because of its possible effect on her bone status with an additional positive effect on her hypoparathyroidism, carefully evaluating the risk/benefit ratio. Therapy with TP may also avoid the risk of a new event of BPs-related osteonecrosis of the jaw.
A few months after having begun the therapy with TP – December 2022, our last evaluation - the patient referred an improvement in her clinical condition, with a reduction of the bone pain and an improvement of her range of movements and a severe positive effect on bone turnover markers.
TP may represent a noteworthy treatment in TM patients, especially when long-term sequential therapy is needed and resulting in an increase of hip and femoral neck BMD [12,13]. Notwithstanding, TP has not yet been sufficiently studied in TM patients and more data are required to fully understand the efficacy and safety of TP in those patients [9].
Conclusion
This case report proves that the therapy of osteoporosis in patients with TM remains an open problem. TM patients often present multiple comorbidities which create limitations to osteoporosis’s treatment. Moreover, these comoboridites are often risk factors for osteoporosis and that cannot be easily corrected.
- Origa R. β-Thalassemia. Genet Med. 2017 Jun;19(6):609-619. doi: 10.1038/gim.2016.173. Epub 2016 Nov 3. PMID: 27811859.
- Galanello R, Origa R. Beta-thalassemia. Orphanet J Rare Dis. 2010 May 21;5:11. doi: 10.1186/1750-1172-5-11. PMID: 20492708; PMCID: PMC2893117.
- Ali S, Mumtaz S, Shakir HA, Khan M, Tahir HM, Mumtaz S, Mughal TA, Hassan A, Kazmi SAR, Sadia, Irfan M, Khan MA. Current status of beta-thalassemia and its treatment strategies. Mol Genet Genomic Med. 2021 Dec;9(12):e1788. doi: 10.1002/mgg3.1788. Epub 2021 Nov 5. PMID: 34738740; PMCID: PMC8683628.
- Yassin MA, Abdel Rahman MO, Hamad AA, Poil AR, Abdelrazek MT, Hussein RM, Kassem NA, Fadul AM, Elkourashy SA, Nashwan AJ. Denosumab versus zoledronic acid for patients with beta-thalassemia major-induced osteoporosis. Medicine (Baltimore). 2020 Dec 18;99(51):e23637. doi: 10.1097/MD.0000000000023637. PMID: 33371098; PMCID: PMC7748343.
- Gagliardi I, Celico M, Gamberini MR, Pontrelli M, Fortini M, Carnevale A, Napoli N, Zatelli MC, Ambrosio MR. Efficacy and Safety of Teriparatide in Beta-Thalassemia Major Associated Osteoporosis: A Real-Life Experience. Calcif Tissue Int. 2022 Jul;111(1):56-65. doi: 10.1007/s00223-022-00963-3. Epub 2022 Mar 4. PMID: 35243531; PMCID: PMC9232424.
- Giusti A, Pinto V, Forni GL, Pilotto A. Management of beta-thalassemia-associated osteoporosis. Ann N Y Acad Sci. 2016 Mar;1368(1):73-81. doi: 10.1111/nyas.13041. Epub 2016 Apr 6. PMID: 27060977.
- Vall H. Teriparatide. StatPearls. 2022.
- Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002 Oct 16;288(15):1889-97. doi: 10.1001/jama.288.15.1889. Erratum in: JAMA 2002 Dec 11;288(22):2825. PMID: 12377088.
- Stefanopoulos D, Papaioannou NA, Papavassiliou AG, Mastorakos G, Vryonidou A, Michou A, Dontas IA, Lyritis G, Kassi E, Tournis S. A contemporary therapeutic approach to bone disease in beta-thalassemia - a review. J Frailty Sarcopenia Falls. 2018 Mar 1;3(1):13-25. doi: 10.22540/JFSF-03-013. PMID: 32300690; PMCID: PMC7155348.
- Fixen C, Tunoa J. Romosozumab: a Review of Efficacy, Safety, and Cardiovascular Risk. Curr Osteoporos Rep. 2021 Feb;19(1):15-22. doi: 10.1007/s11914-020-00652-w. Epub 2021 Jan 7. PMID: 33409990.
- Carsote M, Vasiliu C, Trandafir AI, Albu SE, Dumitrascu MC, Popa A, Mehedintu C, Petca RC, Petca A, Sandru F. New Entity-Thalassemic Endocrine Disease: Major Beta-Thalassemia and Endocrine Involvement. Diagnostics (Basel). 2022 Aug 9;12(8):1921. doi: 10.3390/diagnostics12081921. PMID: 36010271; PMCID: PMC9406368.
- Tournis S, Dede AD, Savvidis C, Triantafyllopoulos IK, Kattamis A, Papaioannou N. Effects of teriparatide retreatment in a patient with β-thalassemia major. Transfusion. 2015 Dec;55(12):2905-10. doi: 10.1111/trf.13237. Epub 2015 Jul 14. PMID: 26174236.
- Feichtinger X, Kocijan R, Resch H, Muschitz C. Bone microarchitecture deteriorations and a fragility fracture in a patient with beta and alpha heterozygous thalassemia: a case report. Wien Klin Wochenschr. 2017 Mar;129(5-6):212-216. doi: 10.1007/s00508-016-1032-7. Epub 2016 Jun 30. PMID: 27363996; PMCID: PMC5346126.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
