International Journal of Clinical Endocrinology and Metabolism
Type A Insulin Resistance Syndrome- Novel insulin receptor gene mutation and familiar phenotypic variability
Analía V Freire1*, Paula Scaglia1,2, Mirta G Gryngarten1, Mariana Gutiérrez1, Andrea J Arcari1, Laura Suarez1, María Gabriela Ballerini1, Laura Valinotto2,3, Mónica I Natale2,4, Kenny Y Del Toro Camargo5, Ignacio Bergadá1, Rodolfo A Rey1,2 and María Gabriela Ropelato1,2
2Laboratorio de Medicina Traslacional-Hospital de Niños Ricardo Gutiérrez
3Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET)
4Facultad de Medicina, Universidad de Buenos Aires
5Unidad Médica Villa Country, Barranquilla, Colombia
Cite this as
Freire AV, Scaglia P, Gryngarten MG, Gutiérrez M, Arcari AJ, et al. (2019) Type A Insulin Resistance Syndrome- Novel insulin receptor gene mutation and familiar phenotypic variability. Int J Clin Endocrinol Metab 5(1): 016-019. DOI: 10.17352/ijcem.000037Type A Insulin Resistance Syndrome is due to heterozygous mutations in the insulin receptor (INSR) gene or its signaling pathway.
We present a premenarcheal 14 year-old girl with normal BMI, severe hirsutism, acanthosis nigricans, clitoral hypertrophy, deep voice, enlarged polycystic ovaries, severe hyperinsulinemia and biochemical hyperandrogenism.
We identified a novel heterozygous missense variant in the tyrosine kinase domain of INSR (p.Leu1150Pro) and an heterozygous missense variant in SH2B adapter protein 1 involved in the insulin pathway (p.Ala663Val). Interestingly, the patients’ mother and brother had the same INSR mutation although of a milder phenotype, reason why their IR went undiagnosed.
The novel heterozygous p.Leu1150Pro mutation in the INSR gene appears to be the cause of the type A insulin resistance syndrome; the SH2B1 mutation, likely to synergistically affect the insulin pathway, may contribute to explain the more severe presentation of the phenotype in the patient and the phenotypic variability of the syndrome within this family.
Introduction
Insulin resistance (IR) generally occurs in obese individuals with a favorable genetic background; IR is rare in lean individuals. Most cases of this syndrome are due to mutations in the insulin receptor gene (INSR) or its signaling pathway [1,2]. Patients with Type A IR syndrome present severe IR, hyperandrogenism and acanthosis nigricans in the absence of obesity or lipoatrophy. Molecular studies help characterize this mostly monogenic condition.
Patient report
A 14 year-old caucasian girl consulted for hirsutism, acanthosis nigricans and absence of menarche despite thelarche start at age 9. She was born from non-consanguineous parents at term and with normal weight. Developmental milestones and her past medical history were normal; the patient´s health had been good and she had always had good appetite. No family history of diabetes was reported.
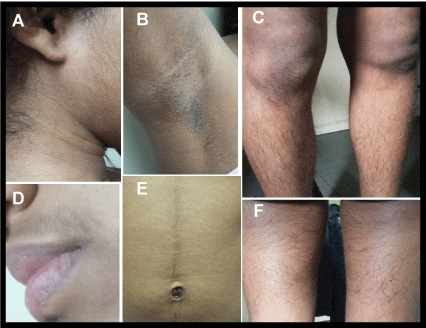
Two years before admission the patient began to complain of progressive whole body and facial hirsutism associated to seborrhea and a deepening of her voice. Her height was 158.5 cm (50th centile), weight 42.5 kg (10th centile) and BMI was 14.3 kg/m2 (-1.3 SD). At physical examination she showed normal muscular habitus, severe hirsutism (Ferriman-Gallwey score=32) and acanthosis nigricans on the neck, axillae and knees (Figure 1), facial and capillary seborrhea, clitoromegaly and male like voice. Her breast staging was Tanner 4, her pubic hair 6 and bone age was 15 years.
A trans-abdominal pelvic ultrasound showed a normal configuration of the uterus. Both ovaries were enlarged with polycystic morphology (14.2 cm3 and 16.3 cm,3) with normal adrenal glands; liver was also normal, without steatosis.
Routine (glucose, hepatic and lipid profile) and hormonal analyses at baseline and a standard 75-g oral glucose tolerance test (OGTT) were performed. Adrenal hyperfunction was ruled out by ACTH test and suppression test with Dexamethasone. The patient showed severe biochemical ovarian hyperandrogenism, normal hepatic and lipid profiles and increased serum levels of adiponectin (Table 1A). OGTT showed fasting glucose level of 73 mg/dL and 136 mg/dL at 120 min, with severe hyperinsulinemia (47.7 and 600 µUI/mL, respectively) (Table 1B).
After these molecular findings, the family was evaluated. The mother (39 y-o) presented normal weight (BMI 23 kg/m2), her menarche had been at age 16, she had never been hirsute and had had no problems in conceiving. A mild acanthosis nigricans on the armpits was found at the physical exam. The father (38 y-p) was overweight (BMI 27.9 kg/m2) without any relevant findings. The prepubertal brother (11.8 y-o) was overweight (BMI 25.5kg/m2) and presented acanthosis nigricans on the neck and hands.
The mother and brother underwent similar baseline laboratory evaluations and OGTT; the father could not be evaluated due to social reasons. Hyperandrogenia (testosterone: 87 ng/dl and androstenodione: 441 ng/dL) and severe hyperinsulinemia were found in the mother, with normal glucose responses to the OGTT. Severe hyperinsulinism was found for the brother at OGTT (Table 1B) although with normal glucose responses. His androgenic profile was normal.
With the diagnosis of Type A IR syndrome, the patient was started on Metformin 1500 mg/day. Her menarche occurred 3 months later. She was lost to follow up due to social reasons.
Molecular study
Genomic DNA was isolated from peripheral blood by CTAB method [3]. The patient´s clinical exome was studied by next generation sequencing (NGS) using the TruSight One assay in a NextSeq500 system Illumina. Resulting variants were prioritized, considering mutation impact, population frequency, and a list of candidate genes according to the pathway suspected to be altered (list of genes available upon request). The variants identified were confirmed in the patient and her relatives by PCR amplification and Sanger sequencing to evaluate its segregation (primers available upon request).
In silico bioinformatic analysis: Missense variants on candidate genes identified by NGS sequencing were classified based on their potential impact on protein function or structure using five In silico bioinformatics tools (Polyphen-2, Mutation Taster, SIFT, MutPred, and CADD prediction). Sequence variants were classified according to ACMG guidelines [4].
A novel heterozygous missense variant in exon 19 of INSR gene (NM_000208.3: c.3449T>C, p.Leu1150Pro) was identified in the proband. This variant is in the tyrosine kinase domain of the insulin receptor. Sanger sequencing confirmed the findings in the patient and revealed that her mother and her brother were heterozygous for the same variant.
The variant found in the INSR gene is classified as likely pathogenic according to ACMG guidelines. This mutation has not been reported in publicly available 1000G, ExAC, EVS and NCBI dbSNP databases and was predicted to be pathogenic by five In silico bioinformatics tools. By the multiple alignment analysis of the insulin receptor sequences in several species, we found that Leu1150 was conserved http://www.ibi.vu.nl/programs/pralinewww/(Supplementary Figure 1). Introduction of proline in position 1150 is predicted to have helix-breaking effects according to PSIPRED [5]. Furthermore, In silico molecular 3D modeling of β-Subunit of IR showed that Proline 1150 breaks the alpha-helix E and changes the bonding angle of the polypeptide chain, yielding a helix distortion (Supplementary Figure 2). This amino acid change could alter the displacement from the catalytic site of the activation loop, therefore probably causing loss of kinase activity.
In the proband, exome analysis also revealed a heterozygous missense variant in exon 11 of SH2B1 gene (NM_0011457951:c.1988C>T, p.Ala663Val). This variant was paternally-inherited. SH2B1 gene encodes an adaptor protein (SH2-B) that is involved in the insulin pathway.
The variant found in SH2B1 gene is classified as a variant of unknown significance according to ACMG guidelines. It has a population frequency lower than 1% in ExAC and 1000G. The potential effect of this variant on protein function has variable classification from benign (Polyphen-2 and Mutation Taster) or tolerated (SIFT) to potential high impact (CADD score=2.5).
Discussion
We describe a premenarcheal patient that presented with severe hyperandrogenism, acanthosis nigricans and severe IR. Although the criteria of severe IR in OGTT is not formally established, a peak insulin higher than 260 µUI/mL in individuals with BMI < 30 kg/m is the value considered in practice [1,2]. The patient was diagnosed as a classical phenotype of the Type A IR syndrome (OMIM # 610549*).
One of the differential diagnosis of this syndrome is the polycystic ovarian syndrome. The pelvic ultrasound showed a very important enlargement of both ovaries with polycystic morphology. The natural history of Type A IR syndrome in a cohort followed for up to 30 years showed that 6 out of 8 female patients needed surgical intervention to control the hyperandrogenism including partial or total removal of the ovaries due to massive ovarian enlargement [6]. However, the pathophysiological mechanisms of hyperinsulinemia within the ovaries and other tissues when insulin receptor is defective remain unexplained. It is well established that the insulin receptor and the IGF1 receptor (IGF1R) act through similar post-receptor pathways. High insulin concentrations can cross-react with the IGF1R or IGF1R–IR hybrid receptors promoting the clinical and biochemical manifestations of the syndrome [7]. According to the results of the largest cohort study of this rare condition, hyperinsulinemia correlates with the degree of hyperandrogenism, ovarian enlargement [6] and the degree of acanthosis nigricans [8]. The pathogenesis of this skin disorder is not completely clear; it has been proposed that high levels of insulin activate IGF1R to increase the mitotic activity of keratinocytes causing darkening and thickening in folds such as neck, armpit, and extension areas such as knees and fingers knuckles [8].
Our patient´s laboratory results support the absence of fatty liver and show a normal lipid profile. This is an element of differential diagnosis with lipodystrophy where the patients showed severe dyslipidemia [9]. Interestingly, the patient showed increased serum levels of adiponectin. This finding is usually described in patients with severe IR due to receptor defects in contrast to prevalent forms of IR associated with obesity where adiponectin levels are reduced. Hyperinsulinemia is able to suppress the adiponectin gene expression in adipose tissue if the elements of the cellular insulin signal network are intact [10].
Although no functional data were reported for the variant identified in this family, we assume that it is likely to be causal of the severe IR for several reasons. The Leu1150 residue affects the tyrosine kinase activity and functional studies have shown that mutations in tyrosine kinase domain impair insulin action [11,12], this very rare variant is absent from the controls provided by public databases including the ExAC browser covering the exomes of > 65,000 individuals. Furthermore, the variant shows co-segregation with disease in the affected family members.
Defects in the INSR gene cause a wide spectrum of congenital diseases associated with IR. In contrast with biallelic mutations of INSR that result in the more severe Donohue syndrome or Rabson–Mendenhall syndrome, Type A IR syndrome is typically due to heterozygous mutations in the INSR gene with autosomal dominant pattern of inheritance. The clinical manifestation is related to a dominant-negative mechanism that has been proposed in other mutations affecting the tyrosine kinase activity of the insulin receptor [13]. Mutants and wild type heterodimers of insulin receptor associated randomly might lead to a 75% reduction in insulin receptor tyrosine kinase activity [8].
Sexual dimorphism is associated with type A IR syndrome, which is more often diagnosed in females during infancy or adolescence than in males, since females show more health problems associated with this condition (especially hyperandrogenism) while males only present acanthosis nigricans, symptomatic postprandial hypoglycemia and commonly go undiagnosed, or simply diagnosed with “type 2 diabetes” in mid-life [1]. In the case of this family, if the proband had not been diagnosed, the patient’s brother would probably have gone undiagnosed.
Our patient presented more severe phenotype than her mother, who had had no clinical manifestations of hyperandrogenism during adolescence and nowadays only shows a mild acanthosis nigricans. We assume that the phenotypical differences could be explained by the coexistence of two variants in different genes of the insulin pathway.
The insulin pathway, in our patient, would sinergically have been affected by the INSR mutation and the SH2B1 gene variant found. SH2B adapter protein 1 adaptor binds to the activation loop of IR via its SH2 domain. It is functionally a physiological enhancer of insulin action required for glucose homeostasis. The systemic deletion of SH2B1 impaired the insulin receptor activation. SH2B1-/- knockout mice developed hyperinsulinemia and glucose intolerance and their insulin levels were 20-fold higher than normal while blood glucose levels were 3.3-fold above those of age-matched wild-type controls [14].
In our patient, the additional variant may translate into a more severe phenotype, compared to her mother who only had the insulin gene variant defect but did not present hirsutism during adolescence.
Conclusion
We identified a novel heterozygous mutation (p.Leu1150Pro) in the INSR gene using an NGS approach in an adolescent with Type A IR syndrome. The finding of other variant of the SH2B1 gene, likely to synergistically affect the insulin pathway, may contribute to explain the more severe presentation of the phenotype in the patient and the phenotypic variability of the syndrome within this family.
We thank Dr. Saul Malozowski for critically reading this manuscript.
Disclosure statement
The authors have nothing to disclose.
M.G.G, M.G.B, I.B and M.G.R. are research members of the Carrera de Investigador en Salud, Ministerio de Salud, Gobierno de la Ciudad Autónoma de Buenos Aires. This research was supported by a grant of Consejo de Investigación en Salud, Ministerio de Salud, Gobierno de la Ciudad Autónoma de Buenos Aires (CIS-GCBA), Argentina to M.G.R.
This work was partially supported by a grant FS BIO 05/17 of Fondo Argentino Sectorial (FONARSEC), Agencia Nacional de Promoción Científica y Técnica (ANPCYT), Argentina.
- Parker VE, Semple RK (2013) Genetics in endocrinology: genetic forms of severe insulin resistance: what endocrinologists should know. Eur J Endocrinol 69: R71-R80. Link: https://bit.ly/2XD7FqF
- Semple RK, Savage DB, Cochran EK, Gorden P, O'Rahilly S (2011) Genetic syndromes of severe insulin resistance. Endocr Rev 32: 498-514. Link: https://bit.ly/2IA0JEs
- Del SG, Manfioletti G, Schneider C (1989) The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques 7: 514-520. Link: https://bit.ly/2ZqfTmx
- Richards S, Aziz N, Bale S, Bick D, Das S, et al. (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405-424. Link: https://bit.ly/2G8sEts
- Buchan DW, Minneci F, Nugent TC, Bryson K, Jones DT (2013) Scalable web services for the PSIPRED Protein Analysis Workbench. Nucleic Acids Res 41: W349-W357. Link: https://bit.ly/2F6cZeY
- Musso C, Cochran E, Moran SA, Skarulis MC, Oral EA, et al. (2004) Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): a 30-year prospective. Medicine (Baltimore) 83: 209-222. Link: https://bit.ly/2Xayeq8
- Mani AM, Fenwick MA, Cheng Z, Sharma MK, Singh D, et al (2010) IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction 139: 139-151. Link: https://bit.ly/2IDp24h
- Accili D, Cama A, Barbetti F, Kadowaki H, Kadowaki T, et al (1992) Insulin resistance due to mutations of the insulin receptor gene: an overview. J Endocrinol Invest 15: 857-864. Link: https://bit.ly/2ZhZsZe
- Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, et al. (2009) Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 119: 315-322. Link: https://bit.ly/2WGOZdk
- Cook JR, Semple RK (2010) Hypoadiponectinemia--cause or consequence of human "insulin resistance"? J Clin Endocrinol Metab 95: 1544-1554. Link: https://bit.ly/2XJEja0
- Cama A, de la Luz SM, Quon MJ, Ottini L, Gorden P, et al. (1993) Substitution of glutamic acid for alanine 1135 in the putative "catalytic loop" of the tyrosine kinase domain of the human insulin receptor. A mutation that impairs proteolytic processing into subunits and inhibits receptor tyrosine kinase activity. J Biol Chem 268: 8060-8069. Link: https://bit.ly/2KeTzbZ
- Formisano P, Sohn KJ, Miele C, Di FB, Petruzziello A, et al. (1993) Mutation in a conserved motif next to the insulin receptor key autophosphorylation sites de-regulates kinase activity and impairs insulin action. J Biol Chem 268: 5241-5248. Link: https://bit.ly/2Kf8Nhb
- Ardon O, Procter M, Tvrdik T, Longo N, Mao R (2014) Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol Genet Metab Rep 1: 71-84. Link: https://bit.ly/2KGgGvw
- Duan C, Yang H, White MF, Rui L (2004) Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol 24: 7435-7443. Link: https://bit.ly/2WAWAVU
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
