International Journal of Clinical Endocrinology and Metabolism
Intrasellar Parasitic Infection: Finding the Culprit - How to Get the Right Diagnosis
Buravej Assavapongpaiboon1 and Behrouz Salehian2*
2Department of Clinical Diabetes, Endocrinology and Metabolism, City of Hope Comprehensive Cancer Center, Duarte, California, USA
Cite this as
Assavapongpaiboon B, Salehian B (2017) Intrasellar Parasitic Infection: Finding the Culprit - How to Get the Right Diagnosis. Int J Clin Endocrinol Metab 3(1): 008-015. DOI: 10.17352/ijcem.000022Purpose: Parasitic diseases can involve central nervous system presenting with various manifestation. However, parasitic infection of pituitary is very rare, unexpected and can be missed diagnosis with tumor. Organisms, clinical features and diagnostic method of parasitic infection in sella turcica were described in this review.
Methods: Case reports or case series from MEDLINE, PubMed, and Scopus updated to June 2016 were searched. Only full text papers from original publication which allows data abstraction on clinical, laboratory and neuroimaging findings of intrasellar parasitic infection were included.
Results: Out of 28 adult patients and 6 children, neurocysticercosis, toxoplasmosis, malaria, hydatid disease and amebiasis have been reported with pituitary involvement. All pediatric cases were involved in congenital toxoplasmosis. For adults, the two most frequent symptoms were headache (86%) and visual symptoms (61%). Sixty-eight percent of adults had hormonal disturbance which panhypopituitarism and hyperprolactinemia were most common. The majority of the cases had a definitive diagnosis of infection from indentifying parasitic organism from pathology except all cases of congenital toxoplasmosis, malaria and 4 cases of cysticercosis which diagnosis was established on the basis of neuroimaging findings and immune diagnosis tests.
Conclusion: History of risk for exposure and clinical manifestations of headache, visual disturbance, endocrine disturbance and eosinophilia are initial clues that lead to diagnosis of parasitic pituitary infection. In addition, hormonal dysfunction should be carefully evaluated. Definite diagnosis requiring parasitic identification is occasionally invasive. Immunodiagnosis or molecular diagnosis with neuroimaging can be applied to achieve the correct diagnosis.
Introduction
Parasitic diseases have worldwide distribution with increased prevalence in areas of poor sanitation. In nonendemic areas, cases also occur because of an increase in international travel and host immunosuppression caused by HIV infection or immunosuppressive therapy. Despite less common than bacterial or viral infection, parasitic infection of central nervous system (CNS) can be fatal if undetected. Especially, parasitic infection of pituitary gland is very rare and unexpected. It can be difficult to make a correct diagnosis which can lead to delay in treatment and be associated with the risk of life-threatening conditions. Although many reviews have been published about parasitic infection of the brain, parasitic infection in sella turcica area has received little attention. In this review, we bring parasitic infection of pituitary gland to light, emphasizing on clinical features, significance and diagnosis of this entity.
Methods
A literature search of case reports or case series of patients with parasitic infection of pituitary was performed using the electronic database of MEDLINE, PubMed, and Scopus. The search was updated to June 2016. The keywords used in the literature were the combinations of name of organism or parasitic diseases such as toxoplasma/toxoplasmosis, cysticercosis/neurocysticercosis and words associated with pituitary gland such as‘‘sellar’’, ‘‘intrasellar’’, ‘‘hypophysis’’, ‘‘hypohyseal’’, and ‘‘pituitary’’. Full articles written in English and relevant to the topic were included in this review. We included the original publication which allows data abstraction on clinical, laboratory and neuroimaging findings whether intrasellar parasitic infection occurred as the only presentation of the disease or as the part of a more disseminated infection.
Results
We assessed information of 23 full-text papers (24 case reports and 3 case series). The total 34 patients were analyzed and information was included in this review [1-24].
Demographic data
For infection in 28 adults, patient age ranged in age from 18 to 81 years (median 42 years); 13(46%) of patients were male. For infection of children, 6 cases of congenital toxoplasmosis were included; patient age ranged from at birth to 1 year (median 2 months); 4(67%) of patients were male. The patients were reported from the USA, Mexico, Germany, Turkey, China, Colombia, India, Thailand, Korea, Belgium, Switzerland, Greece and Saudi Arabia.
Parasitology
Out of 28 adult patients and 6 children, twenty patients were infected with Taenia solium presenting with intrasellar neurocysticercosis. Ten of patients were infected with Toxoplasma gondii (6) cases involved with congenital toxoplasmosis and 3 cases associated with pituitary adenoma and the other case presenting as complication from HIV infection). Two of patients had cerebral malaria. The others were one case of pituitary amebiasis and one case of pituitary hydatid disease. Life cycles, route of transmission, and frequent clinical presentation of each parasitic organism are presented in Table 1.
Clinical presentation
In adult group, headache was the most common presenting symptom (64%) and the most common symptom (86%). Second most common symptom was ophthalmological disturbance (61%) defined by decrease visual acuity, visual field defect (bitemporal hemianopia and superior hemianopsia) and bilateral exophthalmos. Endocrine abnormalities were noted in 19 patients (68%) including 7 with panhypopituitarism (1with co-existing diabetes insipidus), 7 with hyperprolactinemia, 2 with isolated diabetes insipidus, 3with partial hypopituitarism (isolate hypothyroidism, isolate adrenal insufficiency with diabetes insipidus and one not classified). Other symptoms included seizure, fever, nausea and focal neurological symptoms. Fever was present in 2 patients (7%) who both had malarial infection.
From 6 pediatric cases of congenital toxoplasmosis, sepsis-like symptoms and seizure are common (50%). Other symptoms included microcephaly, hydrocephalus and polyuria. Endocrine abnormalities included 2 cases with isolated diabetes insipidus, four with panhypopituitarism (2 cases with co-existing diabetes insipidus and one with precocious puberty during follow-up visits). The most common endocrine disturbance was diabetes insipidus (67%).
Diagnosis
The majority of the patients have a definitive diagnosis of infection from identifying parasitic organism from pathology except all cases of congenital toxoplasmosis, malaria and 4 cases of cysticercosis which diagnosis was established on the basis of neuroimaging findings and immune diagnosis tests. Duration from onset of symptoms to diagnosis varied from each parasitic disease. Acute to subacute onset included amebiasis, malaria (1 week) and toxoplasmosis in HIV person (3 weeks). Chronic onset included cysticercosis (4 months), hydatid cyst (7 months) and toxoplasmosis with pituitary adenoma (3.5years).
Discussion
Neurocysticercosis
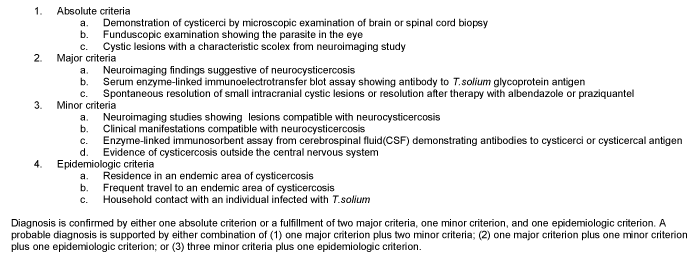
Taenia solium is the most common parasite which involves sella turcica causing neurocysticercosis. Over 50 million people around the world are estimated to have cysticercosis infection, although estimates are probably low since many infections are subclinical. Central and South America, sub-Saharan Africa, India, and Asia are endemic areas for T. solium [25]. Neurocysticercosis is divided into parenchymal and extraparenchymal forms. Extraparenchymal forms include intraventricular, subarachnoid space of brain and spinal and intraocular. However, sellar involvement is rare. Definite diagnosis can be difficult without the presence of parasite (see the absolute criteria below). Therefore, diagnostic criteria using clinical presentation, radiographic studies, serologic tests, and exposure history was delineated by a consensus conference (Figure 1) [26].
The characteristic imaging of neurocysticercosis includes cystic lesions with or without eccentric scolex which can be associated with perilesional edema and enhancing lesion depending on the stages. One or more nodular calcifications can be seen (Figure 2). Parenchymal cysticerci are usually round and 10–20 mm in diameter. In contrast, cystic lesions in the subarachnoid space are larger and various in size as there is no limiting parenchymal tissue in these sites [27]. The form of subarachnoid neurocysticercosis has distinguishing characteristic of grape-liked clusters of cysts in the cisterns called “racemosecysticercus”. This is a proliferating form of larval tapeworm containing multiple interconnected cysts of different sizes without scolices [28]. Racemosecysticercosis occurs primarily in parts of the nervous system where the parasite is not closely enclosed by host tissues, such as the cisterns at the base of the brain including suprasellar cistern which may extend into the sella turcica [2,8]. Diagnosis is more challenging when the sellarcysticerci are the sole manifestation of the disease and no densities within the cysts that indicated the presence of scolices. In our review, nearly 50% of lesions confined to the sellar region which should be differentiated from other causes of cystic lesions such as craniopharyngioma, cystic pituitary adenoma, arachnoid cyst, Rathke’s cleft cyst, epidermoid cyst and hydatid cyst. Extrasellar finding such as subarachnoid cysticercosis involving the basal CSF cisterns (associated or not with hydrocephalus) and the brain parenchymal cysts may facilitate diagnosis. Specific antibodies to cysticerci were included in criteria. An immunoblot assay using lentil lectin purified glycoproteins is 98% sensitive and 100% specific [29]. However, patients with single intracranial lesions or with calcifications may be seronegative [30]. With this assay, it is 10% more sensitive using serum than for CSF specimens. On the other hand, enzyme-linked immunosorbent assays for CSF, not serum, are reliable and interpretation should be done with caution due to high false-positive and false-negative rates [31].
Toxoplasmosis
Interestingly, toxoplasmosis has been incidentally found in 3 patients diagnosed with prolactinoma presenting with amenorrhea and MRI suggestive of pituitary adenomas [13,16]. Due to the recurrent of pituitary adenoma after surgery and inadequate response to dopamine agonist therapy; surgical removal of tumor was needed. The presence of toxoplasma bradycyst was revealed in all cases, 2 of them had prolactin producing adenoma and one with nonfunctioning adenoma. It seems this is a coincidental toxoplasmosis of pituitary and pituitary adenoma, which was detected at the time of the surgery; the prevalence of pseudocyst of toxoplasmosis in normal pituitary or microadenoma is unknown.
Approximately 25 to 30% of the world’s human population is infected by Toxoplasma gondii [32]. Europe, Central America, Brazil, and Central Africa are the areas which the highest rates of infection with T. gondii have been reported [33]. T.gondii infection presents a wide range of clinical manifestation. In congenital form, majority of cases are asymptomatic. However, acute manifestations may occur as nonspecific septic-like features with fever, anemia, jaundice, seizure and hydrocephalus. Occasionally, few infants have late presentation with neurologic symptoms such as sensory-neural deafness, microcephaly and developmental delay [34]. Differential diagnosis includes other intrauterine infections that have similar manifestation in the newborn such as rubella, cytomegalovirus, syphilis and congenital lymphocytic choriomeningitis virus syndrome and Zika virus [35,36]. Computed Tomography (CT) without contrast is preferred because it is fast, less expensive and can be done without sedation. Intracranial calcifications scattered all over the brain, hydrocephalus and cortical atrophy are characteristic findings. Usually, serologic testing has become the routine method of diagnosis. For accurate diagnosis, testing of blood sample from both the infant and the mother is required. Results from mother typically have positive Toxoplasma IgG and IgM antibody. For diagnosis of newborn, presence of Toxoplasmic IgM is diagnostic [37]. When IgM titers are negative or unequivocal, IgA and IgE ELISA should be performed [38,39]. Serial serologic testing during the first year of life is required for diagnosis when the initial results are equivocal. T. gondii polymerase chain reaction (PCR) on amniotic fluid and infant CSF are also useful for diagnosis in the case with characteristic clinical findings (chorioretinitis, intracranial calcification and/or hydrocephalus) [40]. In this review, diabetes insipidus is the most common endocrine (67%). Therefore, polyuria in the background of congenital toxoplasmosis raises concern of pituitary involvement.
Reactivation of toxoplasmosis commonly seen in HIV-infected person and other immunocompromised conditions is another manifestation presenting with encephalopathy. For HIV-infected patients , a presumptive diagnosis can be made if the patient has a CD4 count <100 cells/microL, has not been receiving proper prophylaxis to prevent toxoplasmosis and has all of the following:1) compatible clinical syndrome: fever, headache, focal neurologic deficits, seizures, confusion and mental status change 2) positive T. gondii IgG antibody 3) Brain imaging (preferably magnetic resonance imaging) that demonstrates a typical radiographic appearance (e.g., single or multiple ring-enhancing lesions (<2 cm) with the basal ganglia and cortico-medullary junction most commonly involved) Toxoplasma encephalitis can be diagnosed with a 90 percent probability with the presence of these criteria [41,42]. CSF analysis for T. gondii PCR should also be performed to establish the diagnosis, when the patient is safe to undergo lumbar puncture, due to high specificity (96 to 100%) [43,44]. Moreover, CSF analysis can suggest alternative diagnoses such as primary CNS lymphoma (Epstein-Barr virus positive), progressive multifocal leukoencephalopathy (JC virus positive), mycobacterial infection ( culture for mycobacterial positive), cryptococcosis (cryptococcal antigen positive) and bacterial abscess [45], Also, a therapeutic trial of anti - Toxoplasma medications (pyrimethamine plus sulfadiazine or clindamycin) is frequently used to assess the diagnosis. If patients fail to respond or have lymphoma, clinical signs and symptoms worsen by day 7. These patients require brain biopsy by a stereotactic CT-guided method with or without a change in therapy.
Malaria
In general, up to 21% of severe Plasmodium falciparum infections are complicated with polyuria. However, about 66% of polyuria cases has central diabetes insipidus (DI) after laboratory investigation [46]. Other 2 case reports showing cerebral malaria with documented central DI [20,21]. Pathogenesis of DI in cerebral malaria could have been a result of obstruction of neurohypophyseal microvasculature by the malarial parasites. With treatment of the parasitemia, the microvascular obstruction gradually resolves and hence the requirement of desmopressin is temporary. In addition, severe falciparum malaria is associated with secondary adrenal insufficiency. Blunted ACTH response to CRH stimulation was found in patients with severe malaria. This may reflect impairment of corticotroph function due to parasitic erythrocyte sequestration in hypothalamic-pituitary portal system or somatostatin-like peptide produced by parasite [47].
Malaria prevails throughout most of the tropical regions of the world, with P. falciparum causing the largest burden of disease predominating in Africa, New Guinea, and Hispaniola (Haiti and the Dominican Republic) [48]. One of the pictures of severe P. falciparum infection is cerebral malaria. Malaria should be suspected in the setting of fever and history of exposure to endemic area (residence in or travel to an area where malaria is endemic) [49]. Clinical tools for parasite-based diagnosis include microscopy (visualization of parasites in stained blood smears) and rapid diagnostic tests (RDTs; which detect antigen or antibody). Detection of parasites on Giemsa-stained blood smears by light microscopy is the standard tool for diagnosis of malaria [50]. However, microscopy cannot reliably detect very low parasitemia (<5 to 10 parasites/mcL) or cases where the majority of the parasite biomass is sequestered (e.g., in the case of placental sequestration during pregnancy) [51]. RDTs should be used if microscopy is not readily available due to their accuracy and ease of use. RDTs usually detect Plasmodium antigens including histidine-rich protein 2 (HRP2), Plasmodium lactate dehydrogenase (pLDH), and aldolase. The approach to RDT selection for Plasmodium species depends on the epidemiology of infection and goals for control in the region where the test is used [52]. Molecular techniques for detection of genetic material are limited to research settings.
Hydatid disease
Cystic echinococcosis is a disease caused by tapeworm Ecchinococcus granulosus infection. These infections are prevalent in most areas where livestock is raised in association with dogs. These parasites are found on all continents which areas of high prevalence include China, central Asia, the Middle East, the Mediterranean region, eastern Africa, and parts of South America [53]. Echinococcal cysts enlarge slowly and patient is usually asymptomatic. Their expanding size and space-occupying effect in an involved organ elicit symptoms. The liver and the lungs are the most common sites of these cysts. Common symptoms are right upper quadrant pain, nausea, and vomiting in liver involvement or cough, chest pain, dyspnea and hemoptysis in lung involvement [54]. Cerebral cysts are approximately present in 2–3% of all cases. Headache is the most common presenting symptom of cerebral hydatid cysts (70–75%), followed by weakness in the extremities. Other symptoms are epilepsy, mental changes, skull deformities and, more rarely, dyskinetic phenomena [55]. Complication includes cyst rupture presenting with fever and acute hypersensitivity reactions, including anaphylaxis.
For untreated and uncomplicated hydrated brain lesion, MRI brain usually reveals well-defined large, non-enhancing cystic lesion with no perilesional edema. [56]. the cyst may be single or multiple and may or may not show septation. Calcifications are rare and occur in less than 1 % [57]. However, the pathognomonic finding, daughter cysts within the larger cyst is rare [58]. Infection can complicate the cysts which may turn to have a multilocular appearance with septations with perilesional edema. Infected cysts resemble gliomatous tumors, but gliomas usually have thick and nodular enhancing walls [59]. Serology is both useful for diagnosis and for follow-up after treatment [60,61]. Antibody detection is more sensitive than antigen detection for diagnosis of E. granulosus. The methods most frequently employed for initial screening tests are ELISA, most sensitive and specific of the available assays, and indirect hemagglutination (IHA). These tests use crude antigens such as hydatid fluid or protoscolex extracts. Confirmatory tests using specific antigens can then be performed, such as immunoelectrophoresis and immunoblotting [62].
Amoebic disease
Amoebic disease is a result of Entamoeba histolytica infection. The disease is 7 to 10 times more common among adult men, generally seen in migrants from and long-term travelers to endemic areas including India, Africa, Mexico, and parts of Central and South America [63,64]. Worldwide, approximately 50 million people develop colitis or extraintestinal disease, with over 100,000 deaths annually [65]. Extraintestinal manifestations include amebic liver abscess and other more rare manifestations such as liver, pulmonary, cardiac, or brain involvement. Cerebral amebiasis results from with hematogenous spread of infection. Abrupt onset of symptoms such as meningeal signs, facial nerve palsy, motor paralysis, and seizure and rapid progression to death if untreated is characteristic [66]. There is no pathognomonic finding on CT scan. Irregular foci without a capsule or surrounding enhancement, however, are suggestive [67]. Serology is helpful for diagnosis in amebiasis involving the brain especially cases with liver abscess. Enzyme-linked immunosorbent assays or agar gel diffusion assays positive result with appropriate clinical syndrome suggests active disease. Sensitivity of enzyme immunoassays (EIA) for E. histolytica is approximately 95% in patients with extraintestinal amebiasis, 70% in patients with active intestinal infections, and 10% in patients with asymptomatic intestinal E. histolytica infections [68]. Interpretation of indirect hemagglutination test should be aware because it may remain positive for as long as 10 years. In addition, PCR of brain abscess aspirate or CSF is adjunctive for diagnosis [66]. The definitive diagnosis of amebic colitis is made by the demonstration of E.histolytica trophozoites ingesting red blood cell. However, most cases have not been microscopically observed in cerebral amebiasis. At least three fresh stool specimens should be collected because trophozoites are killed rapidly by water, drying, or barium. For circumstances in which amebic brain abscess is suspected based on epidemiology, clinical manifestations, and radiographic findings, treatment (metronidazole) should be started immediately. Surgical intervention for decompression and tissue biopsy may be required.
Conclusion
History of risk for exposure and clinical manifestations of headache, visual disturbance, endocrine disturbance and eosinophilia are initial clues that lead to diagnosis of parasitic pituitary infection. Further investigation should include visual evaluation, hormonal evaluation for hypopituitarism and neuroimaging. Attention should be given to correcting endocrine deficiencies in particular adrenal insufficiency and diabetes insipidus which is more prevalent in cases of severe malaria. Although identification of the causative parasite is difficult, immunodiagnostics and molecular diagnosis can be applied to achieve the correct diagnosis. Neuroimaging plays an important role to differentiate diagnosis, while serological and molecular tests can confirm the diagnosis. Treatment of these parasitic diseases is complex, involving surgery, and different modalities based on each parasitic organism. In addition, drug resistance is reported worldwide. In Table 2, we summarized the parasitological and serological methods for the diagnosis of associated parasitic infection and commonly used drugs for treatment of these infections in non tropical area help of tropical medicine and infectious-disease specialist is crucial for correct diagnosis and management.
- Zhang X, Huang G, Ji T, Liu W, Gao Y, et al. (2014) Sellar cysticercosis and septum pellucidum cyst: a case report and review of the literature. Southeast Asian J Trop Med Public Health 45: 584-587. Link: https://goo.gl/HEv9OI
- Dutta D, Kumar M, Ghosh S, Mukhopadhyay S, Chowdhury S, et al. (2013) Pituitary hormone deficiency due to racemose neurocysticercosis. Lancet Diabetes Endocrinol1: e13. Link: https://goo.gl/0v3rnU
- Ramirez Mdel P, Restrepo JE, Syro LV, Fabio Rotondo, Francisco Londoño J, et al. (2012) Neurocysticercosis, meningioma, and silent corticotroph pituitary adenoma in a 61-year-old woman. Case Rep Pathol2012: 340840. Link: https://goo.gl/0ypso5
- Kelesidis T, Tsiodras S (2011) Hypopituitarism caused by neurocysticercosis. Am J Med Sci341: 414-416. Link: https://goo.gl/HlGB9h
- Cheong JH, Kim JM, Kim CH (2010) Neurocysticercosis involving the pituitary stalk : case report and literature review. J Korean Neurosurg Soc48: 91-93. Link: https://goo.gl/I4hHFk
- Ruangkanchanasetr P, Bangchuad T, Sithinamsuwan P, Yupin Benjasuratwong, Warin Chuankrerkkul, et al. (2006) Hypothalamic neurocysticercosis presenting with polyuria: a first report of an unusual manifestation. Nephrol Dial Transplant 21: 2308-2310. Link: https://goo.gl/Hu0RLV
- Arriada-Mendicoa N, Celis-Lopez MA, Higuera-Calleja J, Corona-Vazquez T (2003) Imaging features of sellar cysticercosis. AJNR Am J Neuroradiol 24: 1386-1389. Link: https://goo.gl/TwFmNc
- (1993) Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 8-1993. A 62-year-old Cape Verdean woman with blurred vision, diplopia, a suprasellar mass, and lymphocytic meningitis. N Engl J Med328: 566-573. Link: https://goo.gl/4PMN5D
- Boecher-Schwarz HG, Hey O, Higer HP, Perneczky A (1991) Intrasellar cysticercosis mimicking a pituitary adenoma. Br J Neurosurg5: 405-407. Link: https://goo.gl/5GLxMB
- Del Brutto OH, Guevara J, Sotelo J (1988) Intrasellar cysticercosis. J Neurosurg 69: 58-60. Link: https://goo.gl/5UekYG
- Rafael H, Gomez-Llata S (1985) Intrasellar cysticercosis. Case report. J Neurosurg63: 975-976. Link: https://goo.gl/CTV7NK
- Prosser PR, Wilson CB, Forsham PH (1978) Intrasellar cysticercosis presenting as a pituitary tumor: successful transsphenoidal cystectomy with preservation of pituitary function. Am J Trop Med Hyg27: 976-978. Link: https://goo.gl/1vH9z1
- Berkmann S, Fischer I, Sonderegger B, Fischli S, Fandino J, et al. (2015) Sellar Toxoplasmosis and Nonfunctioning Pituitary Adenoma. World Neurosurg84: 1491-1494. Link: https://goo.gl/udKnnn
- Mohamed S, Osman A, Jurayyan NA Al, Nemri A Al, Salih MA, et al. (2014) Congenital toxoplasmosis presenting as central diabetes insipidus in an infant: a case report. BMC Res Notes7: 184. Link: https://goo.gl/EiRjRr
- Siahanidou T, Tsoumas D, Kanaka-Gantenbein C, Mandyla H (2006) Neuroendocrine abnormalities in a neonate with congenital toxoplasmosis. J Pediatr Endocrinol Metab19: 1363-1366. Link: https://goo.gl/n20Aci
- Zhang X, Li Q, Hu P, Cheng H, Huang G (2002) Two case reports of pituitary adenoma associated with Toxoplasma gondii infection. J Clin Pathol 55: 965-966. Link: https://goo.gl/kWZRBz
- Oygur N, Yilmaz G, Ozkaynak C, Guven AG (1998) Central diabetes insipitus in a patient with congenital toxoplasmosis. American journal of perinatology 15: 191-192. Link: https://goo.gl/S7IwkS
- Massa G, Vanderschueren-Lodeweyckx M, Van Vliet G, Craen M, de Zegher F, et al. (1989) Hypothalamo-pituitary dysfunction in congenital toxoplasmosis. Eur J Pediatr148: 742-744. Link: https://goo.gl/j6azKZ
- Milligan SA, Katz MS, Craven PC, Strandberg DA, Russell IJ, et al. (1984)Toxoplasmosis presenting as panhypopituitarism in a patient with the acquired immune deficiency syndrome. Am J Med 77: 760-764. Link: https://goo.gl/KpDKAT
- Premji R, Roopnarinesingh N, Cohen J, Sen S (2016) Cerebral Malaria: An Unusual Cause of Central Diabetes Insipidus. Case Rep Endocrinol2016:2047410. Link: https://goo.gl/9dtmJ8
- Schubert S, Achenbach H, Engelmann L, Borte G, Stumvoll M, et al. (2006) Central diabetes insipidus in a patient with malaria tropica. J Endocrinol Invest 29: 265-266. Link: https://goo.gl/gkG9CU
- Becker GL Jr, Knep S, Lance KP, Kaufman L (1980) Amebic abscess of the brain. Neurosurgery 6: 192-194. Link: https://goo.gl/iQLzz0
- Ozgen T, Bertan V, Kansu T, Akalin S (1984) Intrasellar hydatid cyst. Case report. J Neurosurg 60: 647-648. Link: https://goo.gl/OVrbCw
- Choi HJ, Cornford M, Wang L, Sun J, Friedman TC (2004) Acute chagas' disease presenting with a suprasellar mass and panhypopituitarism. Pituitary 7: 111-114. Link: https://goo.gl/uZlGkF
- Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, et al. (2010) A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis4: e870. Link: https://goo.gl/4EUFaw
- OR Del Brutto, V Rajshekhar, AC White, VCW Tsang, TE Nash, et al. (2001) Proposed diagnostic criteria for neurocysticercosis. Neurology 57: 177-183. Link: https://goo.gl/8HSrVm
- Venkat B, Aggarwal N, Makhaik S, Sood R (2016) A comprehensive review of imaging findings in human cysticercosis. Jpn J Radiol34: 241-257. Link: https://goo.gl/uIFISy
- Machado DC, Camilo GB, Alves UD, de Oliveira CE, de Oliveira RV, et al. (2002) Imaging aspects of the racemose neurocysticercosis. Arch Med Sci 11: 1356-1360. Link: https://goo.gl/z0a3Al
- Tsang VC, Brand JA, Boyer AE (1989) An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium). J Infect Dis159: 50-59. Link: https://goo.gl/IYeOYd
- Wilson M, Bryan RT, Fried JA, et al. (1991) Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis164: 1007-1009. Link: https://goo.gl/gQoxYy
- Ramos-Kuri M, Montoya RM, Padilla A, Govezensky T, Díaz ML, et al. (1992) Immunodiagnosis of neurocysticercosis. Disappointing performance of serology (enzyme-linked immunosorbent assay) in an unbiased sample of neurological patients. Arch Neurol 49: 633-636. Link: https://goo.gl/JCl79B
- Montoya JG, Liesenfeld O (2004) Toxoplasmosis. Lancet 363: 1965-1976. Link: https://goo.gl/D513W0
- Berger F, Goulet V, Le Strat Y, Desenclos JC (2009) Toxoplasmosis among pregnant women in France: risk factors and change of prevalence between 1995 and 2003. Rev Epidemiol Sante Publique57: 241-248. Link: https://goo.gl/peOiIY
- Robert-Gangneux F, Darde ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev25: 264-296. Link: https://goo.gl/mEhY5z
- Peyron F, Wallon M, Kieffer F, Garweg J (2016) Toxoplasmosis. In: Wilson CB, Nizet V, Maldonado YA, Remington JS, Klein JO, eds. Infectious Diseases of the Fetus and Newborn Infant 8 ed. Philadelphia: Elsevier Saunders.
- Musso D, Gubler DJ (2016) Zika Virus. Clin Microbiol Rev 29: 487-524. Link: https://goo.gl/p9OQVf
- McAuley JB (2008) Toxoplasmosis in children. The Pediatric infectious disease journal 27: 161-162. Link: https://goo.gl/7B6vU9
- Wong SY, Hajdu MP, Ramirez R, Thulliez P, McLeod R, et al. (1993) Role of specific immunoglobulin E in diagnosis of acute toxoplasma infection and toxoplasmosis. J Clin Microbiol31: 2952-2959. Link: https://goo.gl/RddC7F
- Stepick-Biek P, Thulliez P, Araujo FG, Remington JS (1993) IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis162: 270-273. Link: https://goo.gl/HF5AM7
- Olariu TR, Remington JS, Montoya JG (2014) Polymerase chain reaction in cerebrospinal fluid for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J33: 566-570. Link: https://goo.gl/s6Dbfw
- Luft BJ, Remington JS (1992) Toxoplasmic encephalitis in AIDS. Clin Infect Dis15: 211-222. Link: https://goo.gl/LFYmcD
- Cohn JA, McMeeking A, Cohen W, Jacobs J, Holzman RS (1989) Evaluation of the policy of empiric treatment of suspected Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Am J Med86: 521-527. Link: https://goo.gl/1eq4l6
- Nogui FL, Mattas S, Turcato Junior G, Lewi DS (2009) Neurotoxoplasmosis diagnosis for HIV-1 patients by real-time PCR of cerebrospinal fluid. Braz J Infect Dis13: 18-23. Link: https://goo.gl/rQWum6
- Mesquita RT, Ziegler AP, Hiramoto RM, Vidal JE, Pereira-Chioccola VL (2010) Real-time quantitative PCR in cerebral toxoplasmosis diagnosis of Brazilian human immunodeficiency virus-infected patients. J Med Microbiol 59: 641-647. Link: https://goo.gl/XcExcX
- Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A (1997) Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. Aids 11: 1-17. Link: https://goo.gl/hdxusR
- Grimwade K, French N, Mthembu D, Gilks C (2004) Polyuria in association with Plasmodium falciparum malaria in a region of unstable transmission. Trans R Soc Trop Med Hyg98: 255-260. Link: https://goo.gl/dCQPOv
- Davis TM, Li TA, Tran QB, Robertson K, Dyer JR, et al. (1997) The hypothalamic-pituitary-adrenocortical axis in severe falciparum malaria: effects of cytokines. J Clin Endocrinol Metab 82: 3029-3033. Link: https://goo.gl/1P4S6h
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005) The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434: 214-217. Link: https://goo.gl/M15euq
- (2015) Guidelines for the Treatment of Malaria 3rd ed Geneva2015. Link: https://goo.gl/DYoQvC
- Abanyie FA, Arguin PM, Gutman J (2011) State of malaria diagnostic testing at clinical laboratories in the United States, 2010: a nationwide survey. Malar J10: 340. Link: https://goo.gl/bhzBT7
- Murray CK, Gasser RA Jr., Magill AJ, Miller RS (2008) Update on rapid diagnostic testing for malaria. Clin Microbiol Rev21: 97-110. Link: https://goo.gl/HnVYa7
- Bell D, Peeling RW, Pacific/TDR WH-ROftW (2006) Evaluation of rapid diagnostic tests: malaria. Nat Rev Microbiol4: S34-38. Link: https://goo.gl/bchC0V
- Jenkins DJ, Romig T, Thompson RC (2005) Emergence/re-emergence of Echinococcus spp.--a global update. Int J Parasitol 35: 1205-1219. Link: https://goo.gl/R6P9HR
- Santivanez S, Garcia HH (2010) Pulmonary cystic echinococcosis. Curr Opin Pulm Med16: 257-261. Link: https://goo.gl/lZKbyu
- Nourbakhsh A, Vannemreddy P, Minagar A, Toledo EG, Palacios E, Nanda A (2010) Hydatid disease of the central nervous system: a review of literature with an emphasis on Latin American countries. Neurol Res 32: 245-251. Link: https://goo.gl/N9TT7w
- Tuzun M, Hekimoglu B (1998) Hydatid disease of the CNS: imaging features. AJR Am J Roentgenol171: 1497-1500. Link: https://goo.gl/sYvixc
- Aksungur EH, Oguz M, Bicakci K, Altay M (1994) Cerebral hydatid lesion with daughter cysts: MR and CT findings. AJR Am J Roentgenol163: 1269-1270. Link: https://goo.gl/OuXARY
- Haliloglu M, Saatci I, Akhan O, Ozmen MN, Besim A (1997) Spectrum of imaging findings in pediatric hydatid disease. AJR Am J Roentgenol169: 1627-1631. Link: https://goo.gl/Zpo1vp
- Kovoor JM, Thomas RD, Chandrashekhar HS, Jayakumar PN, Pillai S, et al. (2007) Neurohydatidosis. Australas Radiol51: 406-411. Link: https://goo.gl/JrSGiz
- McManus DP, Zhang W, Li J, Bartley PB (2003) Echinococcosis. Lancet362: 1295-1304. Link: https://goo.gl/mczl6t
- R Riganò, E Profumo, S Ioppolo, S Notargiacomo, E Ortona, et al. (1995) Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin Exp Immunol102: 281-285. Link: https://goo.gl/wYALSk
- Elena Ortonaa, Rachele Riganòa, Brigitta Buttaria, Federica Delunardoa, Salvatore Ioppolo, et al. (2003) An update on immunodiagnosis of cystic echinococcosis. Acta tropica 85: 165-171. Link: https://goo.gl/VIsib3
- Acuna-Soto R, Maguire JH, Wirth DF (2000) Gender distribution in asymptomatic and invasive amebiasis. Am J Gastroenterol95: 1277-1283. Link: https://goo.gl/W9ihax
- Aucott JN, Ravdin JI (1993) Amebiasis and "nonpathogenic" intestinal protozoa. Infect Dis Clin North Am 7: 467-485. Link: https://goo.gl/bSbBNF
- Bercu TE, Petri WA, Behm JW (2007) Amebic colitis: new insights into pathogenesis and treatment. Curr Gastroenterol Rep9: 429-433. Link: https://goo.gl/02K6sy
- Petri WA, Haque R (2013) Entamoeba histolytica brain abscess. Handb Clin Neurol114: 147-152. Link: https://goo.gl/v1pSZl
- Maldonado-Barrera CA, Campos-Esparza Mdel R, Muñoz-Fernández L, Victoria-Hernández JA, Campos-Rodríguez R, et al. (2012) Clinical case of cerebral amebiasis caused by E. histolytica. Parasitol Res110: 1291-1296. Link: https://goo.gl/ffi7se
- Wilson M, Schantz PM, Nutman T (2006) Molecular and immunological approaches to the diagnosis of parasitic infection. In: Detrick B, Hamilton RG, Folds JD, eds. Manual of Molecular and Clinical Laboratory Immunology. Washington, DC: American Society for Microbiology 557–568.
- Fogang YF, Savadogo AA, Camara M, Toffa DH, Basse A, et al. (2015) Managing neurocysticercosis: challenges and solutions. Int J Gen Med8: 333-344. Link: https://goo.gl/hcz0UG
- Garcia HH, Nash TE, Del Brutto OH (2014) Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol13: 1202-1215. Link: https://goo.gl/MpTakK
- Liu Q, Wang ZD, Huang SY, Zhu XQ (2015) Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasit Vectors 8:292. Link: https://goo.gl/4PeUTP
- Kieffer F, Wallon M (2013) Congenital toxoplasmosis. Handbook of clinical neurology112: 1099-1101. Link: https://goo.gl/Y8x2QH
- Dard C, Fricker-Hidalgo H, Brenier-Pinchart MP, Pelloux H (2016) Relevance of and New Developments in Serology for Toxoplasmosis. Trends Parasitol 32: 492-506. Link: https://goo.gl/BwZtpq
- Sahu PK, Satpathi S, Behera PK, Mishra SK, Mohanty S, Wassmer SC (2015) Pathogenesis of cerebral malaria: new diagnostic tools, biomarkers, and therapeutic approaches. Front Cell Infect Microbiol5: 75. Link: https://goo.gl/GEyLIi
- Agudelo Higuita NI, Brunetti E, McCloskey C (2016) Cystic Echinococcosis. J Clin Microbiol 54: 518-523. Link: https://goo.gl/pSf96S
- Manzano-Roman R, Sanchez-Ovejero C, Hernandez-Gonzalez A, Casulli A, Siles-Lucas M (2015) Serological Diagnosis and Follow-Up of Human Cystic Echinococcosis: A New Hope for the Future? Biomed Res Int2015: 428205. Link: https://goo.gl/rgpzN6
- Choudhuri G, Rangan M (2012) Amebic infection in humans. Indian J Gastroenterol 31: 153-162. Link: https://goo.gl/aeGRvm
- Wilkins PP (2013) Immunodiagnosis of CNS parasitic infections. Handb Clin Neurol114: 23-36. Link: https://goo.gl/hwUbsP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
