International Journal of Clinical Endocrinology and Metabolism
The Value of Positron Emission Tomography/Computerized Tomography Imaging in the Restaging of Differentiated Thyroid Cancers and Evaluation of its Association with Thyroglobulin Levels
Rana Kaya Doner1, Sait Sager1*, Fatma Arzu Gortan1, Ozge Vural Topuz1, Resit Akyel1, Cihan Gündogan2, Gamze Tatar2, Serkan Teksoz3 and Kerim Sonmezoglu1
2Istanbul Training and Research Hospital, Department of Nuclear Medicine, Turkey
3Istanbul University Cerrahpasa Medical Faculty, Department of Endocine Surgery, Turkey
Cite this as
Doner RK, Sager S, Gortan FA, Topuz OV, Akyel R, et al. (2015) The Value of Positron Emission Tomography/Computerized Tomography Imaging in the Restaging of Differentiated Thyroid Cancers and Evaluation of its Association with Thyroglobulin Levels. Int J Clin Endocrinol Metab 1(1):040-047. DOI: 10.17352/ijcem.000009Purpose: Recently, there have been an increasing number of studies indicating that Florine-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is a sensitive method in the evaluation of thyroid cancer. This retrospective study aims to assess the value of FDG PET/CT in the evaluation of thyroid cancer and determine the association between serum thyroglobulin (Tg) levels and FDG PET/CT.
Methods: We reviewed FDG PET/CT images of 104 patients with differentiated thyroid cancer (DTC) (28 men, 76 women) whose: (1) iodine-131 (131I) whole-body scanning (WBS) was negative but had elevated Tg levels or (2) Tg level was low, but showed abnormal findings using various imaging methods.
Results: The overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of FDG PET/CT findings were found to be 95.92%, 87.27%, 87.04%, 96.00%, and 91.35%, respectively. The best Tg cut-off value was found to be 10.4 ng/ml. In the Tg level <10.4 ng/ml group, the sensitivity, specificity, PPV, NPV, and accuracy of FDG PET/CT were found to be 94.12%, 91.30%, 88.89%, 95.45%, and 92.50%, respectively. In the Tg level ≥10.4 ng/ml group, sensitivity, specificity, PPV, NPV, and accuracy of FDG PET/CT exams were found to be 96.88%, 84.38%, 86.11%, 96.43%, and 90.63%, respectively.
Conclusion: The best predictive Tg cut-off value for FDG PET/CT is not yet to be determined. Since FDG PET/CT can identify residual disease and metastatic foci in cases that lack the ability of 131I accumulation, it should be included in algorithms for DTC follow-up. FDG PET/CT seems to be valuable imaging method in the evaluation of patients with highly elevated serum Tg levels and abnormal lesions identified using different imaging modalities. Furthermore, it has potential utility in the elimination of active foci that are present, and in assessing optimal decision making during follow-up.
Introduction
Thyroid cancer accounts for 94.5% of new endocrine malignancies, is responsible for 65.9% of deaths from endocrine cancers, and has a slow progression with a long survival [1]. Although differentiated thyroid cancer (DTC) is generally curable with favourable prognosis and recurrences, local and distant metastases may occur over the years. As serum thyroglobulin (Tg) measurement is the most sensitive and specific biochemical method to determine persistent disease, it is the most valuable and routinely used tumour marker [2]. Serum Tg concentration is expected to be too low to measure following surgery or surgery combined with radioactive iodine (RAI) when a total thyroid ablation has been performed. At follow-up, Tg levels can be a better predictor of disease recurrence if thyroid-stimulating hormone (TSH)-stimulated or TSH-suppressed Tg levels become detectable.
Iodine-131 (131I) whole-body scanning (WBS) plays a significant role in the management of disease after ablation. Its sensitivity and specificity to demonstrate recurrences and residual disease are 50–90% and 80–100%, respectively [3]. 131I WBS can be used to identify tumour tissue that is likely to cause elevated Tg levels in patients with TSH stimulation or TSH suppression. In one study, a diagnostic 131I WBS yielded negative results in 15–50% of patients with elevated serum Tg levels [3]. Possible reasons why 131I WBS can be negative while Tg levels are high include: (1) Iodide trapping defects: Many molecules that are responsible for the uptake and organification of iodine such as sodium/iodide symporter protein, thyroid peroxidase, and pendrin, are defective in malignant thyrocytes [4]. Here, thyrocyte-specific functions are lost, decreasing the uptake of iodine [4]. (2) Loss of differentiation: It is known that roughly 33% of patients with DTC have less differentiated tumours and 1.8% have anaplastic transformations. (3) Small-size metastases: Even if malignant thyrocytes have the ability to concentrate iodine, they may be too small in size to be detected scintigraphically [4]. (4) Improper preparation: Prior to 131I WBS, patients have to go through the preparation phase to increase RAI uptake in the cells. Improper preparation may yield false negative (FN) results [4].
Neck ultrasonography (USG) is the most sensitive imaging method in the assessment of on-going or recurrent disease in long-term follow-up. It is very frequently used in the management of patients with thyroid cancer because it is non-invasive, easy to apply, and is an inexpensive imaging method. Often, ultrasonography is specifically utilized in the investigation of local recurrence, lymphatic involvement, and in the postoperative period for the determination of residual thyroid tissue. Although USG is the only imaging modality for control in patients with low-level serum Tg during follow-up, it has to be the first choice to investigate local recurrence and lymph node metastases noting increase in Tg levels [5]. Patients with detectable Tg values and 30 mIU/L TSH levels, but no 131I WBS uptake, should be evaluated with sophisticated imaging methods. These methods include Tl-201, Tc-99m MIBI, Tc-99m-tetrofosmin, computed tomography (CT), magnetic resonance imaging, or scans with radiopharmaceuticals such as florine-18fluorodeoxyglucose (18FDG) [6,7].
Numerous studies have shown that 18FDG has the highest sensitivity [6,7]. It has been demonstrated that 18FDG positron emission tomography (PET)/CT has a 92% positive predictive value (PPV) for establishing the foci of recurrence/metastases when serum Tg levels increase, and it has a 93% negative predictive value (NPV) for excluding the disease when Tg levels are lower [8]. F-18FDG PET is the metabolic imaging modality that has been increasingly used in clinical practice. This method has routinely been applied in various cancers with pinpoint accuracy. In recent years, 18FDG PET has become a common imaging modality for thyroid cancer patients with a positive Tg test and negative 131I results. Pioneering studies have demonstrated that 131I WBS and 18FDG PET imaging play a complementary role in the detection of tumour recurrence and metastases [9]. The sensitivity of 18FDG PET imaging alone to detect metastases in patients with DTC was 50%, while that of 18FDG PET together with 131I was 95% [6]. An inverse relationship was detected between uptake of 131I and that of 18FDG, in metastases. It was suggested that 131I uptake continued to exist in DTC metastases and that 18FDG accumulation was low. However, dedifferentiated metastases and diminished 131I accumulating capacity could be visualized by 18FDG, demonstrating higher metabolic activity.
Consistent with this inverse relationship, the so-called “flip-flop” model was first described by Feine et al. and 18FDG PET/CT has been reported to contribute to cases with positive serum Tg levels and negative 131I results [6,10]. Our study population consisted of thyroid cancer patients with negative 131I WBS findings. However, Joensuu and Ahonen reported that some metastases accumulated both 18FDG and 131I imaging [11]. Hung et al. have shown that in some recurrent or metastatic cases of DTCs, incidental focal uptake of 18FDG and iodine can be found. Therefore, in order to optimize the detection of disease sites, both methods have to be used together and the sensitivity can be increased up to 95% [12].
It is possible that the same patient may have multiple metastatic foci with varying degrees of differentiation, therefore, some foci show 131I uptake while the others do not. 18FDG PET/CT is more efficacious in situations where the necessary measures can be taken prior to RAI-negative metastases developing and promoting metastatic disease [13]. A number of studies have emphasized that 18FDG PET/CT findings can be significant when Tg measurements are ≥10 ng/ml [5,14]. Additionally, in the revised American Thyroid Association (ATA) guidelines, the sensitivity of 18FDG PET/CT to detect recurrence/metastatic disease is as low as 11–13% when Tg levels are <10 ng/ml [5]. The aim of this study was to identify the value of 18FDG PET/CT in re-staging DTC, and to evaluate the association between serum Tg levels and 18FDG PET/CT in DTC patients with: (1) a negative diagnostic 131I WBS and elevated Tg or (2) lower serum Tg levels, but abnormal findings detected by other imaging modalities.
This study was a retrospective study and it was accepted by institutional ethical committee. The aim of this study is from a retrospective observation of the results of FDG PET/CT to find the cut-off value of thyroglobulin in the restaging of differentiated thyroid cancers.
Materials and Methods
We retrospectively analysed 104 patients (76 women and 28 men) with histopathologically proven DTC after total thyroidectomy and radioiodine ablation between April 2007 and September 2012. 18FDG PET/CT images were evaluated retrospectively. Inclusion criteria for the present 18FDG PET/CT study were elevated serum Tg levels and negative 131I WBS, or patients whose Tg level was low, but showed abnormal findings in various imaging methods.
The age of patients at the time of 18FDG PET/CT scans ranged from 11 to 79 years (mean age 46.37 ± 15.69 years). Ninety-four patients (90.4%) were diagnosed with papillary thyroid carcinoma (PTC) and 10 patients (9.6%) were diagnosed with follicular thyroid carcinoma compatible with pathology findings. The disease stage at diagnosis was defined according to the TNM Classification System of Malignant Tumours. The pathologic tumour stages are listed in Table 1.
Radioactive 131I therapy was applied to all patients at least once before 18FDG PET/CT imaging was performed. The number of therapy regimens of patients ranged from 1 to 7 times, with the mean being 2.55 ± 1.31 and the median being 2. The average dose of 131I was 351.59 ± 243.32 and the median was 300 mCi (11.100 MBq). Laboratory results (TSH, Tg, anti-Tg) at the time of 18FDG PET/CT scans are shown in Table 2. Serum TSH, Tg, and anti-Tg values were measured by radioimmunoassay or enzyme-linked immunosorbent assay in all cases. The tests were repeated in a different centre to increase accuracy.
Tg levels ranged 0.1–1000 ng/ml, the average being 14.40 ± 4.71. The distribution of Tg serum levels were as follows: 17.3% (n = 18) <5 ng/ml; 18.3% between 5 and 9.9 ng/ml; 28.8% (n = 30) between 10 and 19.9 ng/mL and 35.6% ≥20 ng/ml. Anti-Tg measurement ranged 0–120 (average = 18.87 ± 9.55). TSH levels were between 0 and 100 (ng/ml) (average = 63.86 ± 38.98; median = 74.7). Forty patients (39%) underwent operation following 18FDG PET/CT imaging, 45 patients (43%) were included in the clinical-radiological follow-up, and 19 patients (18%) were treated with RAI.
18FDG PET/CT images were acquired with a 6-slice multidetector CT integrated high-resolution PET scanner (Biograph LSO HI-REZ PET/CT; Siemens, Chicago, Illinois, USA). 18F-FDG (444-629 MBq; 12–17 mCi) was intravenously injected into patients fasting a minimum of 4 hours with blood glucose levels <150 mg/dL. Following injection of 18FDG, patients rested for 60–90 minutes in a quiet room. Next, they emptied their bladders and were taken onto PET/CT scanner beds to assess the bio distribution of the radiopharmaceutical.
Tomogram images, non-contrast low-dose CT images from the vertex to the upper third portion of the thigh, as well as PET images, were acquired. PET data were acquired from 7–9 bed positions, for 3 minutes per bed position. PET images were reconstructed by iterative methods and attenuation correction was performed using a computed tomography attenuation correction series generated by CT images. Sagittal, coronal, and transverse images of PET were obtained. PET images, CT images, and retrospectively fused PET/CT images were simultaneously analysed. The main criterion for review was to identify increased focal 18FDG uptake above the background by visual assessment. Each focal 18FDG uptake on PET images was further analysed on corresponding CT images. Focal 18FDG uptake (e.g., the salivary gland, muscle, adipose tissue, and normal lymphoid tissue) on CT scans was considered to be a physiological manifestation. The presence of abnormal, focal 18FDG uptake in the lymph nodes or soft tissue mass on corresponding CT images was considered significant in terms of recurrence or metastasis. Mild 18FDG uptake in inflammatory/infectious lesions on corresponding CT was not regarded as recurrence/metastasis.
Findings obtained from 18FDG PET/CT scan imaging were correlated with histological examination, other imaging modalities such as USG, CT, and MRI; post-therapeutic 131I WBS, subsequent Tg titres, and clinical follow-up. 18FDG PET/CT scan findings were classified as follows:
(1) true negative (TN), significant 18FDG accumulation on 18FDG PET/CT in assessing for recurrence or metastasis not noted, no foci noted by various diagnostic methods, and improved or stable Tg titre without the need for further treatment; (2) true-positive (TP), histopathology findings of 18FDG accumulation confirmed to be positive by re-operation or by biopsy, enhanced Tg level detected in aspiration fluid and confirmed by other imaging techniques, or persistently abnormal follow-up Tg titre and increased Tg titre that does not decrease after empirical high-dose radioiodine treatment; (3) FN, negative PET/CT findings in terms of recurrence or metastatic disease, but histopathologically established carcinoma, or any pathological focus determined by other methods; and (4) false positive (FP): any carcinoma not detected by means of histological examination of a lesion that has been evaluated as recurrence or metastasis by 18FDG PET/CT, or 18FDG accumulation is not confirmed by another method.
For statistical analyses, Number Cruncher Statistical System (NCSS) 2007 & Power Analysis and Sample Size (PASS) 2008 Statistical Software (Utah, USA) was used. Student’s t test was used for the comparison of descriptive statistical methods (mean, standard deviation, median, rate, and frequency) and normally distributed parameters; Mann-Whitney U test was used for the comparisons of non-normally distributed parameters. Fisher’s exact test and Yates Continuity Correction (Yates-corrected chi-squared) test were used for qualitative comparison. The diagnostic screening tests (sensitivity, specificity, PPV, NPV, and the ROC curve analysis were used for determining the cut-off value for parameters. P < 0.05 was considered significant.
Results
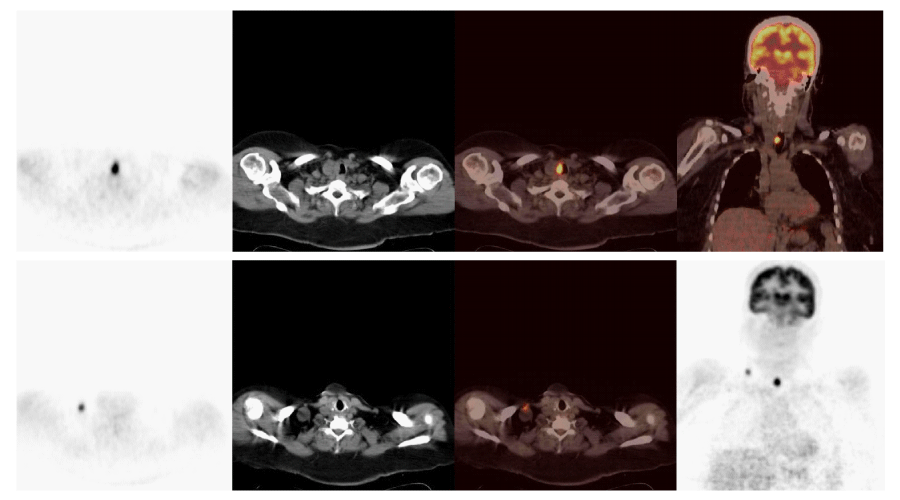
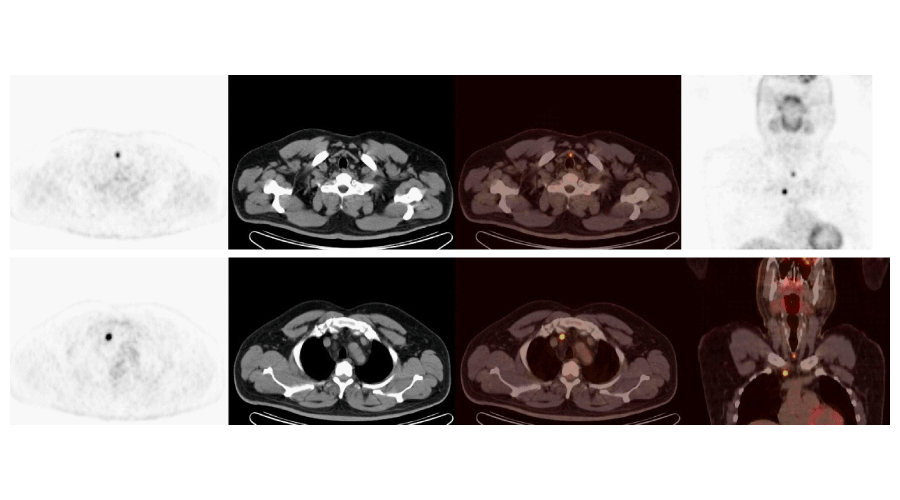
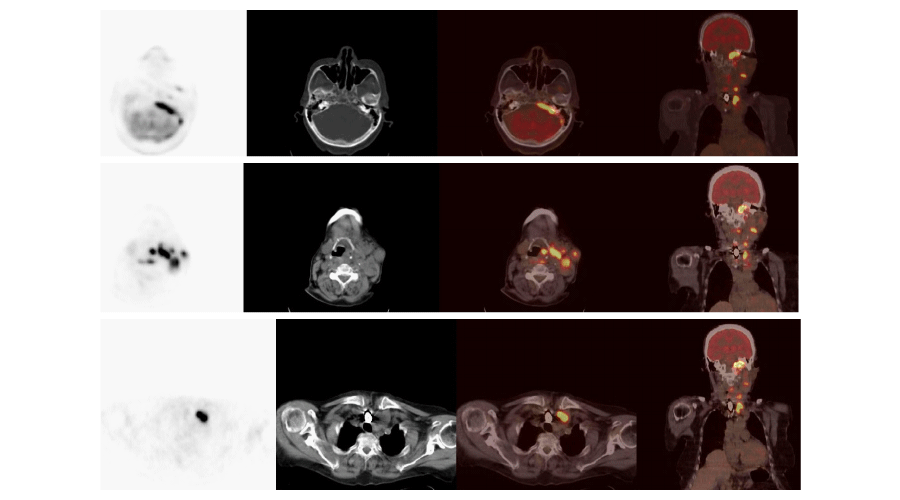
Using 18FDG PET/CT, 54 out of a total of 104 patients, were identified to have abnormal metabolic activity consistent with metastatic and/or local disease. Thirty-nine of these patients underwent surgery (Figures 1, 2), of which, 6 were followed-up clinically/radiologically and 9 received RAI therapy (Figure 3). Empirical RAI therapy was given to 10 patients despite having no lesions accumulating 18FDG. Only one patient without any lesions accumulating 18FDG, as determined by 18FDG PET/CT, was operated upon. This was due to an abnormal soft tissue lesion on the right side of the thyroid gland through the neck USG, but the histopathology findings were benign. Overall, 45.2% of patients (n = 47) were determined to have TP findings, 6.7% (n = 7) had FP findings, 46.2% (n = 48) had TN findings, and 1.9% (n = 2) had FN findings. Cases of recurrence/metastasis on PET/CT are summarized in Table 3.
The mean serum Tg levels of patients showing recurrence/metastasis on PET/CT was 23.03 ± 4.27 while that of cases without recurrence/metastasis was 8.67 ± 4.50 (Table 4). Accordingly, there was a statistically significant difference with regard to serum Tg levels based on recurrence/metastasis on PET/CT (p < 0.01). The serum Tg levels were found to be significantly higher in cases with recurrence/metastasis on PET as compared to cases without recurrence/metastasis (Table 4).
Calculation of cut-off values of Tg was considered, and ROC analysis and diagnostic screening tests were used according to recurrence/metastasis on PET for determining the cut-off value. The best cut-off value was determined to be 10.4 for Tg serum levels, and the area under ROC curve was 62.7% with a standard deviation 5.4% (Tables 5, 6).
For the 104 patients included in this study, the sensitivity, specificity, PPV, NPV, and the accuracy of PET/CT examinations were found to be 95.92%, 87.27%, 87.04%, 96.00%, and 91.35%, respectively. In patients with Tg levels <10.4; the sensitivity, specificity, PPV, NPV, and the accuracy of PET/CT were established to be 94.12%, 91.30%, 88.89% 95.45% and 92.50%, respectively. In patients with Tg levels ≥10.4; the sensitivity, specificity, PPV, NPV, and the accuracy of PET/CT were detected to be 96.88%, 84.38%, 86.11%, 96.43%, and 90.63%, respectively. The statistical analyses of our patients are summarized in Table 7.
Discussion
In 104 DTC cases with a negative 131I WBS despite elevated serum Tg levels, or those with abnormal lesions identified by other imaging modalities, we found that the overall sensitivity and specificity of 18FDG PET/CT in the detection and localization of recurrence, or in establishing the diagnosis of metastatic thyroid cancer, were 95.92% and 87.27%, respectively. In addition, our PPV, NPV, and accuracy were 87.04%, 96.00%, and 91.35%, respectively. In a study by Na et al., performed on patients diagnosed with DTC with elevated serum Tg levels despite negative 131I WBS, the overall sensitivity, specificity, accuracy, PPV, and NPV of 18FDG PET/CT were 69.4%, 66.7%, 69.1%, 95.6%, and 17.4%, respectively [15]. The sensitivity of 18FDG PET/CT was 68.4% in another study performed on 61 patients with suspected recurrence [16]. In a multicentre study conducted in a cohort of 220 German patients, the sensitivity of 18FDG PET/CT was 75%. However, the sensitivity increased to 85% when only those with negative 131I WBS were taken into account. In that study, 18FDG PET/CT was reported to be superior to other imaging methods in a group of patients with negative diagnostic 131I WBS, but with positive serum Tg levels [17].
Our findings have also suggested that 18FDG PET/CT is a potentially helpful method in detecting loco-regional recurrence or distant metastasis as well as precise localization in patients with DTC. We obtained TP results in 87.0% (n = 47/54) of patients with lesions accumulating 18FDG, suggesting recurrence/metastasis on PET/CT scans. The lungs and bone marrow are the most common loco-regional metastases of thyroid cancer [18]. Similarly, in 10 patients, we observed loco-regional and pulmonary metastases to be the first and second most frequent site of metastasis, respectively. The rate of TN results of our study was 96.0%. These findings were confirmed by neck USG, CT/MRI, and clinical follow-up. In a study by Jeong et al., regarding staging of cervical lymph nodes in patients with PTC, 18FDG PET/CT neck USG and contrast-enhanced neck CT were performed and the authors reported no significant difference with regard to lymph node imaging [19]. However, it must be kept in mind that reactive changes in the neck region after thyroidectomy and radioiodine treatment is likely to result in misinterpretation of radiological data. Therefore, 18FDG PET/CT should be the method of choice during follow-up [19].
We identified false positive results in 7 patients (13.0%). Since 3 out of 7 patients had foci suggesting recurrence in the thyroid region of the neck on the 18FDG PET/CT, 2 were identified to have granulomatous inflammation based on the pathological result of excision, and 1 had dyskaryotic cells according to biopsy findings; 18FDG PET/CT results were considered to be FP. The images of lymph nodes in the neck of another 4 cases were evaluated as DTC metastasis on the 18FDG PET/CT. However, the result of a neck dissection performed in 2 of the cases proved to be reactive hyperplasia, and histology findings of a lymph node biopsy of the other 2 cases proved to be mature and immature lymphoid cells. The literature describes reasons that may yield FP findings, including a secondary malignancy, parathyroid adenoma, focal abscesses, and thyroiditis [16]. A variety of studies reported that visualizing physiologic uptake of FDG in non-specific brown fat, muscle, vocal cord, salivary glands, tonsils, and other lymphoid tissues using 18FDG PET in lieu of 18FDG PET/CT, yields FN findings [20,21]. Instead of 18FDG PET, we used 18FDG PET/CT, which provides both anatomical and metabolic information, and was able to differentiate the physiological 18FDG uptake from pathological 18FDG uptake.
Another recent study reported an increase in specificity from 50% to 89% when comparing 18FDG PET results with those of 18FDG PET/CT, respectively [16]. Two out of our 50 cases (4.0%) without any lesions on 18FDG PET/CT, showed FP findings. One of these patients RAI-WBS with a 5-mCi dose of 131I had been negative, showed pathological uptake in the thyroid region on 131I WBS, after empiric high dose 131I was administered following 18FDG PET/CT. As seen with this patient, high-dose RAI therapy should be empirically administered to patients in whom no foci is detected with advanced imaging techniques; it is likely to assess whether pathologic RAI uptake exists or not on screening following therapeutic dose. This is important for determining further treatment options. The other FP case had a lesion suggestive of recurrence in the thyroid region, which was detected on MRI performed after 18FDG PET/CT, with a significant elevation in Tg levels (from 17.4 ng/ml to 71.3 ng/ml) during follow-up. An increase in Tg levels indicated the presence of active disease; therefore, it was evaluated to be FN.
131I negative cases as well as anti-Tg antibody positivity is one of the important dilemmas in the follow-up of DTC. As antibodies cause alterations in Tg measurements, calculating Tg levels does not have any contribution to follow-up in these cases. 131I WBS has been used in residual tumour research, and in the presence of antibody positivity, it is assumed to indicate a residual tumour. 131I WBS performed with low-dose especially for diagnosis has relatively low sensitivity therefore, 18FDG PET is considered invaluable for routine follow-up algorithms. The mean of anti-Tg levels of our patients was 18.87 ± 9.55 and we found no suspected increase in any patient.
In our study, the average serum Tg level was 23.03 ± 4.27 ng/ml, in cases showing recurrence/metastasis on 18FDG PET/CT, while it was 8.67 ± 4.50 ng/ml in those without recurrence/metastasis on 18FDG PET/CT. There was a statistically significant difference between Tg measurements based on recurrence/metastasis (p < 0.01), i.e., the rate of Tg measurements in cases with recurrence/metastasis was higher than those without recurrence/metastasis. Choi et al., found a considerably higher difference between sensitivity of 18FDG PET and serum Tg levels (p < 0.0005) [22]. We calculated the Tg cut-off value and considered the best Tg cut-off value to be 10.4 based on recurrence/metastasis on 18FDG PET/CT exams. Schluter et al., reported that rising Tg levels were positively correlated with TP 18FDG PET findings, and proposed that 18FDG PET is beneficial when the level of Tg is >10 ng/ml [23]. Although most studies have emphasized that 18FDG PET/CT identifies the foci of recurrence/metastasis with great accuracy when the Tg value is ≥10 ng/ml [23-25], a limited number of studies have reported that no association exists between 18FDG PET/CT findings and Tg titres [26].
We found that a large number of 18FDG PET/CT findings were TP in DTC patients with Tg levels ≥10.4 ng/mL. When Tg levels surpass the threshold i.e., ≥10.4 ng/mL, 18FDG PET/CT has a high impact in locating the foci of diseases in accordance with the literature. In a study by Ma et al., a threshold value of 10 ng/ml was used for Tg measurements for administering 131I, and this value has been proposed in the ATA manual [5,27]. At this point, however, it should be noted that clinicians may consider 18FDG PET/CT scans necessary in the presence of stable disease in order to rule out focal active disease and to decide follow-up. We reported the number of TP 18FDG PET/CT findings to be 21 out of 40 DTC patients with Tg levels <10.4 ng/ml and we calculated NPV to be 95.45%; therefore, this suggests that use of 18FDG PET/CT is appropriate for the aforementioned reasons.
It has been reported that NPV of the 18FDG PET/CT examination is 93% in the presence of lower serum Tg levels [8]. We classified patients as having TN findings if they exhibited a long disease-free period during follow-up. Previous studies, as well as ours, should be interpreted carefully because DTC progression is slow. In the current study, the number of DTC patients with FP findings (n = 7) is consistent with the literature [16]. Of these 7 cases, 5 had serum Tg levels ≥10.4 ng/ml. This explains why specificity, PPV, and accuracy in this group are lower in patients with serum Tg levels <10.4 ng/ml. The reason why specificity of 18FDG PET/CT in cases with Tg levels ≥10.4 ng/ml was relatively lower than those with levels <10.4 ng/ml was attributed to 5 of the 7 patients with false-positive findings, having Tg levels ≥ 10.4 ng/ml.
One of the potential indications for 18FDG PET/CT that is significant, but does not draw enough attention, is the evaluation of lesions suspected of metastasis via imaging methods. Ultrasonography is a conventional imaging modality that provides the most significant contribution to the follow-up of DTC, and is a rapid and valuable test method in the evaluation of local recurrence and regional lymph node metastasis. 131I WBS together with 18FDG PET/CT is beneficial for the follow-up evaluation of lesions detected by imaging methods, but not suitable for needle biopsy. Although serum Tg level was <2 ng/ml in our 12 patients, USG findings proved to be suspicious. Hence, 18FDG PET/CT examination was performed and revealed that 6 patients had abnormal lesions accumulating 18FDG. Of these lesions, 3 raised doubts about metastasis in cervical lymph nodes. The other 3 lesions were suspected of recurrence in the thyroid region, and metastases in the proximal left clavicle and in the lungs. Chest CT scans confirmed suspicious metastatic lesions found in the lungs, and the other 5 cases underwent operation. Histopathological findings confirmed bone metastasis of the follicular carcinoma in the left clavicle and the others were metastases from PTC. The findings of 18FDG PET/CT on these 6 patients were accepted as TP. In the other 6 cases with serum Tg level <2 ng/ml, no lesion accumulating FDG was detected on 18FDG PET/CT and TN findings were confirmed during clinical follow-up.
Given the findings of our study, we showed that 18FDG PET/CT is useful in detecting both recurrence/metastasis and revealing TP findings. Therefore, it is safe and valuable to follow-up patients with suspicion of recurrence by radiological/clinical findings, despite lower serum Tg levels. Altenvoer et al., reported that18FDG PET/CT is a potentially useful method to detect metastatic lesions/recurrence, when a serum Tg cut-off level is around 20 ng/mL [28]. Bertagna et al., demonstrated that for patients with Tg levels >21 ng/mL, 18FDG PET/CT has the highest accuracy [29]. Dahel et al., studied 16 patients with DTC, and reported that it is also possible to detect recurrence by means of 18FDG PET/CT imaging in patients with Tg levels <10 ng/mL [30]. Kim et al., suggested that independent of serum Tg levels, 18FDG PET/CT has good sensitivity and specificity in the detection of recurrent PTC in 61 patients [31].
In 2011, Bannas et al., analysed 18FDG PET/CT findings of 30 DTC patients who had high serum Tg levels and negative radioiodine WBS, after a total thyroidectomy followed by radioiodine ablation. FDG accumulating lesions were identified by 18FDG PET/CT in 19/30 patients. 18FDG PET/CT results were TP, FP, TN, and FN in 17, 2, 3, and 8 patients, respectively. Overall sensitivity, specificity, and accuracy were 68.0%, 60.0%, and 66.7%, respectively. The sensitivity, specificity, and accuracy were considerably elevated in the subgroups by 70.0%, 100.0%, and 71.4%, respectively [32]. Na et al., examined 18FDG PET/CT images of 60 DTC patients with elevated serum Tg levels as well as negative radioiodine WBS in 2010. They divided the patients into 4 sub-groups (2 ng/ml ≤ stimulated Tg [sTg] < 5 ng/ml; 5 ng/ml ≤ sTg < 10 ng/ml; 10 ng/ml ≤ sTg < 20 ng/ml; and ≥ 20 ng/ml)] and found that the sensitivity of PET/CT according to Tg levels was 28.6% when Tg was between 2 and 5, 57.1% between 5 and 10, 60.0% between 10 and 20, and 85.7% when Tg was ≥ 20 ng/ml [15]. In a study by Shammas et al., comprising of 61 DTC patients, the overall sensitivity, specificity, and accuracy of 18FDG PET/CT were 68.4%, 82.4%, and 73.8%, respectively. The sensitivity of the F-18FDG PET/CT was observed to be 60%, 63%, and 72% in the groups with serum Tg levels <5, 5 to 10, and >10 ng/ml, respectively [16].
Conclusion
A precise Tg cut-off value of 18FDG PET/CT is yet to be identified. Since 18FDG PET/CT enables identification of residual disease and metastatic foci in cases that lack capability of 131I accumulation, it should be included in routine follow-up algorithms for DTC. While metastatic foci accumulating 131I respond well to treatment with high doses of 131I, foci not accumulating 131I will have to be evaluated primarily for surgery. Therefore, determination of the 131I negative foci may be of critical importance for both the treatment approach and ultimately for survival. In patients with DTC, 18FDG PET/CT is a valuable imaging method in the evaluation of cases with abnormally high serum Tg levels, in patients with abnormal lesions identified with other imaging modalities, in eliminating the presence of active foci, and in optimizing appropriate decision-making during follow-up.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
