International Journal of Clinical Endocrinology and Metabolism
Clinical Aspects of C-Type Natriuretic Peptide on the Cardiovascular System
Silvana M Cantú1,2, Adriana S Donoso1,2, Nicolás M Kouyoumdzian2,3, Natalia L Rukavina Mikusic2,3, Ana M Puyó1,2 and Marcelo R Choi1,2,3*
2INFIBIOC-UBA, Argentina
3ININCA-UBA-CONICET, Argentina
Cite this as
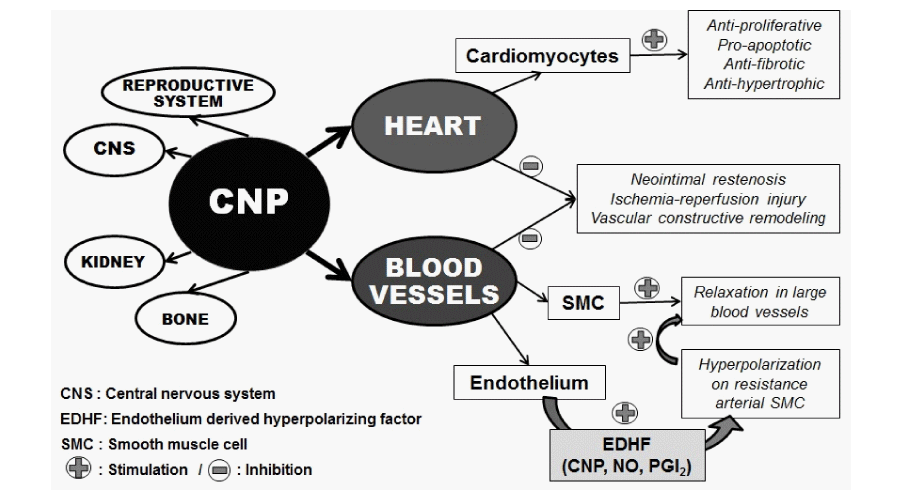
Cantú SM, Donoso AS, Kouyoumdzian NM, Rukavina Mikusic NL, Puyó AM, et al. (2015) Clinical Aspects of C-Type Natriuretic Peptide on the Cardiovascular System. Int J Clin Endocrinol Metab 1(1):031-036. DOI: 10.17352/ijcem.000007C-type natriuretic peptide (CNP) is one of the three most important members of the human natriuretic peptide family, sharing with them a highly conserved 17-aminoacid ring structure, essential to elicit its biological actions by binding to its specific receptor NPR-B. CNP acts in a paracrine or autocrine way and it is cleared from plasma circulation by the NPR-C receptor. This is one of the reasons why its plasma levels are usually low. CNP is widely spread in human tissues, being localized in a variety of cells such as cardiac and vascular smooth muscle cells, chondrocytes, macrophages, glial cells, and renal, brain and reproductive tissues. In the cardiovascular system, CNP is stored in endothelial cells and exert different actions like vasodilation in large conduction vessels, and anti-proliferative, pro-apoptotic, anti-fibrotic and anti-hypertrophic properties in cardiomyocytes; it also inhibits neointimal restenosis and reduces cardiac ischemia-reperfusion injury and vascular constrictive remodeling. In addition, it has been suggested that CNP acts as an endothelium derived hyperpolarizing factor as a consequence of its hyperpolarizing action in resistance arterial smooth vessels muscle. Regarding its clinical aspects, it has been demonstrated the relation between CNP and different cardiovascular pathologies such as heart failure, myocardial infarction, cardiac remodeling, arterial hypertension, angiogenesis and revascularization, restenosis, atherosclerosis, pulmonary hypertension, and myocardial aging.
Abbreviations
Ang II: Angiotensin II; ANP: Atrial Natriuretic Peptide; BNP: B-type Natriuretic Peptide; BZ: Border Zone; CHF: Chronic Heart Failure; CNP: C-type Natriuretic Peptide; EDHF: Endothelium Derived Hyperpolarizing Factor; HF: Heart Failure; I/R: Ischemia/Reperfusion; LV: Left Ventricle; MI: Myocardial Infarction; N: Healthy Myocardium; NO: Nitric Oxide; NP: Natriuretic Peptide; NPR-A: Natriuretic Peptide Receptor A; NPR-B: Natriuretic Peptide Receptor B; NPR-C: Natriuretic Peptide Receptor C; NPs: Natriuretic Peptides; NYHA: New York Heart Association; RZ: Remote Zone
Introduction
The endocrine properties of the heart were described for the first time by de Bold et al. in 1981, who demonstrated that the right atrium produces and secretes a natriuretic and diuretic compound, characterized and named three years later as atrial natriuretic peptide (ANP) [1]. This was the first member of the natriuretic peptide (NP) family to be identified [1,2]. Natriuretic peptides (NPs) are hormones produced by the heart in response to myocardium stretching and cardiac overload [3]. This family includes atrial or A-type natriuretic peptide, brain or B-type natriuretic peptide (BNP), C-type natriuretic peptide (CNP), and urodilatin, which is synthesized by the kidney and is present in human urine [4]. There are two other NPs apparently not produced by humans: DNP, isolated from the venom of Dendroaspis angusticeps or eastern green mamba, and TNP, isolated from the venom of Oxyuranus microlepidotus, also known as inland or western taipan [4]. The aim of this work is to review the latest evidence about the clinical role of CNP on the cardiovascular system.
Natriuretic peptides family and receptors
ANP and BNP are important factors synthesized mainly in the heart and contribute to the regulation of hydroelectrolytic balance and maintenance of vascular smooth muscle tone [5]. These effects are exerted directly by these hormones or indirectly through antagonism of the renin-angiotensin-aldosterone system [5]. They also play a role in adipose tissue generation and lipid metabolism [5-7]. Like other NPs, CNP is secreted as a pro-hormone and needs to be cleaved by proteases to become biologically active [2]. Under normal conditions, ANP is usually synthesized by the atrial myocardium while BNP is synthesized primarily by the ventricular myocardium [2]. On the other hand, CNP is produced by endothelial cells, but it is also present at the central nervous system [2,8]. The NPs are able to bind to three distinct cell surface receptors, natriuretic peptide receptor A (NPR-A), natriuretic peptide receptor B (NPR-B) and natriuretic peptide receptor C (NPR-C) [3]. NPR-A and NPR-B are transmembrane receptors coupled to particulate guanylate cyclase and are responsible in mediate the NPs biological actions, while NPR-C is coupled to an inhibitory G protein and is considered a clearance receptor through internalization and degradation of NPs [9] (Table 1).
C-Type natriuretic peptide
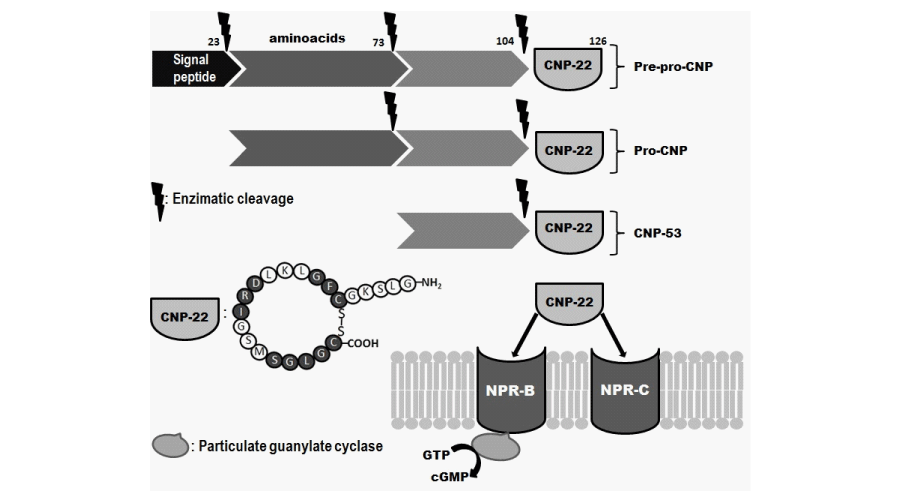
CNP was the third NP to be identified, and it was first purified in 1990 from porcine brain tissue [10]. It is the most spread NP in humans, localized in cardiac and vascular smooth muscle cells, chondrocytes, macrophages, glial cells, renal and brain tissues, and reproductive tissues [6,11,12]. CNP shares a highly conserved 17-aminoacid disulfide ring structure with other members of the natriuretic peptide family but lacks of the carboxy-terminal extension present in both ANP and BNP [2]. The ring structure sequence is essential for receptor binding and to exert its biological activity [13]. Encoded in chromosome 2, CNP gene has two different forms, both derived from a pre-pro-hormone with a 126-aminoacid structure [11]. The enzymatic cleavage of this molecule generates a 53-aminoacid compound called CNP-53, and the active biological form of 22-aminoacid called CNP-22, primarily found in plasma and cerebrospinal fluid [14] (Figure 1).
Transcription of CNP gene is regulated by different factors such as several pro-inflammatory cytokines including tumor necrosis factor alpha, interleukin-1, transforming growth factor beta, and basic fibroblast growth factor [13]. CNP secretion is also stimulated by bacterial lipopolysaccharides, shear stress and by ANP and BNP, being BNP much more potent than ANP [11,13]. CNP triggers a variety of important functions that are not related between them, such as regulation of endochondral bone ossification, central nervous system functions (in particular brain and pituitary), female and male reproductive systems, and cardiovascular function maintenance through endothelium and vascular smooth muscle regulation [5]. Although considered a natriuretic peptide, CNP apparently has no relevant effects on renal water and sodium excretion [2].
CNP acts in a paracrine or autocrine way, while ANP and BNP act by an endocrine mechanism [5]. Consequently, CNP plasma levels are usually low (femtomole per milliliter range) and it is rapidly cleared out from blood flow (t1/2 ~ 3 minutes), being its expression restricted to those organs and tissues that are regulated by CNP [5,10,11]. CNP acts by specific binding to its receptor NPR-B, mainly localized in different human tissues such as brain, myocardium, endothelium, vascular smooth muscle cells and renal tubular cells [14]. The CNP clearance depends on its binding to NPR-C receptors [13] (Figure 1).
CNP and the cardiovascular system
CNP is widely expressed along the vascular bed and is highly expressed in endothelium where is stored and plays an important role by inducing vasorelaxation in large conduction vessels through activation of NPR-B receptor subtype [12]. As a vasodilator agent, CNP inhibits the release of endothelin stimulated by angiotensin II (Ang II) [15]. CNP can also activate NPR-C receptor that leads to an hyperpolarizing effect in resistance vascular bed, suggesting that CNP acts as an endothelium derived hyperpolarizing factor (EDHF) together with other paracrine endothelial vasorelaxant mediators such as nitric oxide (NO) and prostacyclin [2,13,16,17].
CNP exhibit several actions on cardiomyocytes such as anti-proliferative, pro-apoptotic, anti-fibrotic and anti-hypertrophic effects [18,19]. It also inhibits neointimal restenosis and reduces cardiac ischemia-reperfusion injury and vascular constrictive remodeling [18]. These beneficial effects should be related to the inhibition of cardiac fibroblasts collagen synthesis due to an inhibitory effect on DNA [12,19]. Furthermore, CNP could regulate blood pressure by promoting a potent endothelium-independent vasodilatory action in small resistant arteries [7]. The expression of CNP in injured arterial vessels stimulates inducible nitric oxide synthase secretion leading to NO production [14]. It also inhibits angiotensin I vasoconstrictive effect but not that of Ang II, suggesting that CNP may exert local endogenous regulatory actions on vascular angiotensin converting enzyme (ACE) [14,15] (Figure 2).
The abundant location of CNP to endothelial cells and the fact that transcription of its gene may be regulated by several pro-inflammatory cytokines could explain its important role in modulating the activity of immune cells. Kiemer et al. demonstrated that CNP suppressed in vitro production of pro-inflammatory cyclo-oxygenase 2- derived prostaglandin E2 in isolated cells [20]. ANP also inhibits vascular inflammation in vivo. It has been reported that perivascular application of CNP inhibits neointimal hyperplasia of vein grafts in mouse, rabbit, and pig models [21-23].
Heart failure
In 1993, using immunohistochemistry techniques, Wei et al. found higher CNP tissue concentration in atrial and ventricular myocardium of patients undergoing coronary bypass graft surgery and from patients with end-stage heart failure undergoing cardiac transplantation, compared to hearts from normal donors [24]. In 2003, another study demonstrated that CNP plasma levels significantly increased in coronary sinus in patients undergoing right and left heart catheterization as part of their congestive heart failure treatment (coronary sinus: 4.59±1.54 pg/mL versus aortic root: 3.55±1.53 pg/mL; p<0.035) [25]. These findings correlated directly with CNP myocardial production [25]. Moreover, del Ry et al. demonstrated that plasma CNP levels were significantly higher in chronic heart failure (CHF) patients compared to healthy subjects in relation to clinical severity evaluated according to the New York Heart Association (NYHA) [26-28]. In this study, CNP plasma level in healthy subjects was 2.7±0.2 pg/ml; in NYHA class I patients it was 4.9±0.7 pg/ml; in NYHA class II patients it was 7.0±0.4 pg/ml (p<0.001 versus controls); in NYHA class III patients it was 9.6±0.7 pg/ml (p<0.001 versus controls and class I and II), and in NYHA class IV patients it was 11.8±2.0 pg/ml (p<0.001 versus controls, class I and II) [26]. These data suggests that elevated CNP from cardiac tissue could be related to an increasing myocardial dysfunction [12]. Another study reported that CNP is synthesized and secreted by cardiac fibroblasts in order to inhibit cardiomyocytes hypertrophy [5]. Taking all these considerations together, it seems that the heart produces and secretes CNP during heart failure triggering an important compensatory physiological response to cardiac remodeling [13]. In this setting, the high levels of CNP secretion are apparently related to the severity of the disease, as it was showed by the progressive increment in CNP plasma levels with worsening of symptoms, as well as the negative relationship between plasma CNP levels and left ventricular ejection fraction [3,25]. Passino et al demonstrated that CNP plasma concentration can be positively affected by a therapeutic intervention, such as physical training in heart failure (HF) patients [29]. They hypothesized that the decrease in plasma concentrations of the hormone observed at the end of a period of aerobic training could be due to improvement of the endothelial function achieved after an aerobic exercise-training program [29]. On the other hand, Zakeri et al. observed that urinary excretion of CNP is elevated in acute decompensated heart failure patients [30]. Nevertheless its utility as a biomarker for adverse outcomes prediction in HF patients is still unknown [30].
Myocardial infarction and cardiac remodeling
Experimental studies performed by Hobbs et al demonstrated that both CNP and NPR-C contribute to regulate the coronary blood flow in ischemic cardiac tissue and protect the heart against ischemia/reperfusion (I/R) injury by reducing the size of the infarction zone [31]. They also demonstrated that CNP maintains the levels of left ventricular developed pressure and coronary perfusion pressure as be observed in pre-ischemic situation [31]. In addition, Soeki et al. showed that in vivo administration of CNP improved cardiac function and that it might reduce cardiac remodeling post myocardial infarction (MI) by its anti-hypertrophic and anti-fibrotic effects [32]. They observed that CNP considerably reduced the enlargement of the left ventricle (LV) after MI with no effects on arterial pressure (LV end-diastolic dimension: MI+CNP: 7.7±0.1 mm; MI+vehicle: 8.3±0.1 mm; sham: 6.7±0.1 mm; p< 0.01) [32]. Moreover, they observed a markedly reduction in cardiac fibrosis, demonstrated by an important attenuation of the morphometrical collagen volume fraction augmentation in the heart regions non-affected by MI (MI+CNP: 3.9±0.3%; MI+vehicle: 5.7±0.5%; sham: 3.1±0.2%, p<0.01) [32]. They also showed that CNP markedly attenuated the increase in cardiomyocytes cross-sectional area [32]. Del Ry et al. reported that the increased levels of cardiac CNP after MI could be related to an increase in vascularization nearby the infarct zone and to the I/R process [33]. The density of the cardiovascular network (overall length of capillaries and the number of branch points) was markedly higher in the infarct border zone (BZ) compared with the non-infarcted remote zone (RZ) and with healthy myocardium (N) (BZ: 20.40±1.47; RZ: 17.40±2.80; N: 11.40±0.85; p<0.001 N vs. BZ; p=0.04 N vs. RZ) [33]. The vasculogenesis effect could be mediated by an increase in CNP levels that triggers a positive stimulation for vascular endothelial growth factor secretion [33].
These results obtained from experimental models correlates with those observed at clinical setting. In humans, endogenous an exogenous CNP could act as a protector factor for damage caused by ventricular remodeling and I/R after a MI, because of its anti-hypertrophic effect over cardiomyocytes and its anti-proliferative action over cardiac fibroblasts [3,11].
Arterial hypertension
One of the features of arterial hypertension is the presence of an abnormal function of the endothelium that includes shear stress and the loss of NO bioactivity, causing vascular remodeling and dysfunction [14]. Given the fact that CNP secretion is increased in response to shear stress and that it triggers anti-remodeling effects on endothelial cells and vascular smooth muscle, it is possible that endothelial CNP produced a protective effect against arterial hypertension associated vascular dysfunctions, which could lead to consider the possible use of this peptide as pharmacological therapy [11]. Additional studies showed that a significant increase in CNP plasma level is related to hypertension in patients requiring coronary angiography [11]. It has been proposed that CNP produces a chronic regulation of blood pressure through different mechanisms such as suppression of vasopressin, ACTH, aldosterone synthesis and sympathetic tone. CNP could act as a protection agent for hypertension through NPR-B activation [11,14]. Results from different experimental models carried out by Costa et al. demonstrated that exogenous administration of CNP can reduce mean arterial pressure and increase nitric oxide synthase activity leading to an increased production of NO [34,35]. This mechanism could be mediated by the interaction of CNP with its receptor NPR-C [34,35]. Experiments performed on spontaneously hypertensive rats demonstrated that 14-days CNP infusion (0.75 mg/h/rat, via subcutaneously implanted osmotic pumps) reduces systolic blood pressure in 15 mmHg compared with control group [36]. These findings suggest a possible use of CNP as a new strategy in anti-hypertensive therapy.
Cellular mechanisms that mediate CNP vasodilator effects include direct opening of K+ channel of the GIRK family, in smooth muscle vessels cells, and on the other hand, an indirect stimulation of NO release by vascular endothelium [9,34-36].
Angiogenesis and revascularization
CNP derived from endothelium can activate endothelial cells promoting reparation of damaged blood vessels according to its pro-angiogenic activity [11]. In damaged carotid arteries it was shown that systemic CNP infusion exalts endothelial growth and prevents intimal thickening [11]. Experimental studies showed that animals infused with CNP regenerate their endothelium during inflammatory events and cardiac tissue damage, as it occurs in acute myocarditis [37]. Treatment with CNP significantly improved cardiac function determined by an increase in maximum dP/dt and fractional shortening (%FS) and by a decrease in left ventricular end diastolic pressure [37]. This effect could be beneficial to prevent cardiac failure as well as myocardium injury induced by cardiac inflammatory processes [37].
Restenosis
As CNP inhibits vascular smooth muscle hyperplasia, it promotes endothelial cell proliferation, and it has anti-thrombotic and anti-inflammatory actions, CNP appears to be an optimal candidate to be released from drug eluting stents [11]. Other beneficial action of CNP can be apply for coronary artery bypass surgery in patients requiring for auto-transplantation [11].
Atherosclerosis
Casco et al. demonstrated the presence of NPs in human atherosclerotic plaques, with an important increase in CNP levels and its receptor NPR-B, while Kuehnl et al. demonstrated that NPs are also markedly expressed in advanced atherosclerotic plaques of internal carotid arteries [38]. Although CNP and NPR-B expression were observed at endothelial cells and vascular smooth muscle cells, CNP secretion was also seen at atherosclerotic plaques associated macrophage cells, which seems to be an important source of the peptide in this pathologic structure [39].
Under physiological conditions, CNP secretion occurs at endothelial and vascular smooth muscle cells [11]. While growth of atherosclerotic plaque progresses the secretion pattern changes, showing less production in the previous mentioned cells and increased production in macrophages [11]. It is also important to know that there exists a suppression of CNP endothelial cells secretion causing by the oxidized form of LDL molecule found in the foam cells of atherosclerotic plaques [11]. In addition, it has been demonstrated that CNP inhibits oxidized LDL-stimulated vascular smooth muscle migration and proliferation, at least through a cGMP-dependent process, showing that CNP may play a role as a local antimigration factor during the process of intimal thickening in coronary atherosclerosis [40].
Pulmonary hypertension
In pulmonary hypertension, the bioactivity of NO is reduced and the same effect happens with prostacyclin and other endothelium-dependent vasodilators, while production and secretion of endothelin-1 and other endogenous vasoconstrictors are increased [11]. Considering this scenario, exogenous CNP administration could have positive therapeutic effects for the treatment of pulmonary hypertension [11].
Postural orthostatic tachycardia syndrome
Clinical studies carried out by Li et al. [41] in children with postural orthostatic tachycardia syndrome (POTS) showed higher plasma C-type natriuretic peptide levels compared to control group (32.8±9.7 pg/mL vs 24.2±8.4 pg/mL; p<0.01). The samples were obtained from patients in supine position [41]. No differences were observed in plasma CNP levels obtained from POTS patients in upright and in supine position compared to control group [41]. Researchers suggest that CNP could be responsible for the vasodilation evidenced in children with POTS [41].
As metoprolol is an important drug for the treatment of POTS, Lin et al. [42] investigated the variation in CNP plasma levels with and without this drug in children and adolescent patients. In POTS patients without metoprolol, plasma CNP levels were higher than in control group (51.9±31.4 pg/ml vs. 25.1±19.1 pg/ml; p< 0.001) [42]. CNP plasma levels from metoprolol responder patients were significantly higher than non-responder one (59.1± 33.5 pg/ml vs. 34.8±16.7 pg/ml; p< 0.05) [42]. Investigators concluded that plasma CNP concentration could be used as a possible predictor of metoprolol therapeutic efficacy on POTS in children [42].
Vascular calcification
Huang et al. demonstrated that CNP inhibited calcification in rat vascular smooth muscle cells (VSMCs) through the cGMP/PKG pathway [43]. Also, clinical studies in human aortic valves showed that CNP is down-regulated in calcified human aortic valves [44]. Calcific aortic valve disease (CAVD) is characterized thickening of the valve leaflets, caused by fibrosis and calcification that lead to impairment of their motion [44]. In this sense, Yip et al. proved that CNP inhibited differentiation of valvular interstitial cells (VICs) to myofibroblasts and osteoblasts, phenotypes associated with CAVD [45]. Altogether, this evidence suggests a novel mechanism to explain the protective effect of CNP in the aortic valve and provide a new target for prophylaxis and therapy for cardiovascular diseases associated with ectopic calcification [43,45].
Myocardial aging
Evidence suggests that myocardial aging is a process characterized by LV fibrosis caused by a reduction in cardiomyocyte number, cardiac fibroblast proliferation, and collagen deposition, leading to ventricular dysfunction [46]. In this sense, it has been demonstrated that a decline in CNP bioavailability could be a contributor to age-related LV fibrosis [46]. They demonstrated in a Fischer rat model of aging, that a progressive decline in circulating CNP characterizes natural aging and is strongly associated with a reciprocal increase in LV fibrosis [46]. By using adult human cardiac fibroblasts, they also demonstrated that the antiproliferative actions of high-dose CNP may involve a non-cGMP pathway via NPR-C [46]. These results open the possibility to develop a CNP-based therapy for myocardial aging as well as pathophysiological conditions with impaired LV function [46].
Conclusions
C-type natriuretic peptide is directly related to a variety of cardiovascular pathologies and processes. High levels of plasmatic and urinary CNP were found in patients suffering from heart failure, myocardial infarction and arterial hypertension, compared with healthy patients. It was also demonstrated by several researchers that CNP increases angiogenesis and revascularization. Different processes such as vascular smooth muscle hyperplasia inhibition, stimulation of endothelial proliferation, anti-inflammatory and anti-thrombotic effects suggest that CNP could be considered as a pharmacological agent for vascular restenosis prevention to be used in patients with coronary surgery, and as a preventive agent for intima thickening in injury arterial vessels. Also, a modification in CNP secretion pattern was found in atherosclerotic plaques, changing from endothelial cells and smooth muscle cells that normally secreted it, to macrophages cells associated to those plaques. From a clinical diagnostic point of view, plasmatic and urinary levels are not yet established as a standard value to be implemented as a biomarker, and more studies are required in this field. Finally, and considering all the studies undergoing to the date, CNP research must keep moving forward to the study of cardiovascular pathologies from a patophysiological, pharmacological and diagnostically points of view.
The authors thank to the Universidad de Buenos Aires (UBACYT 20020130200105BA and 20020130100019BA), ANPCYT (PICT 2012-01775) and INFIBIOC for supporting our research.
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H (1981) A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89-94.
- Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, et al. (2014) Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther 144: 12–27.
- Del Ry S (2013) C-type natriuretic peptide: A new cardiac mediator. Peptides 40: 93–98.
- Fernández BE, Cavallero S, Puyó AM (2010) Capítulo 15: Péptidos natriuréticos: origen, síntesis y secreción por el corazón endócrino y parácrino. Funciones fisiológicas y participación en patologías cardio-vasculares. Libro: Fisiopatología cardiovascular. Bases racionales para la terapéutica 237-249.Directores: R.J. Gelpi, M. Donato. Co-directores: C. Morales, L. Grinfeld. Editorial Corpus, Rosario, Santa Fé, Argentina. ISBN Nº 950-9030-02-3.
- Sellitti DF, Koles N, Mendonça MC (2011) Regulation of C-type natriuretic peptide expression. Peptides 32: 1964–1971.
- Del Ry S, Cabiatia M, Vozzi F, Battolla B, Caselli C, et al. (2011) Expression of C-type natriuretic peptide and its receptor NPR-B in cardiomyocytes. Peptides 32: 1713–1718.
- Savoia C, Volpe M, Alonzo A, Rossi C, Rubattu S (2010) Natriuretic peptides and cardiovascular damage in the metabolic syndrome: molecular mechanisms and clinical implications. Clin Sci 118: 231–240.
- Volpe M, Rubattu S, Burnett Jr J (2014) Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J 35: 419–425.
- Rose RA, Giles WR (2008) Natriuretic peptide C receptor signaling in the heart and vasculature. J Physiol 586: 353–366.
- Sudoh T, Minamino N, Kangawa K, Matsuo H (1990) C-type natriuretic peptide (CNP): a new member of the natriuretic peptide family identified in porcine brain. Biochem Biophys Res Comm 168: 863–870.
- Lumsden NG, Khambata RS, Hobbs AJ (2010) C-Type natriuretic peptide (CNP): cardiovascular roles and potential as a therapeutic target. Curr Pharm Des 16: 4080–4088.
- Palmer SC, Prickett TCR, Espiner EA, Yandle TG, Mark Richards A (2009) Regional Release and Clearance of C-Type Natriuretic Peptides in the Human Circulation and Relation to Cardiac Function. Hypertension 54: 612-618.
- Del Ry S, Passino C, Emdin M, Giannessi D (2006) C-type natriuretic peptide and heart failure. Pharmacol Res 54: 326–333.
- Kalra PR, Anker SD, Struthers AD, Coats AJS (2001) The role of C-type natriuretic peptide in cardiovascular medicine. Eur Heart J 22: 997–1007.
- Das BB, Solinger R (2009) Role of Natriuretic Peptide Family in Cardiovascular Medicine. Cardiovasc Hematol Agents Med Chem 7: 29-42.
- Simon A, Harrington HO, Liu GX, Koren G, Choudhary G (2009) Mechanism of C-type natriuretic peptide-induced endothelial cell hyperpolarization. Am J Physiol Lung Cell Mol Physiol 296: L248–L256.
- Villar IC, Panayiotou CM, Sheraz A, Sheraz A, Madhani M, et al. (2007) Definitive role for natriuretic peptide receptor-C in mediating the vasorelaxant activity of C-type natriuretic peptide and endothelium-derived hyperpolarising factor. Cardiovasc Res 74: 515–525.
- Kuehnl A, Pelisek J, Bruckmeier M, Safi W, Eckstein HH (2013) Comparative measurement of CNP and NT-proCNP in human blood samples: a methodological evaluation. J Negat Results Biomed 12: 7.
- Cacciapuoti F (2010) Natriuretic peptide system and cardiovascular disease. Heart Views 11: 10–15.
- Kiemer A, Lehner M, Hartung T, Vollmar A (2002) Inhibition of cyclooxygenase-2 by natriuretic peptides. Endocrinology 143: 846–852.
- Schachner T, Zou Y, Oberhuber A, Mairinger H, Tzankov A, et al. (2004) Perivascular application of C-type natriuretic peptide attenuates neointimal hyperplasia in experimental vein grafts. Eur J Cardiothorac Surg 25: 585–590.
- Ohno N, Itoh H, Ikeda T, Ueyama K, Yamahara K, et al. (2002) Accelerated reendothelialization with suppressed thrombogenic property and neointimal hyperplasia of rabbit jugular vein grafts by adenovirus-mediated gene transfer of C-type natriuretic peptide. Circulation 105: 1623–1626.
- Rotmans J, Verhagen H, Velema E, de Klejin DP, van der Heuvel M, et al. (2004) Local overexpression of C-type natriuretic peptide ameliorates vascular adaptation of porcine hemodialysis grafts. Kidney Int 65: 1897–1905.
- Wei CM, Heublein DM, Perrella MA, Lerman A, Rodeheffer RJ, et al. (1993) Natriuretic Peptide System in Human Heart Failure. Circulation 88: 1004-1009.
- Kalra PR, Clague JR, Bolger AP, Anker SD, Poole-Wilson PA, et al. (2003) Myocardial Production of C-Type Natriuretic Peptide in Chronic Heart Failure. Circulation 107: 571-573.
- Del Ry S, Passino C, Maltinti M, Emdin M, Giannessi D (2005) C-type natriuretic peptide plasma levels increase in patients with chronic heart failure as a function of clinical severity. European J Heart Fail 7: 1145–1148.
- Del Ry S, Maltinti M, Cabiati M, Emdin M, Giannessi D, et al. (2008) C-type natriuretic peptide and its relation to non-invasive indices of left ventricular function in patients with chronic heart failure. Peptides 29: 79-82.
- Del Ry S, Cabiati M, Stefano T, Catapano G, Caselli C, et al. (2011) Comparison of NT-proCNP and CNP plasma levels in heart failure, diabetes and cirrhosis patients. Regul Pept 166: 15–20.
- Passino C, Del Ry S, Severino S, Gabutti A, Prontera C, et al. (2008) C-type natriuretic peptide expression in patients with chronic heart failure: effects of aerobic training. Eur J Cardiovasc Prev Rehabil 15: 168–172.
- Zakeri R, Sangaralingham SJ, Sandberg SM, Heublein DM, Scott CG, et al. (2013) Urinary C-type Natriuretic Peptide: A New Heart Failure Biomarker. JACC Heart Fail 1: 170-177.
- Hobbs A, Foster P, Prescott C, Scotland R, Ahluwalia A (2004) Natriuretic Peptide Receptor-C Regulates Coronary Blood Flow and Prevents Myocardial Ischemia/Reperfusion Injury. Novel Cardioprotective Role for Endothelium-Derived C-Type Natriuretic Peptide. Circulation 110: 1231-1235.
- Soeki T, Kishimoto I, Okumura H, Tokudome T, Horio T, et al. (2005) C-Type Natriuretic Peptide, a Novel Antifibrotic and Antihypertrophic Agent, Prevents Cardiac Remodeling After Myocardial Infarction. J Am Coll Cardiol 45: 608-616.
- Del Ry S, Cabiati M, Martino A, Cavallini C, Caselli GD, et al. (2013) High concentration of C-type natriuretic peptide promotes VEGF-dependent vasculogenesis in the remodeled region of infarcted swine heart with preserved left ventricular ejection fraction. Int J Cardiol 168: 2426–2434.
- Costa MA, Elesgaray R, Caniffi C, Fellet A, Arranz C (2007) Role of cardiovascular nitric oxide system in C-type natriuretic peptide effects. Biochem Biophys Res Commun 359: 180-186.
- Caniffi C, Elesgaray R, Gironacci M, Arranz C, Costa MA (2010) C-type natriuretic peptide effects on cardiovascular nitric oxide system in spontaneously hypertensive rats. Peptides 31: 1309-1318.
- Caniffi C, Sueiro L, Bouchet G, Romero M, Barrionuevo E, et al. (2015) Respuesta cardiovascular a la administración crónica de péptido natriurético tipo C en ratas espontáneamente hipertensas. Revista Argentina de Cardiología 83: 94-100.
- Obata H, Yanagawa B, Tanaka K, Ohnishi S, Kataoka M, et al. (2007) CNP infusion attenuates cardiac dysfunction and inflammation in myocarditis. Biochem Biophys Res Commun 356: 60–66.
- Casco VH, Veinot JP, Kuroski de Bold ML, Masters RG, Stevenson MM, et al. (2002) Natriuretic Peptide System Gene Expression in Human Coronary Arteries. J Histochem Cytochem 50: 799–809.
- Kuehnl A, Pelisek J, Pongratz J, Eckstein HH (2012) C-type Natriuretic Peptide and its Receptors in Atherosclerotic Plaques of the Carotid Artery of Clinically Asymptomatic Patients. Eur J Vasc Endovasc Surg 43: 649-654.
- Kohno M, Yokokawa K, Yasunari K, Kano H, Minami M, et al. (1997) Effect of natriuretic peptide family on the oxidized LDL induced migration of human coronary artery smooth muscle cells. Circ Res 81: 585–590.
- Li H, Han Z, Chen S, Liao Y, Wang Y, et al. (2015) Total Peripheral Vascular Resistance, Cardiac Output, and Plasma C-Type Natriuretic Peptide Level in Children with Postural Tachycardia Syndrome. J Pediatr 166:1385-1389.
- Lin J, Han Z, Li H, Chen SY, Li X, et al. (2015) Plasma C-Type Natriuretic Peptide as a Predictor for Therapeutic Response to Metoprolol in Children with Postural Tachycardia Syndrome. PLoS One 10: e0121913.
- Huang Z, Li J, Jiang Z, Qi Y, Tang C, et al. (2003) Effects of Adrenomedullin, C-type Natriuretic Peptide, and Parathyroid Hormone–Related Peptide on Calcification in Cultured Rat Vascular Smooth Muscle Cells J Cardiovasc Pharmacol 42: 89-97.
- Peltonen TO, Taskinen P, Soini Y, Rysa J, Ronkainen J, et al. (2007) Distinct downregulation of C-type natriuretic peptide system in human aortic valve stenosis. Circulation 116: 1283–1289.
- Yip CY, Blaser MC, Mirzaei Z, Zhong X, Simmons CA (2011) Inhibition of pathological differentiation of valvular interstitial cells by C-type natriuretic peptide. Arterioscler Thromb Vasc Biol 31: 1881-1889.
- Sangaralingham S, Huntley B, Martin F, McKie PM, Bellavia D, et al. (2011) The Aging Heart, Myocardial Fibrosis, and its Relationship to Circulating C-Type Natriuretic Peptide. Hypertension 57: 201-207.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
