International Journal of Agricultural Science and Food Technology
Determination of some heavy metals and microbial profile of raw Sugar samples collected from Sudanese Sugar Industries in season 2017 in relation to EU.1998 and ICUMSA,1974 standards
AM Babeker1*, AR Ahmed2, GI Mastafa1, AA Abdalla3 and A Bakur1
2Ahfad University for Women, Omdurman, Sudan
3Faculty of Agricultural Technology, Elneelin University, Khartoum, Sudan
Cite this as
Babeker AM, Ahmed AR, Mastafa GI, Abdalla AA, Bakur A (2022) Determination of some heavy metals and microbial profile of raw Sugar samples collected from Sudanese Sugar Industries in season 2017 in relation to EU.1998 and ICUMSA,1974 standards. Int J Agric Sc Food Technol 8(3): 202-208. DOI: 10.17352/2455-815X.000164Copyright License
© 2022 Babeker AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The study was conducted in Sudanese Sugar Industries during the period 2017 to aim the determination some heavy metals and microbial profiles of raw Sugar samples collected from Sudanese Sugar Industries in season 2017 in relation to EU.1998 and ICUMSA,1974 standards. The samples were obtained from all Sudanese Sugar Industries namely, (Kenana, White Nile, Assalaya, Sennar, Guneid, and New Halfa). After that the samples were transferred to the laboratory to assess the quality parameters of raw sugar which include the determination of some heavy metals content of sugar cane per mg/kg in Sudanese sugar companies which include Mercury, Cadmium, Lead, and Arsenic, Determination Microbial analysis for final sugar which includes Detection of Thermopolis Bacteria (Sporeforming Bacteria, Bacteria producing H2S and H2 swell) and Determination Methophilic Bacteria (Total Count of bacteria CFU/10 gm, Determination Coliform Bacteria CFU/ 10 gm, Determination of Leuconstoc Mesenteroides Bacteria CFU/10 gm. and Enumeration of Yeasts and Moulds CFU/ 10 gm and Determination the concentration of Dextran. and the data were analyzed by using a Statistical system Complete Randomized Design (CRD) and analysis of variance technique by Least Significant Different Test (LSD) according to Fisher’s LSD method, 2010 at (Probability 0.05). Was applied to compare differences between means. The results showed that the mean concentration of Mercury, Cadmium, Lead and Arsenic was (0.02 mg/ kg grams of sugar), (0.62 mg/ kg sugar), (6.74 mg/ kg sugar), and (2.73mg/ kg sugar). These results Indicate all sample industries were agreeable with critical limit maximum value. It was concluded that the total count of Bacteria for raw Sugar White Nile and Sennar agreed with recommended limit. In the case of total yeasts, Sporeforming Bacteria, and Dextranium Bacteria all samples Industries were highest than the critical limit. While the total Moulds and Bacteria Production H2S all samples Industries were less than the critical limit except Kenana and Guneid respectively. Concerning the Total Count of Leuconstoc Mesenteroides Bacteria in sugar, the results revealed all samples Industries were higher than the recommended limit. And the samples Industries were online with the recommended limit concerning the dextran concentration except Guneid industry was highest than the critical limit (75 ppm). The study recommended that the Sudanese Sugar Industry needs to establish proper quality assurance laboratories to help in monitoring the quality and safety of raw materials and end products.In conclusion, the grain yield of maize increased with increasing N rate and plant density up to the optimum. Therefore, it’s possible to recommend using a high N rate with both low and medium plant density (< 45,000 plants ha-1) and (45,000 to 65,000plants ha-1) to harvest high grain yield.
Introduction
Raw sugar is an intermediate product of the refining and affixation process of sugar manufacturing that consists of pale yellow to brown sugar crystals covered with a film of syrup. It is of yellowish brown color due to the presence of molasses (3.6%) and has a burnt flavor with coarse crystal [1]. The sugarcane (Saccharum officinarum L.) is a commonly distributed plant and is one of the most significant sources of sugar in Sudan. Current reports have shed light on numerous biological properties of sugarcane and its resulting products. Fresh sugarcane juice is widespread in Sudan as an inexpensive and sweet beverage [2]. Sugarcane is grown in the rain season and is one of the main cash crops of Sudan. The keeping quality of sugar was studied keeping in the view that the process of drying played a pivotal role. By keeping the sugar under humid conditions, microbial decomposition along with loss of sugar occurred rendering the quality of sugar impure. If the size of the crystals of sugar becomes enlarged, it will ultimately increase the moisture percentage of the sugar sample [1].
The sugarcane industry is considered one of the organized sectors. This sector is among the countries leading economic enterprises. Sugar is mainly extracted from sugarcane and sugar beet. Studies have indicated that nearly 20-30% of total sucrose synthesized by the sugarcane plant is lost during various stages of raw material handling and sugar mill processing. The post-harvest sugar loss is one of the most alarming problems of the sugar industry and has attracted widespread attention in recent years [3]. Polysaccharides are long chain molecules, either branched or straight. These molecules are derived from two sources: the metabolic activities of the growing plant (e.g .starch) and the metabolic activities of microorganisms (e.g .dextran) growing during its life or at some stage in the subsequent processing [4]. To this amount of sugar loss, we must include the amount consumed for the formation of the 250 ppm of dextran: in the mixed juice, which according to prior data corresponded to 0.022 pounds (0.01 kg) per ton of sugar cane. That is, in the presence of 250 ppm of dextran in the mixed juice a total of 0.282 kg of sugar per ton of processed sugar cane was lost, which linearly extrapolated to the presence of 1 000 ppm of dextran in mixed juice generating a loss of 1.128 kg of sugar /ton cane.
Another study using conservative numbers from different studies around the world showed that each0.1% increase of dextran in the juice (1000 ppm), resulted in the loss of 8.8 pounds (4 kg) of sugar per ton of sugar produced without considering the industrial recovery [5]. This implies the loss of an additional0.77 pounds (0.35 kg) of sugar per ton of ground sugar cane assuming recovery of 88%.
The main aim of this study is to analyze raw sugars and to evaluate the Quality parameter. The specific objectives are to determine the heavy metals and microbial parameters of raw sugar samples collected from Sudanese Sugar Industries in season 2017. After that compared with Quality, the parameter is established by (ICUMSA, 1974) standards.
General objective
Determination of some heavy metals and microbial profile of raw Sugar samples collected from Sudanese Sugar Industries in season 2017 in relation to European Unit EU.1998 and ICUMSA,1974 standards.
Specific objectives
- Determination of some heavy metals content of sugar cane per mg/kg in Sudanese sugar companies which include Mercury, Cadmium, Lead, and Arsenic.
- Determination of Microbial analysis for final sugar which includes Detection of Thermopolis Bacteria (Sporeforming Bacteria, Bacteria producing H2S and H2 swell) and Determination Methophilic Bacteria (Total Bacteria Count Colony Forming Unit (CFU/10 gm), Determination of Coliform Bacteria CFU/ 10 gram, Determination of Leuconstoc Mesenteroides Bacteria CFU/10 gm. and Enumeration of Yeasts and Moulds CFU/ 10 gram.
- Determination of the concentration of Dextran for raw sugar samples collected from Sudanese Sugar Industries in season 2017.
Materials and methods
Sampling
Samples of sugar were obtained from the Sudanese sugar Industries in season 2017. Samples were kept in dry bottles sealed tightly and labeled. All samples were then transferred to the analytical laboratory for assessing the chemical and biological properties with reference to (Poel, et al. 1998) and (ICUMSA, 1974) standards (Figure 1).
Methods of analysis
All the heavy metals analysis of raw sugar was carried out according to the international commission for uniform methods of sugar analysis, ICUMSA [7]. whereas the Microbial profile of raw sugar was carried out according to standard methods described by the Society of Soft Drink Technologists, SSDT (1957) [8], and ICUMSA (1978 and 1974) [7]. And the dextran concentration in raw sugar was Determination according to the [9] method and ICUMSA [10].
Determination of the heavy metals of raw Sugar samples collected from Sudanese Sugar Industries in season, 2017
Sugar sample preparation: Approximately 0.25 g of dried sugar powder was weighed and placed into the polytetrafluoroethylene digestion vessel, 7 ml of concentrated HNO3 and 1 ml of hydrogen peroxide (H2O2) were added, and the contents were digested using a two-step temperature program. During the first step, the temperature was linearly increased to 190°C over 10 minutes; the maximum power of the rotating magnetron was 1000 W. During the second step, the temperature was maintained at 190°C for 30 minutes. After digestion and cooling, each solution was evaporated to 2 ml and diluted with deionized water to 50 ml in a volumetric flask and kept for the different analyses.
Preparation of standard curve for metals: The standard curve for metals in raw sugar and the standard solutions were prepared by dissolving 1.0 gm of metals of Lead, Cadmium, Chromium, and Arsenic in a minimum quantity of aqua regain HCl and HNO3 (1:3, respectively), and made up to one liter in volumetric flask by adding de-ionized water (Stock solution). The working standard solution was then prepared by suitable dilution of the stock solution. The calibration curves for metal ions were drawn by taking 0-40 μg/L as required for the calculations.
The microbial profile of raw sugar samples was collected from Sudanese Sugar Industries in season, 2017
Samples preparation: Fifteen grams raw sugar sample under testing adding 50 grams distilled water then the sample was placed on the mixer to homogenize after that adding NaCl 0.95% after that the samples became ready to culture.
Microbial analysis of raw Sugar: Samples were placed in sterile labeled sterile bottles and were first diluted with distilled water 50% (means in a ratio of 1: 1). The diluted samples were then analyzed for the following:
Microbial content total aerobic Mesophilic Bacteria counts, which include T.B C, Fungal ( Yeasts and Moulds), Coliform, Leuconstoc Mesenteroides and also Detection the Thermophilic Bacteria which include Spore Forming Bacteria, Detection of Bacteria producing H2S and Bacteria producing H2 [9].
Detection of Thermophilic Bacteria which includes: Detection of Thermopolis Spore forming Bacteria.
Preparation media and culturing: Grams from Dextrose Tryptone Agar (D.T.A) Media were weighed and dissolved in 1000 ml distilled water in a conical flask then placed in a water bath at 40 c for one hour to dissolve media. After that media was placed on the autoclave for 20 minutes after reaching a temperature of 121c and presser 15 /inch the media was discarded and cooled at 40c. Before culturing the sample, the vegetative cell was first killed by heat treatment at 10 minutes. Then 6ml from each sample was taken and distributed in three Petri dishes by equal volume (2 ml for each). Then 15 ml of Dextrose Tryptone Agar Media prepared above was poured into each Petri dish near flam then all dishes were left for about one hour at room temperature to solidify and then all Petri dishes were incubated at 52 °C for 72 hours. Yellow Colonies were counted after 72 hours and results were expressed as colony-forming units per milliliter (CFU/1ml). After that was carried out the calculation of stability or percentage of microorganisms present in the sample was (ICUMSA, 1974).
Detection of bacteria producing H2S
Preparation media and culturing: 23 grams from Iron Sulphite Agar Media was weighed and dissolved in 1000 ml distilled water in a conical flask then placed in a water bath at 40 c for one hour to *dissolving media. After that media was placed on the autoclave for 20 minutes after reaching a temperature of 121c and presser 15 /inch the media was discarded and cooled at 40c. 6 ml from each sample was taken and distributed in three test tubes by equal volume (2ml for each). Then 15 ml of Iron Sulphite Agar Media prepared above was poured into each test tube near the flam then all test tubes were left for about one hour at room temperature to solidify and then all test tubes were incubated at 52 °C for 72 hours. Cracks and swells appear after 72 hours and results are expressed as the detection of Bacteria producing H2S.
Detection of bacteria producing and H2
Preparation media and culturing: 52 grams from Reinforced Clostridial Agar Media was weighed and dissolved in 1000 ml distilled water in a conical flask and then placed on a water bath at 40 °c for one hour to dissolve media. After that media was placed on autoclave for 20 minutes after reaching a temperature of 121c and presser 15 /inch the media was discarded and cooled at 40 °c. 6 ml from each sample was taken and distributed in three test tubes by equal volume (2 ml for each). Then 15 ml of Reinforced Clostridia Agar Media prepared above was poured into each test tube near the flam then all tubes were left for about one hour at room temperature to solidify and then all test tubes were incubated at 52 °C for 72 hours. Black Colonies were observed after 72 hours and the results indicated the presence of bacteria producing H2 (ICUMSA, 1974).
Determination of Methophilic Bacteria which includes: Determination of Total Bacteria Count (CFU/ 10 grams).
Preparation media and culturing: 17.5 grams from Plate Count Agar Media was weighed and dissolved in 1000 ml distilled water in a conical flask and then placed in a water bath at 40 c for one hour to dissolve media. After that media was placed on autoclave for 20 minutes after reaching temperature 121c and presser 15 /inch the media was discarded and cooled at 40 °c. 6 ml from each sample was taken and distributed in three Petri dishes by equal volume (2 ml for each). Then 15 ml from Plate Count Agar Media prepared above was poured into each Petri dish near flam then all dishes were left for about one hour at room temperature to solidify and then all Petri dishes were incubated at 37 °C for 48 hours. Colonies were counted after 48 hours and results were expressed as colony-forming units per milliliter (CFU/1ml). After that was carried out the calculation of stability or percentage of microorganisms present in the sample was (ICUMSA, 1974).
Determination coliform bacteria Count (CFU/10 grams)
Preparation media and culturing: 52 grams from Macon key Agar Media was weighed and dissolved in 1000 ml distilled water in a conical flask and then placed in a water bath at 40 °c for one hour to dissolve media. After that media was placed on autoclave for 20 minutes after reaching temperature 121 °c and presser 15 /inch the media was discarded and cooled at 40 °c. 6ml from each sample was taken and distributed in three Petri dishes by equal volume (2 ml for each). Then 15 ml from Macon key Agar Media prepared above was poured into each Petri dish near flam then all dishes were left for about one hour at room temperature to solidify and then all Petri dishes were incubated at 37 °C for 48 hours. Pink Colonies were counted after 48 hours and results were expressed as colony-forming units per milliliter (CFU/1ml). After that was carried out the calculation of stability or percentage of microorganisms present in the sample was (ICUMSA, 1974).
Determination of enumeration of Yeasts and Moulds (CFU/10 grams)
Preparation media and culturing: 16 grams from Rose Bengal Chloramphenicol Agar Media (RBCAM) was weighted and dissolved in 1500 ml distilled water in a conical flask and then placed in a water bath at 40 c for one hour to dissolve media. After that media was placed on the autoclave for 20 minutes after reaching a temperature of 121 c and presser 15 /inch the media was discarded and cooled at 40c. 6ml from each sample was taken and distributed in three Petri dishes by equal volume (2 ml for each). Then 15 ml from Rose Bengal Chloramphenicol Agar Media prepared above was poured into each Petri dish near the flam then all dishes were left for about one hour at room temperature to solidify and then all Petri dishes were incubated at 30 °C for 72 hours. Pink Colonies were counted after 72 hours and results were expressed as colony-forming units per milliliter (CFU/1ml). After that was carried out the calculation of stability or percentage of microorganisms present in the sample was (ICUMSA, 1974).
Detection of Leuconstoc Mesenteroides bacteria producing dextran
Preparation media and culturing: 23.5 grams from Sucrose Agar Media (S.A.M) Media was weighed and dissolved in 1000 ml distilled water in a conical flask and then placed on a water bath at 40 c for one hour to dissolve media. After that media was placed on autoclave for 20 minutes after reaching temperature 121c and presser 15 /inch the media was discarded and cooled at 40c. 6ml from each sample was taken and distributed in three Petri dishes by equal volume (2 ml for each). Then 15 ml from Sucrose Agar Media prepared above was poured into each Petri dish near flam then all dishes were left for about one hour at room temperature to solidify and then all Petri dishes were incubated at 31 °C for 72 hours. Colonies were counted after 24 hours and results were expressed as colony-forming units per milliliter (CFU/1ml), (ICUMSA, 1974).
Determination of dextran in sugar samples collected from Sudanese Sugar Industries at the season, 2017
Dextran in sugar solution was determined by a modified alcohol Haze method of the ICUMSA [10]. The test sample was dissolved in water; soluble starch was destroyed by incubation with a suitable enzyme (Novo Termamyl 120L, Novo Industry A/S, Bagsvaerd, Denmark). Protein is removed by precipitation with Tricolor acetic acid (TCA) followed by filtration with acid-washed Kieselguhr. The dextran haze was produced by diluting an aliquot of the treated, filtered solution to twice the aliquot volume by the addition of ethanol. The turbidity of the dextran haze was measured by the absorbance in a spectrophotometer at a wavelength of 720 nm.
Statistical analysis
The data was analyzed by using system complete randomized design (CRD) and analysis of variance technique by Least Significant Different Test (LSD) according to Fisher’s LSD method, 2010 at Probability 0.05 (equivalent to a 95% confidence level), Was applied to compare differences between mean sample industries.
Results and discussion
Heavy metals content of raw sugar samples collected from Sudanese Sugar Industries in season 2017
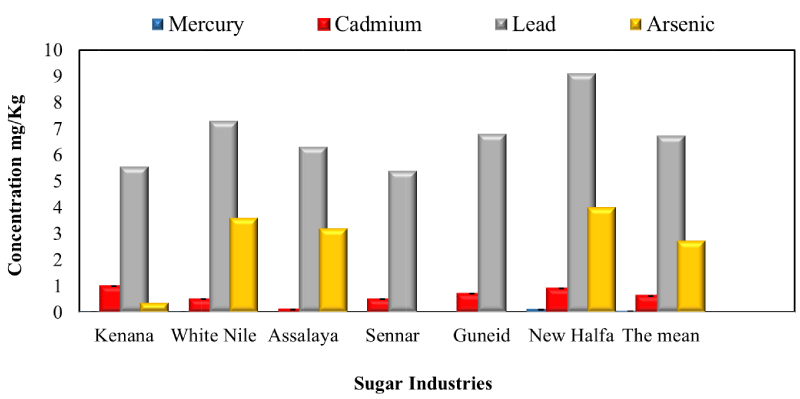
Determination concentration of Mercury mg/ kg for raw sugar samples collected from Sudanese Sugar Industries in season 2017: The study results showed in Figure 2 that the mean concentration of Mercury for all samples collected from Sudanese sugar industries was (0.02 mg/ kg grams sugar). The Mercury was found in three samples of Industries only which include Kenana, White Nile, and New Halfa by concentrations of 0.005.0.002 and 0.1 mg/ kg grams sugar, respectively. And the rest of the samples were free from Mercury. The specification Limits of Mercury concentration for sugar must be ranged between (> 0.02 - 0.10/Kg sugar) according to (Poel, et al. 1998). Through these results, all sample industries were matched with the recommended limit.
Determination concentration of Cadmium mg/ kg for raw sugar samples collected from Sudanese Sugar Industries in season, 2017: The study results revealed that the mean concentration of Cadmium for all sugar samples collected from Sudanese sugar industries was (0.62 mg/ kg sugar). That means lower than 1.5 mg/ kg was obtained [11]. The value ranging between (0.1 to .99 mg/ kg sugar) was recorded by Assalaya and factory Kenana respectively. The specification Limits of Cadmium concentration for sugar must be ranged between (> 0.02 – 1.00 /Kg sugar) according to (Poelk et al. 1998). Through these results, all sample industries were within the recommended ranged.
Determination concentration of Lead mg/ kg for raw sugar samples collected from Sudanese Sugar Industries in season, 2017: The study results revealed that the mean concentration of Lead for all sugar samples collected from Sudanese sugar industries was (6.74 mg/ kg sugar). That means higher than ranging from 0.028 to 0. 27 mg/kg was obtained [11]. The value ranging from (5.4 to 9.1 mg/ kg of sugar) was recorded by Sennar and New Halfa respectively. The specification Limits of Lead concentration for sugar must be ranged between (> 0.10 –10.00/Kg sugar) according to (Poel, et al. 1998). Through these results, all sample industries were within the recommended ranged.
Determination concentration of Arsenic mg/ kg for raw sugar samples collected from Sudanese Sugar Industries in season, 2017: The study results showed in Figure 2 that the mean concentration of Arsenic for all sugar samples collected from Sudanese sugar industries was (2.73mg/ kg sugar). That means higher than ranging from 0.0024 to 0.0107 mg/kg was obtained [11]. The significantly highest concentration of Arsenic value was 4.0 mg/ kg sugar recorded by New Halfa and flowed by White Nile (3.6 mg/ kg sugar). While the significantly lowest concentration of Arsenic value was (0.36 mg/ kg sugar) and was recorded by Kenana. The specification Limits of Arsenic concentration for sugar must be ranged between (>0.10 –4.00/Kg sugar) according to (Poel, et al. 1998). Through these results, all sample industries were within the recommended.
Microbiological analysis of raw sugar samples collected from Sudanese Sugar Industries in season, 2017
Determination of the Total Count of Bacteria (CFU/10grams): The study results showed in Table 1 that the mean Total Count of Bacteria for all samples collected from Sudanese sugar industries was (536.83 Colony Forming Unit/ 10 grams of sugar). this means is highest then (138 Colony Forming Unit/ 10 grams sugar) was obtained by [12]. The highest value was (900 CFU/ 10 grams sugar) recorded in Guneid and the lowest value was (300 CFU/10 grams sugar) recorded in White Nile. The specification Limits of the Total Count of Bacteria in sugar must be less than (400 CFU/ 10 grams of sugar) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample industries were higher than the recommended limit except White Nile and Sennar were in line with the critical limit (less than 400 CFU/ 10 grams sugar).
Detecting the presence of Coliform Bacteria: The study of detecting the presence of Coliform Bacteria in the sugars collected from all Sudanese sugar industries found that there are no bacteria detected in all samples. This result agreed with the specification established by [10].
Determination total count of fungal (Yeasts and Moulds) per CFU/10grams
Determination of the total count of yeasts (CFU/10grams): The mean of the Total Count of Fungal (Yeasts) for all samples collected from Sudanese sugar industries was (47.67 Colony Forming Units/ 10 grams of sugar). The highest value was (90 CFU/ 10 grams sugar) recorded in Guneid and the lowest values were (27- 21 CFU/10 grams sugar) recorded in White Nile and Sennar. This result disagreed with [12] was mentioned the Total Count of Fungal was zero or not found in all samples Industries. The specification Limits of the Total Count of Fungal (Yeasts) in sugar must be less than (20 CFU/ 10 grams of sugar) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample industries were higher than the recommended limit.
Determination of the total count of moulds (CFU/10grams): While mean of the Total Count of Fungal (Moulds) for all samples collected from Sudanese sugar industries was (17.83 Colony Forming Units/ 10 grams of sugar). The highest value was (26 CFU/ 10 grams sugar) recorded in Guneid and the lowest values were (9 CFU/10 grams sugar) recorded in White Nile. This result disagreed with [12] was mentioned the Total Count of Fungal (Moulds) was zero or not found in all samples Industries. The specification Limits of the Total Count of Fungal (Moulds) in sugar must be less than (20 CFU/ 10 grams of sugar) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample industries were less than the recommended limit while the Kenana and Guneid exceeded the critical limit.
Determination total count of thermopolis bacteria (CFU/10grams)
Determination of total count of spore forming bacteria: The study results showed in the Table 1 that the mean Total Count of Spore Forming Bacteria for all samples collected from Sudanese sugar industries was (25.67 Colony Forming Units/ 10 grams of sugar). this result lowest than (163.33 Colony Forming Unit/ 10 grams sugar) was reported by [12]. The highest value was (56 CFU/ 10 grams sugar) recorded in Guneid and the lowest value was (7 CFU/10 grams sugar) recorded in White Nile. The specification Limits of the Total Count of Bacteria in sugar must not exist (Nill) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample Industries were higher than the recommended limit.
Detecting the bacteria production (H2S): The study of detecting the presence of Bacteria Production (H2S) in the sugars collected from all Sudanese sugar industries found that there are bacteria Production H2S detected in Kenana and Guneid respectively. While the rest Industries were free from these bacteria. This result agreed with [12] also not detected Bacteria Production (H2S). The specification of Bacteria Production (H2S in sugar must not exist (Nill) according to the international commission for uniform methods of sugar analysis [10].
Detecting the bacteria production (H2 well): The study of detecting the presence of Bacteria Production (H2S well) in the sugars collected from all Sudanese sugar industries found that there is no bacteria Production H2 Swell detected in all sugar industries. The specification of Bacteria Production (H2 Swell in sugar must not exist (Nill) according to the international commission for uniform methods of sugar analysis [10]. It concluded that all Sudanese sugar industries were completely free from coliform and Production (H2 Swell) bacteria. This result is approved with specification standards.
Determination of the total count of Leuconstoc Mesenteroides bacteria for raw sugar samples collected from Sudanese Sugar Industries at the season, 2017
The study results showed in the Table 2 that the mean of the Total Count of Leuconstoc Mesenteroides Bacteria for all samples collected from Sudanese sugar industries was (36.67 Colony Forming Unit/ 10 grams sugar). The highest value was (83.63 CFU/ 10 grams sugar) recorded in Guneid and the lowest value was (9.12 CFU/10 grams sugar) recorded in White Nile. The statistical analysis revealed there is a significant difference between all Industries respectively at a significant level of 0.05%. The specification Limits of the Total Count of Leuconstoc Mesenteroides Bacteria in sugar must not exist (Nill) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample industries were higher than the recommended limit.
Determination of the concentration of dextran for raw sugar samples collected from Sudanese Sugar Industries in season, 2017
The study results showed in the Table 2 that the mean concentration of the Dextran for all sugar samples collected from Sudanese sugar industries was (47.48ppm/kg sugar). these results lowest than (56.00ppm/kg of sugar) was reported by [13-17]. The significantly highest concentration of Dextran value was 94.09ppm /kg sugar recorded by Guneid. While the significantly lowest concentration of Dextran value was (18.88ppm /kg sugar) recorded by White Nile. The statistical analysis revealed there is a significant difference between all Industries at a significant level of 0.05%. The specification Limits of Dextran concentration in sugar must be less than (75ppm/kg sugar) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample industries were less than the recommended limit except Guneid was highest than the critical limit.
Specification Limits according to European Unit admissible limits in mg/Kg of sugar analysis (Poel, et al. 1998).
Conclusion and recommendations
Conclusion
The study was conducted in Sudanese Sugar Industries which include (Kenana, White Nile, Assalaya, Sennar, Guneid, and New Halfa) to Evaluation the Quality parameters of the Sudanese Sugar Industry according to European Unit admissible limits (Poel, et al. 1998) and ICUMSA [10].
The data were collected through laboratory tests concerned with the quality parameters of raw sugar samples.
In the case of heavy metals, all samples tested were agreeable with critical limit maximum value.
It was concluded that the total count of Bacteria for raw Sugar White Nile and Sennar agreed with recommended limit.
In the case of total yeasts, Sporeforming Bacteria and Dextranium Bacteria all samples industries were highest than the critical limit.
In the case of total Moulds and Bacteria Production H2S, all sample industries were less than the critical limit except Kenana and Guneid respectively.
The specification Limits of the Total Count of Leuconstoc Mesenteroides Bacteria in sugar must not exist (Nill) according to the international commission for uniform methods of sugar analysis [10]. Through these results, all sample industries were higher than the recommended limit.
It was concluded that all sample industries were online with the recommended limit concerning the dextran concentration except the Guneid industry was highest than the critical limit (75 ppm).
Recommendations
The Sudanese Sugar Industry needs to establish proper quality assurance laboratories to help in monitoring the quality and safety of raw materials and end productions.
More research was required to determine the heavy metals, residual fertilizers, herbicides, and pesticides in sugar cane and sugar production.
Sudanese Sugar Industry needs to establish proper time and manner to eliminate, prevent, or reduce the growth of microorganisms in processing lines that can lead to deterioration of sugar production as well as reduction recovery.
I would like to express my deep appreciation to Prof. Abdel Halim Rahama Ahmed, supervisor, and Dr. Ghada Ibrahim Mustafa, co-supervisor for them.
Support and encouragement.
I would like to express my deep appreciation to Prof. Moyad Bilal for his help in completing this study.
The author would like to express gratitude to production management and employees of industries for providing appropriate information for the conduct of this study.
- Javaid GS, Bhatti MB, Rashid K, Khalid M. To Introduce the Raw Sugar Refinery, Its Operational Concept and Quality Prespective in Pakistan. Life Science International Journal. 2011; 5: 2053-2062.
- Ali FG, Chattha AA, Iqbal MA. Some Fundamental Causes of Low Sugar Recovery and Vital Approach for Its Improvement. Pakistan Sugarcane Journal. 2001; 16: 56-61.
- Saxena P, Srivastava RP, Sharma ML. Impact of cut to crush delay and bio-chemical changes in sugarcane. AJCS. 2010; 4(9):692-699.
- James AC, Day DF. The process and financial impact of dextran on a sugar factory. Midland Research Laboratories, Inc. 2000.
- Rodríguez Jiménez E. BiotecnologíaAplicada. 2005; 22.
- El hajwa AM. Effect of variety, soil type, phosphorous and znic Application on Sugar cane performance in North West Sennar. Ph.D. Thesis. University of Gazira,Faculty of Agricltural Sciences, Wad Madeni, Sudan. 2000.
- International commission for uniform methods of sugar analysis, ICUMSA, 1978 - Proceedings of the Seventeenth Session. (1978). Subject 12.
- Society of Soft Drink Technologists (1957). Communication, Hughes, the Present status of the Bacteriological Testing of Sugar for the Bottling Industry, Fourth Annual Meeting,
- Chen JCP, Chi Chou C. Cane Sugar Handbook: A Manual for Cane Sugar Manufacturers and Their Chemists. 12th Edition, ISBN: 978-0-471-53037-4 - 1120 pages, November 1993.
- International commission for uniform methods of sugar analysis, ICUMSA (1994) Polarization was determined by Automatic Saccharometer (Model: AUTOPOL 880, SN: 80851, USA) using a light source 589.44 nm (yellow) according to ICUMSA method GS1/2/3-1 (1994).
- Xueli W, Tongbin C, Mei L, Bo S, Xiaoming W, Yanmei L. Selection of Sugar Cane Varieties with a Low Heavy Metal Accumulation Ability for the Ecological Remediation of Contaminated Farmland, College of Agriculture, Guangxi University, Nanning 530005, China, Journal of Resources and Ecology. 2012; 3(4): 373-378.
- Chittrepol S, Boonyaratanakornkit M, Sriroth K. Enumeration and Identification of Microorganisms in Plantation White Sugar from Factories in Thailand, Department of Applied Microbiology, Institute of Food Research and Product Development, Kasetsart University,Bangkok. 10900, Thailand Kasetsart J. (Nat Sci.) 2000; 42:321-327.
- Bukhari MM, El Khaseh S, Osman A, Eldeen S, Hegazi F. Investigations of the influence of dextran on sugar cane quality and sugar cane processing in Kenana sugar factory. Department of Food Engineering, Faculty of Engineering, University of El Imam Elmahdi, Sudan. Journal of Chemical and Pharmaceutical Research. 2015; 7(4):381-392, ISSN: 0975-7384
- Elhag A, Abouna MA, Mukhtar SA. Response of sugarcane to different levels of nitrogen in four Estates of the Sudanese Sugar Company. Sudan Journal of Agricultural Research. 2007; 8: 123-131.
- GOP (2009-2010) Agricultural Statistics of Pakistan. Ministry of Food,Agriculture and Livestock, Government of Pa-kistan, Islamabad.
- Shanmuganathan M, Annadurai K, Nageswari R, Asokhan M. Evaluation of sugarcane clones for quantitative yield and quality characters in aicrp trials for early season Sugarcane Research Station, Sirugamani – 639 115, Trichy (Dist). Email: shanagri@gmail.com (Received: 12 Sep 2014; Accepted:07 Dec 2014 Electronic Journal of Plant Breeding. 2015; 6(1): 292-297 (Mar 2015) ISSN 0975-928X
- South African Sugar Technologists Association SASTA. Cane Testing Service Tongaat-Hulett Sugar Limited – Technical Engineering Group- Laboratory Manual including the Official Methods ISBN 978-0-620-43586-4 - 5th Edition, August 2009.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
