International Journal of Agricultural Science and Food Technology
Review on postharvest quality and handling of apple
Anbesse Girma Shewa1*, Daniel Alemu Gobena2 and Mikiyas Kebede Ali2
2Food Technology and Process Engineering, Haramaya University, Ethiopia
Cite this as
Shewa AG, Gobena DA, Ali MK (2022) Review on postharvest quality and handling of apple. J Agric Sc Food Technol 8(1): 028-032. DOI: 10.17352/2455-815X.000141Copyright License
© 2022 Shewa AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Apple is a climacteric temperate fruit with high market demand providing essential components to the body. Nowadays, apple production is increasing every year especially in EU member countries are the highest producer. Hence, this paper reviews the postharvest quality and associated changes during handling. Usually, farmers face difficulty in estimating the right harvesting time though Different methods exist to test the maturity stage such as counting the days after full bloom, color change, firmness test, measuring soluble sugar, starch iodine test and Streif index. Depending on different factors, farmers can choose the best maturity estimation method in various locations. Postharvest diseases of apples makeup a major part of the economic losses incurred during apple production. Annual losses of many fruits, including apple, vary from 5 to 35% and for developing countries, it ranges between 20%-50%. The most common causes are poor harvesting methods, transportation and storage methods which can open access for fungal growth species such as Botrytis species. B. cinerea is one of the most important of the species with unique characteristics causing the highest losses.
Introduction
Apple (Malus domestica) is a temperate fruit adapted to the temperate zone of latitude varying between 35 and 50° (Kellerhals, 2009). It consists of various biologically active compounds and certain phenolic compounds that are recognized to act as antioxidants. Apple is available throughout the year and consumed as fresh or after being processed in to juice, slice or other products (Wu, et al. 2007).
The current production of apple worldwide is exceeding 50 million tons each year. EU member states are among the highest producers of apple with high consumption. Jonagold, Jonagored and Golden Delicious are the most widely grown apple cultivars in Belgium for both local consumption and export (Kellerhals, 2009).
Postharvest diseases of apples makeup a major part of the economic losses incurred during apple production. Annual losses vary from 5 to 35% and for developing countries, it ranges between 20- 50% losses before it reaches the consumers [1].
A variety of bacterial and fungal species are responsible for postharvest losses. The most common fungal pathogens are Botrytis cinerea, Penicillium expansum and, Mucor piriformis (Elad, et al. 2007). Among these fungal pathogens, Botrytis species are the most threatening pathogens of many agricultural commodities including ornamentals and field crops (Elad, et al. 2004). B. cinerea is one of the most important of the Botrytis species with unique characteristics. It can live both pathogenically and endophytically [2].
Harvest date prediction and maturity monitoring
The most vital concern in the harvesting procedure is to select the ideal harvesting time. The fruit maturity at harvest directly affects quality and storability of the apple fruit [3,4]. For long-term storage, fruits have to be harvested at earlier maturity stage. Different methods are used to test the maturity stage such as counting the days after full bloom, color change, firmness test, measuring soluble sugar, starch iodine test and Streif index.
Streif index, (firmness/(TSS × starch index)), is used most often in determining apple maturity stage because of its reliability and it is not sensitive to location variation and year [5]. These measurements are easy, rapid and inexpensive. Starch level decreases as the maturity stage of the fruit increases because of the conversion of starch to sugar. It starts to decline before the onset of ripening and this helps to predict the maturity level of the apple. Starch-iodine chart picture is a very simple, cost-effective and quick method to assess the maturity stage of the apple during a harvest and storage time [4-7].
Respiration and ethylene production rate of apple increases as it reaches to maturity. The climacteric minimum point is a good indicator of maturity stage before the onset of ripening. Monitoring respiration and ethylene production is usually done in the laboratories and are a good indication of maturity stage. This method is not feasible and practical for farmers to use them on the field due to the requirement of laboratory setup [8,9].
Postharvest change and apple quality
Fruit ripening is a period associated with physiological and structural changes: fruit softening, respiration, hydrolysis of starch, chlorophyll degradation and membrane changes. These physiological and structural changes after harvesting can be slowed down primarily by Controlled Atmosphere (CA) storage. Due to low concentration of oxygen and higher carbon dioxide, all biochemical processes are suppressed in CA storage [10]. Apple quality is a combination of parameters such as firmness, color, Total Soluble Solid (TSS), sugar content and titratable acidity. These parameters are important in determining apple maturity and consumer pleasure. Apple firmness is associated with texture while TSS, sugar and acidity are related to the taste characteristics of the apple [11].
Pectic substances are the main constituents of both middle lamella and cell wall matrix. They are key regulators of intercellular cell adhesion. Thus, pectic has been considered an important substance determining the fruit texture [12.13]. Various networks of pectin, cellulose microfibril, proteins (structural) and phenolics determine the firmness. Microfibrils are embedded between the matrix of polysaccharides and pectin forming stiff bonds.
Besides these bonds, links between various components such as between xylan and cellulose through hydrogen bond, rhamnogalacturonan attached together and homogalacturonans to each other by ionic calcium bond determines the firmness of the cell wall. Pectin to pectin through ester bond formation and linkage between rhamnogalacturonan and xyloglucan by covalent bond also contributes to the firmness of the cell wall [14].
Cell wall modification is the main reason for the softening during fruit ripening. This loss of firmness is due to change in pectin polysaccharides [15]. Enzymes which are believed to be responsible for cell wall modifications are polygalacturonase, pectate lyase, methylesterases, β-galactosidases and α-L arabinofuranosidases. These enzymes causes debranching (side chain of the pectin), depolymerization and solubilization of the cell wall pectin [3,16]. Consequently, the fruit loses its firmness.
Color is an important quality parameter determining the visual quality and market acceptability by consumers. Color development is associated with ripening process due to associated physiological change. It is characterized by color evolution through breakdown of chlorophyll and other pigments [17]. Different factors influence their synthesis such as light energy and temperature [18]. Since color is the first sensation that consumers use to reject or accept a fruit, adequate storage throughout the postharvest chain is required [6].
During fruit ripening and storage, titratable acid content of the fruit declines gradually leading to reduced fruit flavor [11]. Consumer acceptability depends on the right sugar/acidity ratio of fruit. Soluble solid content of the fruit at maturity stage varies between seasons, cultivar, and chemical application. Soluble solid includes organic acids inorganic salts and sugars. Starch degradation as maturity progresses increases the concentration of soluble sugars leading to a sweet taste. Picking soluble solid content of Delicious apple can vary between 10.8%-12.2% [9] .
Ethylene control
Ethylene regulates many plant responses such as senescence, ripening, growth and abscission. This response is controlled by regulation of either ethylene production or action. Usually controlling the activity of enzymes involved in ethylene biosynthesis, such as ACC synthese and ACC oxidase, are most effective (Figure 1). Amino-ethoxy-vinyl-glycine (AVG), Aminooxyacetic acid (AOA) acid and Naphthaleneacetic are used to control ethylene through inhibition of ethylene biosynthesis. 1-methylcyclopropene (1-MCP) completely arrests the action of the ethylene by binding with ethylene receptors[19].
During biosynthesis of ethylene, the conversion of ACC to ethylene requires both ACO enzyme and oxygen. Thus, anaerobic condition favors the suppression of ethylene production due to the low or absence of oxygen. Storing apples under low oxygen conditions, in other words in CA storage plays an important role to inhibit ethylene biosynthesis.
Physical treatments including hot water dip can suppress ethylene biosynthesis by inactivating ACS and ACO enzymes which have an active role in ethylene synthesis. According to the review of Fallik [20]. Hot water immersion (43-53oC, 2 hours), ethylene production of an apple was significantly reduced. Bai, et al. [21]. Also observed reduced ethylene production after placing the apple in 38°C and >93% RH air for 4 days. Moreover, heat treatment also controls microorganisms and insects attack besides inhibiting ethylene production.
Losses of apple
Losses of apple fruits are a major problem in the postharvest chain and are caused by a wide variety of factors, ranging from pre-harvest conditions to retailer and consumer level. Harvesting before optimal maturity stage is one of the factors. There are a number of reasons that farmers may harvest apples before optimal maturity. For example, early arrival of harvesting labors, incorrect prediction of harvesting date or different environmental factors may delay optimum maturity [23]. Consequently, the apple has less sensory qualities.
Apples undergo various loading actions that can lead to bruising and this at different stages of handling such as on the farm, during grading, storage and distribution. According to Yuwana & Duprat [24] and Gonzalez [25]. Between 20%-50% of the total apples get bruised during different handling steps. Bruise severity of apple mainly depends on the cause of the bruise, the variety and harvesting conditions [25].
In CA storage, optimizing the levels of gasses is essential to protect the fruit from atmospheric induced disorders such as low oxygen or high carbon dioxide injury [26]. Too low oxygen level can result in alcoholic flavor produced through anaerobic fermentation. Too high carbon dioxide levels can induce rough fruit skin and internal browning. Apples are typically stored at 0°C and some varieties are susceptible to internal disorders and are often stored at temperatures above 0°C. This sensitivity of apples to low temperature injury is variety dependent [27].
Postharvest pathogens play a major role in causing losses in the apple production chain. More than 90 fungal species have been described that cause decay of apples during storage. The relative importance of each pathogen depends on climatic and storage conditions [28]. Cold storage temperature is vital for postharvest pathogen control because growth temperatures for these pathogens are much higher. Still, some fungi are able to grow at temperatures as low as -2°C. Consequently, they cannot be entirely controlled unless the storage time is limited [29].
Common fungi species that are responsible for the losses of stored apple fruits include Botrytis cinerea, Penicilliumexpansum and Mucor piriformis [30]. Diseases from these fungi occur regularly during storage and cause high yield and economic losses.
Botrytis cinerea
Botrytis cinerea is the most widely distributed necrothrophic fungal pathogen of fruits, vegetables, transported and stored plant commodities. Over 235 crop species can be affected by Botrytis species and some of the Botrytis species are host specific. Epidemics due to Botrytis species are economically damaging. For this reason, strong effort has to be invested before and after harvesting to protect against Botrytis.
B. cinerea is the most interesting and important Botrytis species with unique characteristics. It can live both pathogenically and also endophytically in the host [2,31].
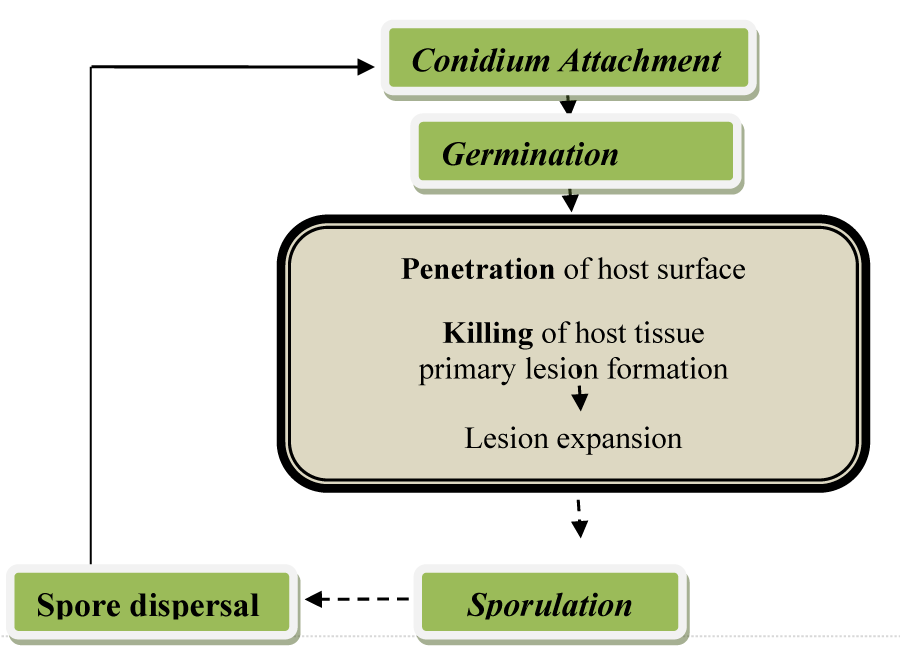
Different stages of infections processes are distinguished in fungus B. cinerea species as indicated in Figure 2. Infection process of B. cinerea comprises major stages: conidium attachment, germination, penetration and killing of the host. Conidia are ubiquitous in the air and transported by different agents such as wind, insects and rain over distances to start a new infection. Once the conidium is attached to the host, it starts to germinate when conditions are good and produces germ tubes. At the tip of the germ tube, appressoria develop which assists penetration. Therefore, the cells are killed and necrotic lesion progresses [2].
- Porat R, Lichter A, Terry LA, Harker R, Buzby J (2018) Postharvest losses of fruit and vegetables during retail and in consumers’ homes: Quantifications, causes, and means of prevention. Postharvest Biology and Technology 139: 135-149. Link: https://bit.ly/3K64X5y
- Prins T (2001) Identification and Functional Analysis of Botrytis cinerea Genes Induced During Infection of Tomato. Link: https://bit.ly/3nmaMSH
- Gwanpua S, Mellidou I, Boeckx J, et al. (2016) Expression analysis of candidate cell wall-related genes associated with changes in pectin biochemistry during postharvest apple softening. Postharvest Bio Technol 112: 176–185. Link: https://bit.ly/3FknlnC
- Reid MS, Watkins CB, Harman JE, Padfield CAS (1982) Starch iodine pattern as a maturity index for Granny Smith apples. New Zeal J Agric Res 25: 587–592. Link: https://bit.ly/3GqrFTN
- DeLong JM, Prange RK, Harrison PA, Schofield RA, DeEll JR (1999) Using the Streif index as a final harvest window for controlled-atmosphere storage of apples. HortScience 34: 1251–1255. Link: https://bit.ly/3renJ2b
- Pathare P, Opara U, Al-Said F (2013) Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Biol Process Technol 6: 36–60. Link: https://bit.ly/3GfRXrP
- Blanpied G, Kenneth J (1992) Predicting Harvest Date Windows for Apples. A cornell Coop Ext 7–9. Link: https://bit.ly/3fe478T
- Belding R (2006) ADP Guide to Post Harvest Handling of Apples: Quality Must be Achieved in the Orchard and Maintained after Harvest. Gov rannual eport 1–40.
- Kingston C (1992) Maturity Indices for Apple and Pear. In Horticultural Review 13: 407-32. Link: https://bit.ly/3qjwo49
- Mehinagic E, Royer G, Symoneaux R, Jourjon F, Prost C (2006) Characterization of odor-active volatiles in apples: Influence of cultivars and maturity stage. J Agric Food Chem 54: 2678–2687. Link: https://bit.ly/3niZNJX
- Kvikliene N, Kviklys D, Vi P (2006) Changes in Fruit Quality During Ripening and Storage in the Apple Cultivar “Auksis.” J Fruit Ornam Plant Res 14: 195–202. Link: https://bit.ly/3qgcTcG
- Billy L, Mehinagic E, Royer G, Renard C, Arvisenet G, et al. (2008) Relationship between texture and pectin composition of two apple cultivars during storage. Postharvest Biol Technol 47: 315–324. Link: https://bit.ly/31QRRYP
- Wakasa Y, Kudo H, Ishikawa R, Akada S, Senda M, et al. (2006) Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Postharvest Biol Technol 39: 193–198. Link: https://bit.ly/34D1on7
- Nara K, Kato Y, Motomura Y (2001) Involvement of terminal-arabinose and -galactose pectic compounds in mealiness of apple fruit during storage. Postharvest Biol Technol 22: 141–150.
- Carpita N, Gibeaut D (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30. Link: https://bit.ly/3FpwaMV
- Ben-Arie R, Kislev N (1979) Ultrastructural changes in the cell walls of ripening apple and pear fruit. J Plant Physiol 64: 197–202. Link: https://bit.ly/3qknJP1
- Lang C, Hübert T (2012) A Colour Ripeness Indicator for Apples. Food Biol Process Technol 5: 3244–3249. Link: https://bit.ly/33t9y0J
- Saure MC (1990) External Control of Anthocyanin Formation in Apple. Scientia Horticulturae 42: 181–218. Link: https://bit.ly/3zRyco1
- Yuan R, Li J (2008) Effect of Sprayable 1-MCP, AVG, and NAA on Ethylene Biosynthesis, Preharvest Fruit Drop, Fruit Maturity, and Quality of “Delicious” Apples. Hortic Sc 43: 1454–1460. Link: https://bit.ly/33tK6IM
- Fallik E (2004) Prestorage Hot Water Treatments: Immersion, Rinsing and Brushing. Postharvest Biol Technol 32: 125–134. Link: https://bit.ly/3HVOQWh
- Bai J, Haven W, Brecht J (2004) Effect of Pretreatment of Intact ʻGalaʼ Apple with Ethanol Vapor, Heat, or 1-Methylcyclopropene on Quality and Shelf Life of Fresh-cut Slices. Am Soc Hortic Sci 129: 583–593. Link: https://bit.ly/3feihqf
- Baur A, Yang S, Pratt H (1971) Ethylene Biosynthesis in Fruit Tissues. Plant Physiol 47: 696–699. Link: https://bit.ly/3K46TM0
- Byers RE, Eno DR (2002) Harvest Date Influences Fruit Size and Yield of “York” and “Golden Delicious” Apple Trees. J Tree Fruit Prod 3: 41–53. Link: https://bit.ly/3K49K7O
- Yuwana Y, Duprat F (1996) postharvest impact brousing of apple as related to the modulus of elasticity. Int Agrophys 10: 131–138. Link: https://bit.ly/336tF5o
- Gonzalez M (2009) Bruising Profile of Fresh “Golden Delicious” apples. Horticulture and landscape architecture. Link: https://bit.ly/3HYeeLb
- Kupferman E (2001) Controlled Atmosphere Storage of Apples and Pears December 2001. Washington State University Tree Fruit Research and Extension Center 1-8. Link: https://bit.ly/3qknGmj
- Kupferman E (2002) Minimizing internal browning in apples and pears.
- Leibinger W, Breuker B, Hahn M, Mendgen K (1997) Control of postharvest pathogens and colonization of the apple surface by antagonistic microorganisms in the field. Phytopathology 87: 1103–1110. Link: https://bit.ly/3fgDSyv
- Mantyka DL (2006) Detection, Quantification and Biological Control of Postharvest Pathogens of Pome Fruit. Victoria. Link: https://bit.ly/31P1CGM
- Sever Z, Tihomir M, Kaliterna J (2012) Losses of apple fruit caused by fungal diseases during storage. Inst Plant Prot Environ Belgrade 313-316. Link: https://bit.ly/3FirI2v
- Rosslenbroich HJ, Stuebler D (2000) Botrytis cinerea- History of Chemical Control and Novel Fungicides for its Management. Crop Prot 19: 557–561. Link: https://bit.ly/3tli0dx
- van Kan JA (2006) Licensed to Kill: The Lifestyle of a Necrotrophic Plant Pathogen. Trends Plant Sci 11: 247–253. Link: https://bit.ly/3zQ1RxS
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
