International Journal of Agricultural Science and Food Technology
Optimization of oxidative improver’s formulation for the wheat flours with different extraction rates
Mahsa Shafiesoltani*, Mania Salehifar and Saeed Baeghbali
Cite this as
Shafiesoltani M, Salehifar M, Baeghbali S (2022) Optimization of oxidative improver’s formulation for the wheat flours with different extraction rates. J Agric Sc Food Technol 8(1): 001-010. DOI: 10.17352/2455-815X.000137Copyright
© 2022 Shafiesoltani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.In this study, the effect of oxidative improvers such as glucose oxidase (10-30 mg/kg) and ascorbic acid (50–150 mg/kg) were compared on the rheological properties of two sets of flours with different extraction rates (75% and 82%). The optimized formulation via the response surface method revealed that the oxidative improvers have a different reaction in different types of flours. In flours with a 75% extraction rate, glucose oxidase played the main role, while in the flours with an 82% extraction rate, ascorbic acid was more effective. Also, this study showed that the effects of both improvers are dose-dependent, for the 75% extraction rate of flour, the optimal dose of glucose oxidase is 23 mg/kg, and for the 82% extraction rate of flour, the optimal dosage is 90 mg/kg of ascorbic acid. Finally, the effect of the optimal formulation was investigated on the bread properties and the results were compared with the control sample.
Introduction
Wheat bread is one of the main sources of human energy in many countries [1]. The most important factor determining the quality of wheat flour is the gluten network. Gluten retains co2 produced by the yeasts in its network and creates a soft plus tender texture in the bread [2]. The quality of wheat depends on the type of soil and geographical conditions [3]. To improve the quality of the gluten network, an oxidative improver is used to enhance dough stability [4]. In general, it has been concluded in all papers that oxidative improvers such as glucose oxidase and ascorbic acid can strengthen the gluten network. Despite this, choosing the right ingredients along with their optimal dose (the formulation) of oxidative improvers are important factors affecting the quality of flour and ultimately in bread. In previous studies, the details of the formulation of the oxidative improvers have not been studied. On the other hand, comparing the effect of oxidative improvers on flour with different extraction rates is also an important issue that has not been studied in previous investigations.
In this research, the effects of glucose oxidase and ascorbic acid on the gluten network of flour with different extraction rates were studied. Additionally, can a fixed formulation be used to strengthen the gluten network in different types of flours?
Materials and methods
Two sets of wheat flours (Fully automatic milling-Buhler Co, Germany) with different extraction rates (75% and 82%) were used. The average value of the moisture, protein (N×5.7), ash, gluten content of the flour with a 75% extraction rate were 14.12gr, 11.37gr, 0.590gr, 26.7gr /100gr flour, respectively, the falling number was 350 s. The average value of the moisture, protein (N×5.7), ash, gluten content of the flour with 82% extraction rate were 13.95gr, 11.81gr, 0.830gr, 27.5gr/100gr flour, respectively and the falling number was 300 s. L(+)- Ascorbic acid (E 300) was purchased from Henan Tech way Co, Zhengzhou, China. Glucose oxidase enzyme (E.C.1.1.304) derived from Aspergillus oryzae with the activity of 10000 u / g was obtained from DSM Co, Heerlen, Netherlands. The additives ranges were (50-150 mg/g) for ascorbic acid and (10-30 mg/g) for glucose oxidase according to their manufacture’s recommendations. Used levels of the additives are given in Table 1.
In this study, six samples of wheat flour were divided into two groups (each group contained three flour samples) according to the milling extraction rates (American classification). The milling extraction rate is determined by the ash content. The ash content of the first set was between 0.5- 0.6% (extraction rate of 75 %), the ash content of the second set was between 0.75-0.9% (extraction rate of 82%).
After preparing two sets of flour, the analytical flour quality was determined (Table 1). Then the effects of ascorbic acid and glucose oxidase on the dough rheological properties were evaluated by Brabender Extensograph instrument (Brabender GmbH Co, Duisburg, Germany). The measuring procedure with the Extensograph has been done according to the international standard( AACC 54-10). Basically the procedure is as follows; preparation of the dough in the Brabender farinograph (Brabender GmbH Co, Duisburg, Germany) according to the ICC method of 114/1, weighting of the dough pieces, homogenization and shaping of the dough into a cylindrical piece, fixing of the sample in the proving cabinet, stretching of the sample until it tears. The force exerted is recorded as a function of time in the extensogram. Subsequently, the extensogram is evaluated by the software program. The reason for choosing the extensograph device, compared to other rheological instruments (Gluten Index, Farinograph, Mixolab instruments) for measuring the rheological properties of the dough is because of the improved ability of this device to measure the quality of flour after adding the improvers because there is a dough resting step in extensograph which give enough time to improvers for having the better activity. After measuring the rheological properties, the optimal oxidative improver for each flour set was determined by the Response Surface Methodology [5]. The bread baking process was done after adding the optimal improver and the results were compared with the control bread sample. In this study, all experiments were performed in triplicate and the average results of each group were reported.
Characterization of wheat flours
The moisture, protein, ash, and gluten content were determined using AACC methods (Approved Methods of the American Association of Cereal Chemists International, 11th edition, 2000), 44-10.01, 46-13.01, 08-01.01, and 38-12-02 (AACC, 2010), respectively.
Alpha-amylase enzyme activity was analyzed according to AACC method 56-81-03 by Falling Number (FN) device (Infracont Co, Pomez, Hungary).
Determination of the rheological properties
A Brabender extensograph was used for determining the rheological properties of flours. The following parameters were determined in the extensograph analysis: resistance to extension (R-value), maximum resistance to extension (Rmax-value), energy, and dough extensibility (E-value).
Bread baking
The bread baking was performed according to the straight dough method according to AACC method 10-09.01 (Approved Methods of the American Association of Cereal Chemists International, 11th edition, 2000), the protocol used was: flour 100%, compressed yeast 2%, salt 2%, sugar 4%, fat 3%, and water as much as necessary for reaching the optimal consistency of 500 BU. The dough was prepared from 300g of flour in a Farinograph (Brabender GmbH Co, Duisburg, Germany). Dough dividing and roll shaping were made by hand and after the standard proofing time, the pieces of dough were baked at 204°C for 25 minutes.
Bread Tests
Specific volume: Specific volume was determined by the seed displacement method (AACC method 10-05.01) and was calculated as the ratio (v/m). Specific volume determination was carried out on bread samples one hour after baking in triplicate.
Shape ratio: The height and width of the central slice of the bread were measured using a pachymeter and the shape was determined by the height/width ratio. A ratio of 0.5 indicates a regular roll shape; a ratio above 0.5 indicates a spherical shape, whilst a ratio below 0.5 indicates a flat shape (Shafiesoltani, et al. 2013).
Sensory evaluation: Sensory evaluation tests were done by ten trained judges including males (5) and females (5) in the age group of 20-50. External characteristics such as (volume, crust, color, shred and symmetry of form and crust characteristics) and internal characteristics such as (grain, crumb color, aroma, and taste, chewability, and crumb texture) were scored for each loaf according to the bread score method developed by the American Institute of Baking and reported by Matz (1991). These scores were converted into a global concept determined as: very good (>90), good (80–90), regular (70–80), and detestable (<70) (Shafiesoltani, et al. 2013).
Statistical analysis
The effects of oxidative improvers on the dough rheological properties were determined by Response Surface Methodology (RSM) using CCD (Khuri and Mukhopadhyay 2010). Response Surface Methodology can be regarded as a collection of statistical and mathematical techniques. The independent variables were ascorbic acid (50- 150 mg/kg) and glucose oxidase (10-30 mg/kg). The dependent variables were: resistance to extension (R-value), maximum resistance to extension (Rmax-value), energy, and dough extensibility (E-value). A central Composite Design (CCD) with five coded levels (- 1.41, -1, 0, +1, and +1.41) was used for studying the effect of independent variables on the dough properties. According to this design, the total number of treatment combinations was 13 (Table 2). The CCD and statistical analysis of the data were done with the Design-Expert software package (version 11, State-Ease Inc., Minneapolis, MN, USA). Coded models were generated to relate independent variables to the dough. Analysis of variance (ANOVA) was performed to evaluate significant differences and check the adjusted and predicted coefficient of determination (R2 values). The significant test level was set at 5% ( p<0.05).
For comparing the results of bread characteristics before and after adding the additives, ANOVA and the T-test method were used by SPSS software.
Results and discussion
Resistance to extension of the flours with low extraction rate
Resistance to extension (R-value) and the maximum resistance to extension (Rmax-value), characterize the force counteracting stretching so this factor indicates the strength of the dough (AACC-10-54, 2012). As shown by the results of the Extensograph test in Table 1, the R-value and the Rmax-value of the control sample are 340 Bu and 362Bu (Brabender unit) respectively, indicating a weak flour.
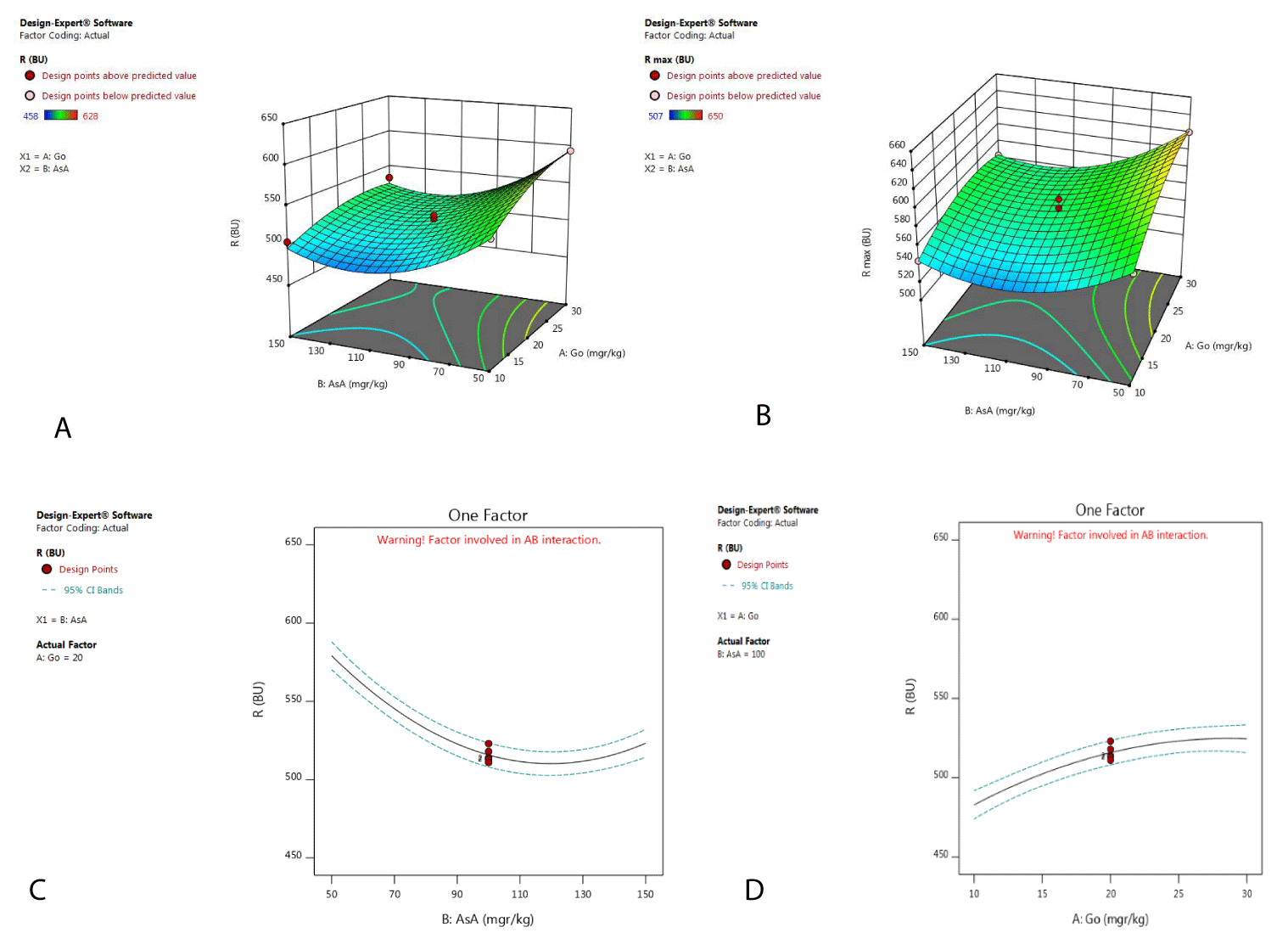
Statistical analysis of the results showed that glucose oxidase and ascorbic acid had a significant effect (p<0.05) on this parameter, so a coded and graph model was obtained (Table 2, Figure 1a,b). According to Figure 1a and Figure 1b, the addition of ascorbic acid up to 100 mg/kg has reduced the strength of the dough (R-value, Rmax value), while the values higher than 100 mg/kg are not statistically significant. This study showed that for increasing the dough stability, ascorbic acid should be used in higher doses (more than 100 mg/kg) in flour with 75% extraction. Less than 100 mg/kg leads to a decrease in the dough stability. Thus, finding the optimal dose of ascorbic acid by Extensograph device at the laboratory before adding it to flour by microfeeder at the product line is important economically because adding values higher than optimal dose are not statistically significant. Ascorbic acid in the presence of oxygen and the ascorbic acid oxidase enzyme converts to dehydroascorbic acid, which is the oxidative factor in the dough. Ascorbic acid in the absence of oxygen acts as a reductive agent and reduces the strength of the dough [6]. However, this study showed that ascorbic acid acts as a reductive agent (at dosages less than 100 mg/kg) in the presence of glucose oxidase enzyme. The reason can be attributed to the competition between glucose oxidase and ascorbic acid for oxygen consumption. Ascorbic acid cannot compete with glucose oxidase and behave as a reductive agent in the dough at concentrations of less than 100 mg/kg [7]. Increasing the dose of ascorbic acid (greater than 100 mg/ kg) enhances its ability to compete with glucose oxidase (Figure 1c). Hence, ascorbic acid is able to act as an oxidative agent in the dough at doses above 100 mg/kg along with glucose oxidase. Reduction of glucose oxidase activity in higher doses (Figure 1d), and the ascorbic acid beginning to act as an oxidative agent in the dough confirms the competition of these two factors in the dough.
Regarding the addition of the glucose oxidase enzyme, as shown in Figure 1a,b there is a direct correlation between the level of glucose oxidase and the R-value, showing that an increase of the dose of glucose oxidase enhances the strength of the dough. This is attributed to the formation of hydrogen peroxide as by-products of glucose oxidation due to the use of glucose oxidase [8,9], hydrogen peroxide are formed is unstable and rapidly converts to water and free radicals of oxygen. Free radicals of oxygen then form disulfide bonds between glutenin and gliadin, thus increasing the strength of the dough [10].
On the other hand, the subunits of glutenin are tyrosine-rich; these amino acids are catalyzed by peroxidases and participate in the formation of dityrosine [11]. Dityrosines are products of tyrosine oxidation that contribute to the structure of the gluten network and act as a kind of stabilizer in the gluten structure [11].
To enhance the stability of the flour with a 75% extraction rate, glucose oxidase has a greater effect than ascorbic acid. Also, according to the ANOVA, the effect of adding the combination of the ascorbic acid and glucose oxidase in flour with a 75% extraction rate was insignificant (p-value>0.05) (Table 2).
Extensibility: Extensibility (E-value) showed the stretching properties of the dough. Analysis of variance showed that there was not a significant difference between the extensibility results of the different flours with a 75% extraction rate (P-value > 0.05) (Table 2).
Energy: The energy parameter describes the work applied for stretching the dough. According to ANOVA, there was not a significant difference between the energy results of the flours with a 75% extraction rate (p-value>0.05) (Table 2).
Extensograph parameter of the flour with a high extraction rate
Resistance to extension: The results in Table 1 show that the R-value and Rmax values of the control sample are 245 BU and 249 BU, respectively which indicates the weakness of the flour. Analysis variance of the results showed that glucose oxidase and ascorbic acid had a significant effect on these parameters. R-value and Rmax-value can be explained mathematically from the variation of ascorbic acid and glucose oxidase (Table 2) and a response surface figure was obtained (Figure 2a,b). According to Figure 2c, the addition of glucose oxidase to the flour with an 82% extraction rate resulted in an increase in the dough stability at doses greater than 20 mg/kg. At doses lower than 20 mg/kg of glucose oxidase, changes in glucose oxidase doses had no affect on the strength of the dough (Figure 2c). This is possibly due to the flour with a high extraction rate, where hydrogen peroxide from the glucose oxidase oxidizes pentosanes instead of the gluten network [12,13]. Also, hydrogen peroxide from the glucose oxide causes the formation of a bond between frolic acid and pentosanes, resulting in the formation of hemicellulose gel in the dough. This gel competes with the gluten network in absorbing water and prevents the formation of the gluten network [14-16]. Thus, the addition of glucose oxidase enzyme at low concentration in flours with an 82% extraction rate had low effect on the dough strength. This is because the high extraction rate of flour lead to a high quantity of pentosanes and frolic acid in response to high quantities of bran in the flour [17]. In flours with a 75% extraction rate, the glucose oxidase had a significant effect at any concentration, but in flours with 82% extraction rate, adding it had less of an impact at doses lower than 20 mg/kg.
Another indirect effect of pentosans could be explained by their ability to form a network that can limit the movement of glutenin. They connect to glutenin proteins covalently, thus hindering the formation of gluten [18]. At the concentration of higher than 20 mg/kg, glucose oxidase caused an increase in the stability of the dough (Figure 2c) due to overcoming the formation of hemicellulose gels in response to the reduction of free pentosans in the dough [18].
There is an optimal dose of ascorbic acid to the flour with 82% extraction rate (Figure 2d). Doses less than 20 mg/kg can increase dough strength. But at a concentration greater than 100 mg/kg (Figure 2d), ascorbic acid induced a decreased effect on the dough stability. The reason attribute to the presence of the glucose oxidase enzyme. At low doses of ascorbic acid, due to the lack of oxidative activity of glucose oxidase enzyme, ascorbic acid has no competitor and acts as an oxidizing agent in the dough, but above 100 mg/kg, due to the activation of glucose oxidase, ascorbic acid becomes a reductive agent in the dough. Glucose oxidase in high doses inhibits the activity of ascorbic acid in the dough and ascorbic acid due to lack of oxygen in the environment (consumption of oxygen by glucose oxidase) becomes a reductive agent and reduces the strength of the dough [19].
The mechanism of ascorbic acid action in increasing dough stability can be attributed to the presence of glutathione in flour [19]. This attributes to the presence of glutathione in flour. Glutathione is a tripeptide composed of the three amino acids glutamine, cysteine and, glycine. This tripeptide is naturally present in wheat flour. Glutathione attenuates the gluten network due to the presence of a sulfhydryl bond on the amino acid cysteine, as it normally tends to bind to the glutenin polymer [6,7]. Gluten, which is the principal protein in wheat flour, is made up of two components, glutenin and gliadin. The polymer of glutenin and the monomer of gliadin form bonds through sulfur bonds in the structure of both components and form the gluten network [20]. The gluten network retain the co2 formed as a result of fermentation, thereby creating a tender texture in wheat bread. Increasing the number of bonds between glutenin and gliadin leads to the greater strength of the gluten network [21]. Thus, any factor that inhibits the binding of glutenin and gliadin weakens the gluten network. Ascorbic acid converts to dehydroascorbic acid in the presence of oxygen and ascorbic acid oxidase enzyme, which oxidizes glutathione. In this way, it prevents the weakening of gluten indirectly [6]. However, the effect of ascorbic acid is dose-dependent. At a concentration greater than 100 mg/ kg, ascorbic acid induced a diminishing effect on the dough stability. The reason for this is the activation of glucose oxidase enzyme at higher concentrations. Glucose oxidase enzyme can limit ascorbic acid oxidative activity [6]. This is because, in the presence of glucose oxidase due to the competition for oxygen consumption, ascorbic acid reacts as a reducing agent and lowers the strength of the dough.
Elasticity: The results of statistical analyses indicated that ascorbic acid had a significant effect on dough elasticity, but the addition of glucose oxidase had no significant effect on this parameter (Table 2). The elasticity of the dough would diminish if ascorbic acid was used (Figure 2e). This is due to the reduction of sulfhydryl bonds due to the oxidizing effect of ascorbic acid because sulfhydryl bonds are one of the factors that can increase dough extensibility [4,22]. Due to the formation of bonds between sulfhydryl and glutenin in the oxidation reaction, the number of sulfhydryl bonds in the dough decrease that result in the reduction of dough flexibility [23,24].
Energy: Statistical analyses of energy indicated that only the addition of glucose oxidase affected this parameter (Table 2). Figure 2f shows that the dough energy increased by adding the glucose oxidase, which was due to the rise in the strength of the dough. Analysis of variance showed that ascorbic acid did not have a signifi cant on this parameter.
Optimization
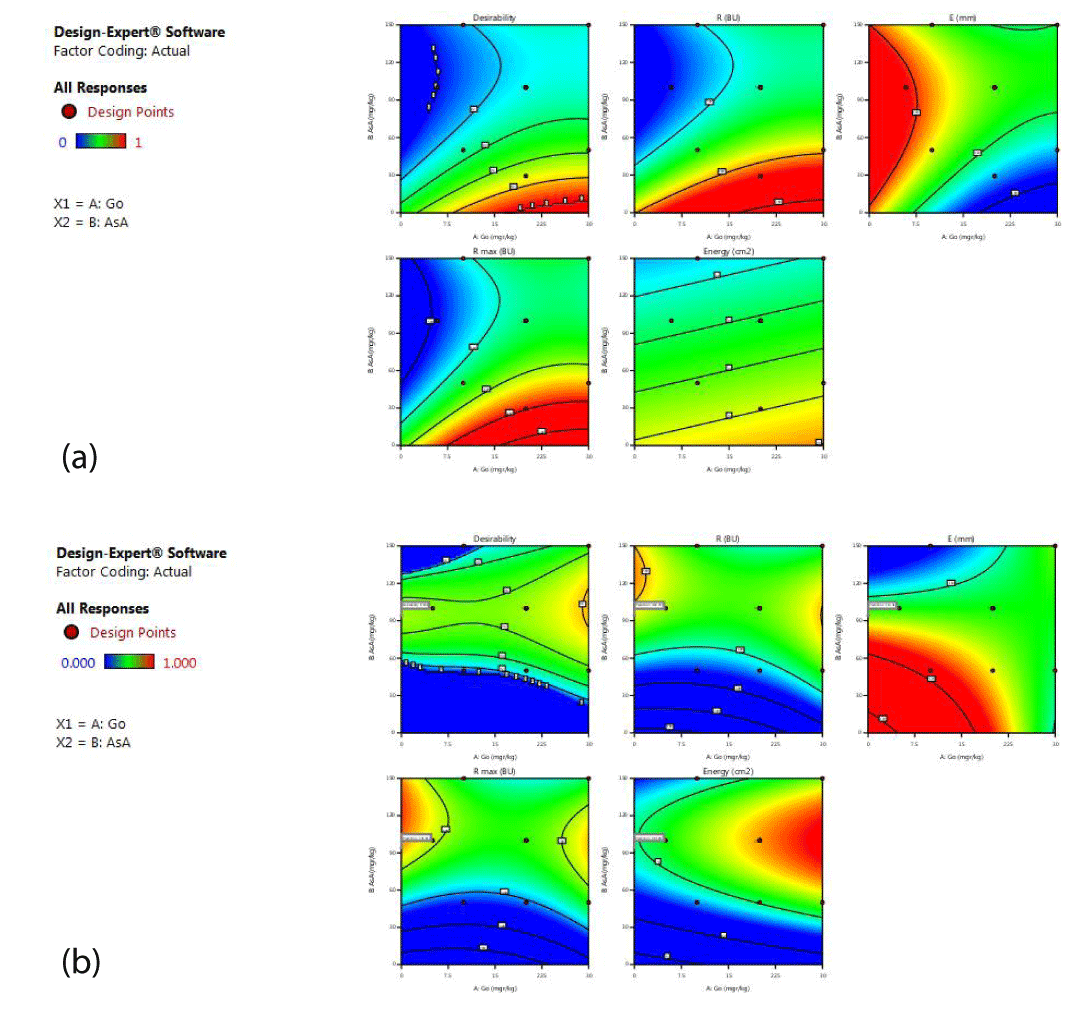
Selecting the optimal samples: Optimal samples were selected in both sets of flours based on the rheological results by RSM. Graphs of numerical optimization by RSM for flour with a 75% extraction rate are presented in Figure 3a demonstrating that the maximum recommended dose for the use of ascorbic acid is 30 mg/kg, which is the minimum quantities in the range as recommended by the supplier. This indicates that the effect of ascorbic acid in flours with a low extraction rate is minimal. The concentration of glucose oxidase suggested is 15-30 mg/kg.
RSM method showed that the use of 23 mg/kg glucose oxidase can create the highest dough strength and its use is more economical than other formulations. This formulation enhances the dough strength up to 711BU in comparison to the control sample with 340 BU. It would cause an acceptable increase in the strength of the dough.
In the case of flour with 82% (Figure 3b) in order to achieve the highest dough strength, it is necessary to use the largest dose of glucose oxidase enzyme while the concentration of ascorbic acid must be at least 90 mg/kg. Thus, in the flours with a high extraction rate, the use of glucose oxidase at minimum dosages is not effective and causes a waste of resources.
According to the combination suggested by the response surface method, the most suitable and economical additive is ascorbic acid and the optimal dose is 90 mg/kg, which increased the maximum strength of the dough, in comparison to the control sample with the dough strength of the 649 BU.
This study showed that the type of oxidative improver for strengthening the gluten network is different for wheat flours with different extraction rates. At low extraction rates of flours, glucose oxidase was a more effective additive while at high extraction rates of flours, adding ascorbic acid was more economical as glucose oxidase should be used at higher doses (greater than 30 mg/kg). Further, since glucose oxidase is more expensive than ascorbic acid, it is not economical to add it at high doses to achieve the result ascorbic acid can produce. Thus, attention should be paid to the type of flour before using the improver. In this study, for the low extraction rate of flour, the best oxidative improver was glucose oxidase at 23 mg/kg. On the other hand, for the high extraction rate of flour, it was better to use 90 mg/kg ascorbic acid. After selecting the best oxidative additives, the optimal oxidative dose was added to the high and low extraction rate flours separately, and after the bread baking process, the properties of the bread were analyzed with results compared to the control bread.
Bread tests
Special volume: The results of the specific volume according to Table 3 show that the specific volume of bread prepared with the oxidized flour was significantly higher than the control sample (T-test, sig- value <0.05). The use of oxidizers improved the gluten network quality, which resulted in better gas retention in the dough and increases the volume of bread as well as its subsequent volume subsequently [21,25].
Shape ratio: As shown in Table 3, the bread containing the optimal dose of oxidized additives has the highest shape ratio number (above 0.5), indicating their spherical shape, which is due to the increase in the height of loaves due to their stronger gluten network. On the other hand, the samples of control bread had a shape ratio below 0.5, suggesting that the bread surface is flat due to the weak gluten network. Weak gluten cannot retain the fermentation gases in its network and the bread surface would collapse in the oven [25].
Sensory evaluation: To evaluate the sensory attributes, one of the most comprehensive methods were used to evaluate all bread characteristics (Table 3).
The results of this evaluation showed that the addition of oxidants did not have a significant effect on characteristics such as grain texture, taste, and smell of the bread (T-test, sig-value>0.05) while exerting a significant positive effect (T-test, sig-value 0<0.05) on properties such as bread volume, crumb color, texture, and chewability in both sets of flours.
The reason for the improved crumb color is the oxidation of bread pigments due to hydrogen peroxide, which is produced by glucose oxidase [4]. The improvement of bread crumbs and their chewability is due to the increased retention of gases resulting from the activity of yeasts in the gluten network after adding oxidative agents [26,27].
However, according to the judges, if an oxidizer improver is used, the symmetry of forms in both groups of the bread samples, as well as the crust color of bread baked by the lower ash content flour, will decrease significantly (T-test, sig-value 0<0.05) compared to control. The negative viewpoint of judges for symmetry of forms was due to the flinty crust of bread (crust breaks like an eggshell), which can be the result of using too much oxidizing improvers [28,29].
In the case of using bread with low ash, the color of the bread crust was pale according to the judges. This color occurs due to the use of glucose oxidase enzyme, which leads to the production of hydrogen peroxide.
The overall score for the bread made with oxidized flour was higher than that of the control bread (Table 3) and in general, according to the judges, better bread can be made with oxidized flour.
Conclusion
In this study, for the 75% extraction rate of flour, the optimal improver is glucose oxidase at the doses of 23 mg/kg, and for the 82% extraction rate of flour, the optimal improver is 90 mg/kg of ascorbic acid.
The results of this study showed that glucose oxidase and ascorbic acid have an optimal dose but excessive use is not economical.
Another finding was the reduction of ascorbic acid activity in the presence of glucose oxidase. This point is important to obtain the optimal formulation for an oxidative improver.
Bread tests showed a higher specific volume and shape ratio for bread containing oxidative improvers compared to the control sample.
Regarding the sensory attributes, adding oxidative improvers had a significant positive effect on most attributes, i.e. volume, color, chewability, and texture of bread crumbs, but it did not have a significant effect on grain, aroma, and taste. It also had a negative significant effect on the crust color and bread symmetry of form.
In general, the total sensory evaluation scores of the bread with the improver were higher than those of the control bread sample.
Declarations
Availability of data and materials: The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Authors’ contributions: All authors (Mahsa Shafiesoltani, Mania Salehifar and Saeed Baeghbali) have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Research highlights:
- Oxidative improvers have different behavior in different types of flours.
- Glucose oxidase is more effective than ascorbic acid at flours with 75% extraction rate.
- Ascorbic acid is more effective than Glucose oxidase at flours with 82% extraction rate
- Ascorbic acid and glucose oxidase are dose-dependent improvers.
This work was supported by the Ard Tak Karaj Company. Thanks are due to the quality control unit of the mentioned company for their skillful assistance in testing the quality of flours and enzymes activity.
- Laskowski W, Górska-Warsewicz HRK, Czeczotko M, Zwolińska J (2019) How important are cereals and cereal products in the average polish diet? Nutrients 11: 679. Link: https://bit.ly/3FUSL5k
- Baratto C, Becker NB, Neves Gelinski JML, Silveira SM (2016) Influence of enzymes and ascorbic acid on dough rheology and wheat bread quality. African Journal of Biotechnology 15: 55-61. Link: https://bit.ly/3eM6PCj
- Kouassi-Koffi JD, Kouassi KH, Yapi A, Ahi AP, Muresan V, et al. (2015) Wheat bread dough rheological properties. Journal of Texture Study 46: 475-486.
- Kouassi-Koffi JD, Sturza A, Paucean A, Man S, Muresan AE, et al. (2019) Effect of glucose oxidase addition on the textural characteristics of wheat-maize dough and bread. Food Science and Technology 39. Link: https://bit.ly/3FXIJjO
- Khuri A , Mukhopadhyay S (2010) Response surface methodology. WIREs Computer Statistics 2: 128-149. Link: https://bit.ly/3pPCU2j
- Koehler P (2003) Effect of Ascorbic Acid in Dough: Reaction of oxidized glutathione with reactive thiol groups of wheat glutelin. J Agric Food Chem 51: 4954–4959. Link: https://bit.ly/3eToKXq
- Koehler P (2003) Concentrations of low and high molecular weight thiols in wheat dough as affected by different concentrations of ascorbic acid. J Agric Food Chem 51: 4948–4953. Link: https://bit.ly/3EMbbUs
- Decamps K, Joye IJ, Courtin CM, Delcour JA ( 2012) Glucose and pyranose oxidase improve bread dough stability. Journal of Cereal Science 55: 380-384. Link: https://bit.ly/332NM41
- Tilley KA, Benjamin RF, Bagorogoza K, Okot-Kotber BM, Prakash O, et al. (2001) Tyrosine crosslinks: Molecular basis of gluten structure and function. J Agric Food Chem 49: 2627–2632. Link: https://bit.ly/3FX1Quu
- Pourmohammadi K, Abedi E (2021) Enzymatic modifications of gluten protein: Oxidative enzymes. Food Chemistry 356: 129679. Link: https://bit.ly/331RfQk
- Peña E, Bernardo A, Soler C, Jouve N (2006) Do tyrosine crosslinks contribute to the formation of the gluten network in common wheat (Triticumaestivum L) dough? Cereal Chemistry 80: 52–55. Link: https://bit.ly/3FWFyc0 .
- Hoseney RC, Faubion JM (1981) A mechanism for the oxidative gelation of wheat flour water soluble pentosans. Cereal Chemistry 58: 421-424. Link: https://bit.ly/32YvMYy
- Wang M, Hamer RJ, Van Vliet T, Oudgenoeg TG (2002) Interaction of water extractable pentosans with gluten protein: Effect on dough properties and gluten quality. Cereal Science 36: 25–37. Link: https://bit.ly/3t0hzFh
- Joye IJ, Lagrain B, Delcour JA ( 2009) Use of chemical redox agent and exogenous enzymes to modify the protein network during bread making a review. Journal of Cereal Science 50: 11–21. Link: https://bit.ly/3ERQxSS
- Martin CP, Wang M, Lichtendonk WJ, Plijter JJ, Hammer RJ (2005) An explanation for the combined effect of xylanase-glucose oxidase in dough systems. Science of Food and Agriculture 85: 1186–1196. Link: https://bit.ly/3pSeANp
- Maa BM, Suna QJ, Lia M, Zhub K (2020) Deterioration mechanisms of high-moisture wheat-based food – A review from physicochemical, structural, and molecular perspectives. Food Chemistry 318: 126495. Link: https://bit.ly/3Hvr1EE
- Banu I, Georgeta S, Violeta I, Aprodu I (2012) Effect of the addition of bran stream on dough rheology and bread quality.Food Technology36: 39-52. Link: https://bit.ly/3mUyCEX
- Wong C M, Wong K H, Chen X D ( 2008) Glucose oxidase: natural occurrence, function, properties and industrial applications. Applied Microbiology Biotechnology 78: 927-938. Link: https://bit.ly/3mXfWnZ
- Kornbrust B A, Forman T, Matveeva I (2012) 19 - Applications of enzymes in Bread making. Bread making 470- 498. Link: https://bit.ly/3pRjSsf
- Almeida EL, Chang YK ( 2012) Effect of the addition of enzymes on the quality of frozen prebaked French bread substituted with whole wheat flour. LWT 49: 64-72. Link: https://bit.ly/3FUSiQC
- Xu X, Luo Z, Yang Q, Xiao Z, Lu X ( 2019) Effect of quinoa flour on baking performance, antioxidant properties and digestibility of wheat bread. Food Chemistry 294: 87–95. Link: https://bit.ly/3FUSiA6
- Ooms N, Jansens KJA, Pareyt B, Reyniers S, Brijs K, et al. (2018) The impact of disulfide bond dynamics in wheat gluten protein on the development of fermented pastry crumb. Food Chemistry 242: 68-74. Link: https://bit.ly/3eTopUE
- Dahiya S, Bajaj BK, Kumar A, Tiwari SK, Singh B ( 2020) A review on biotechnological potential of multifarious enzymes in bread making. Process Biochemistry 99: 290–306. Link: https://bit.ly/3FWs3sV
- Steffolani ME, Ribotta PD, Pérez GT, León AE (2010) Effect of glucose oxidase, transglutaminase, and pentosanase on wheat proteins: relationship with dough properties and bread-making quality. Journal of Cereal Science 51: 366-373. Link: https://bit.ly/3sVeZAh
- Shafisoltani M, Salehifar M, Hashemi M (2014) Effects of enzymatic treatment using Response surface methodology on the quality of bread flour. Food Chem 148: 176-183. Link: https://bit.ly/3mTDni5
- da Silva CB, Almeida LE, Chang YK ( 2016) Interaction between xylanase, glucose oxidase and ascorbic acid on the technological quality of whole wheat bread. Food Technol 46: 2249-2256. Link: https://bit.ly/3mV5PQt
- Malalgoda M, Ohm JB, Meinhardt S, Simsek S ( 2018) Association between gluten protein composition and breadmaking quality characteristics in historical and modern spring wheat. Cereal Chemistry 95: 226–238. Link: https://bit.ly/3zmS3eB
- Miś A, Nawrocka A, Dariusz D (2016) Identification of baking expansion phases of leavened dough using an experimental approach. Food and Bioprocess Technology9: 892–903. Link: https://bit.ly/3JAW0kJ
- Ooms N, Delcour JA (2019) How to impact gluten protein network formation during wheat flour dough making. Current Opinion in Food Science 25: 88–97. Link: https://bit.ly/32NbEc2
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
