International Journal of Agricultural Science and Food Technology
Metallic nutrients in enset (Ensete Ventricosum) corm and soil sample from some West Shoa Zone, Oromia Regional State, Ethiopia
Teressa Bedada* and Alemayehu Abebaw
Cite this as
Bedada T, Abebaw A (2021) Metallic nutrients in enset (Ensete Ventricosum) corm and soil sample from some West Shoa Zone, Oromia Regional State, Ethiopia. J Agric Sc Food Technol 7(1): 073-080. DOI: 10.17352/2455-815X.000091The aim of this study was to investigate the essential and non-essential metals concentration in corm of Ensete ventricosum and soil samples from some West Showa Zone. Ca and Mg were determined complecometric titration method, K and Na were analyzed using Flame Absorption Emission spectroscopy and the other metals with flame atomic absorption spectroscopy after appropriate quality control measures were undertaken to verify and maintain the quality of the data generated. The optimized wet digestion method for corm and soil analysis was found effective for all of the minerals and as it was evaluated through the recovery experiment, a good percentage recovery 95% (Fe concentration in corm) to 112% (Pb concentration in soil) was obtained for the minerals identified. The results of this study showed that the average the metallic nutrient concentrations of elements in the soil sample were ranged in order of decreasing in (mg.kg-1) 3521.11(K) >3497.85(Mg) > 3461.59(Fe) > 3294.93(Ca) >1096.89(Na) > (93.99(Mn) >16.74(Zn) > 3.77(Cu) > 0.26(Cd) but, the concentration of Pb was not detected and the metallic nutrient concentrations of elements in the corm were ranged in order of decreasing in (mg.kg-1) 16425.13(Ca) >13813.33(K) > 1323.55(Mg) > 1131.11(Na) > 76.78(Fe) > 16.16(Zn) >2.77(Cu) >1.94(Mn) but, Cd and Pb were not detected. The nutrient concentrations of the metals were also compared with recommended maximum permissible limits and some international reports; and found to be in a good agreement indicating no exposure risk of using the corm of Ensete ventricosum under the current situation. Statistical test of significance using ANOVA revealed that there were significant differences (P<0.05) between the values of metals in the corm and soil samples obtained from all the sampling sites except Zn concentration for corm and Ca concentration in soil) is not found.
Introduction
Root and tuber crops are widely cultivated in southern Ethiopia, which are supporting a considerable portion of the country’s population as source of food. Prominent among these are potato (Solanum tuberosum L.), sweet potato (Ipomoea batatas L.), Enset (E. ventricosum), Godere (Colacasia esculanta L.), Yams (Dioscorea spp.), Ethiopian dinch (Coleus parviflorus), koteharrie (Diaspora bulbiferous) and Anchote (Coccinia abyssinica). Among these, Enset, Anchote and some yams are endemic to Ethiopia [1]. Enset based farming systems play an important role in food security in Ethiopia [2] and Ensete ventricosum is one of the indigenous root crops widely cultivated in the Central, South and South Western parts of Ethiopia, but recurrent droughts have led to the expansion of Enset cultivation to other parts of the country [3,4].
Ensete ventricosum parts contained high percent of water (85 to 90%), which is beneficial when used as fodder during dry periods. Corm of Ensete ventricosum contained 17 of 20 amino acids. Leaves had 13% protein, among the highest available in Ethiopia, 20% crude fibre and 10% sugar. The pseudostem the main food source, was rich in 80% of soluble carbohydrates and 65% of starch, but has low protein content 4% [5].
Eating the right foods is an important part of maintaining a healthy lifestyle. A single day’s intake of nutrients may affect the body’s organs only slightly but over years and decades the effects of unhealthy diet compounds into disease, shortened lifespan, and a less active lower productive life. The nutrients that we intake today will become part of us tomorrow. The nature and composition of what we eat as determines our future, Enset as a food would have its own influential impact on millions of peoples in Ethiopia. The corm of Enset has rich in essential nutrients and low in non-essential nutrients [6].
The human body requires a number of nutrition to preserve a good health that nutrition accumulated in different parts of plants (FAO, 2004). Thus plants are intermediate reservoirs through which trace elements from soil and partly from water and air, transfer to man and animal (Rogan, et al. 2009). The content of heavy metals is one of the criteria for the use of plant material as food or traditional medicines. Hence determination of mineral compositions in food and medicinal plant is essential for understanding their nutritive importance and health risk [7].
However, no literature report was found on comparative determination the concentration of essential and non-essential metals in corm of Ensete ventricosum with its supporting soil samples. Therefore, the aim of this study to compare the essential and non-essential metals concentration in corm of Ensete ventricosum and soil environment from some different locations of West Shoa Zone, Oromia Regional State, Ethiopia by using flame atomic absorption spectroscopy and flame emission atomic spectroscopy.
Materials and methods
Apparatus and instruments
Polyethylene plastic bags, Electronic analytical balance with 0.0001g sensitivity (AA-200DS Deriver Instrument Company, German), A 250 ml round bottomed flasks fitted and reflux condensers, Whatman filter paper (No.42 150 mm, England), Digestive furnace (Model KDN-20C, China), Flame Photometer (ELICO, CL-378, India) and flame atomic absorption spectrophotometer FAAS (Buck Scientific Model 210 VGG, USA).
Chemicals and reagents
All reagents were analytical reagent grade. The reagents and chemicals used in this study were: HNO3 (65-68%, Uni-Chem® Chemical Reagent, India), HClO4 (70-72%, Uni-Chem® Chemical Reagent, India), H2O2 (30%, Uni- Chem®, India, EDTA-Na2 (98.5-101%, Unic-Chem®, India) and Stock standard of metals.
Description of the study areas
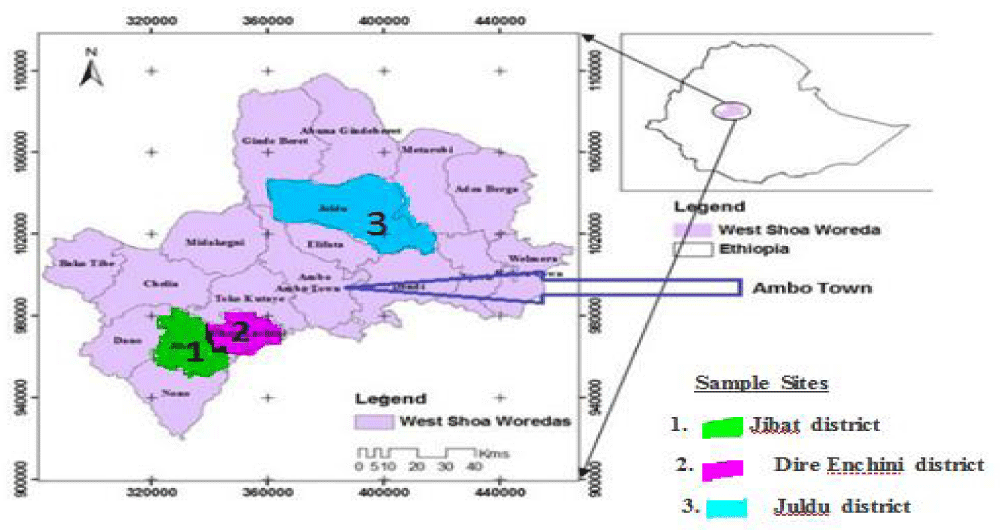
The study was conducted in Oromia National Regional State, West Shoa administrative zone. It was found between 8017’- 9056’N and 3701’-38045’E. The zone was bounded with Amhara Regional State in the Northern part, East Wollega and Horro Guduru Wollega in the West and North West, Jimma Zone in the South West, South West Shoa Zone in the South East, and North Shoa Zone in the North East. West Shoa Zone was 170Km long from North to South and 183Km wide from East to West. For administrative purpose the Zone is divided into eighteen districts Figure 1. Ambo was the capital city of zone and far 114Km to the West of Addis Ababa on the main way from Addis to Nekemte road.
The Zone has variable topography consisting of a high and rugged central plateau, flat and genteel slope, and the peripheral lowlands. The Mean annual temperature of the zone ranges from 10°C to 22°C in the highlands and from 22°C to 30°C in the lowlands. Thereby, Annual average rainfall in the area is ranging from 1200 to over 2000mm. The Zone has three agro climatic zones, namely Dega (57%), Woina dega (25%) and Kolla (18%) (Zone Office Agriculture and Rural Developing, 2015).
Sample collection and protocol
The corm of Ensete ventricosum sample was collected in January, 2016 from the three agricultural areas with its supporting soil. Each sample was collected purposely from four different sub-sites (farm lands) to provide replicate samples. From the three agricultural areas corm can be prepared according to the traditional method. The edible designated plant out of the land was cut into three parts for the separation of pseudostem and Corm with knife (Figure 2). The soil sample was collected from the surface 15cm-25cm depth of the same four sampling areas of Enset by spade. Finally three corm and soil samples one from each stated areas were collected and put into clean cooled.
Sample preparation
Corm and soil samples were collected from each sub-sites (kebeles) were air dried for three days to remove moisture and all clods and clumps. The samples were grinded with a mortar and pestle and then sieved through a 2mm mesh sieve. The four sub-samples were mixed equal proportion together to form a composite sample that represents each sampling areas. The powdered two samples were placed in pre-cleaned screw capped polyethylene container and stored in desiccators containing calcium chloride to keep to constant dry weight till digestion [8].
Optimization of digestion procedure for corm and soil samples
0.5g of air dried and homogenized corm and soil samples were transferred into a 250mL round bottomed flask. To this was added 3.5ml a mixture of HNO3(69-72%) and HClO4 (70%) with a volume ratio of 2:1.5 for corm and 4.5ml a mixture of HNO3 (69-72%), HClO4 (70%) and H2O2 (30%) with a volume ratio of 2:1.5:1 for soil. The mixture were digested on a micro Kjeldahl digestion apparatus by setting the temperature 210 and 230 for 1:45hr and 2:30hr respectively. Then, after the digested solution were allowed to cool for 20min without dismantling the condenser from the flask and for 10min after removing the condenser. To the cooled solution 25ml of distilled water was added to dissolve the precipitate formed on cooling and to minimize dissolution of filter paper by the digest residue while filtering with Whatman filter paper. The round bottom flask was rinsed subsequently with 5ml distilled water until the total volume reached around 45ml. To this final solution, 1% lanthanum nitrate solution was added and the solution was filled to the mark 50ml with distilled water. The digested samples were kept in the refrigerator, until the level of all the metals in the sample solutions were determined by FAAS and FAES.
Method validation and quality control
Precision and accuracy: Precision and accuracy of the analytical method was assessed by repeatability and recovery studies of Matrix Spike (MS) and Laboratory Control Samples (LCS). Recovery study was performed by spiking three replicate of corm and soil samples with a known concentration of metal standard solution (mid-range calibration concentration). The spiked samples were then subjected to the same digestion procedure like the actual sample. Precision was calculated by and accuracy was calculated by this equation [9].
Instrument Detection Limit (IDL)
Instrument Detection Limits (IDLs) was estimated by taking seven replicate measurements of the calibration blank (Distilled water). The IDL is calculated to the concentration equal to three times the standard deviation of seven replicate measurements of blank [10].
Where, Sb is standard deviation of blank (n = 7) and IDL is Instrument detection limit.
Method Detection Limit (MDL)
Method detection Limit is the minimum concentration of analyze that can be identified measured and reported with 99% confidence that the analyze concentration is greater than zero. MDL was based up on seven replicate measurements of a series of calibration blanks (reagent blank) that are carried through the entire sample preparation scheme [11]. The MDL was calculated by:
MDL = S×T- test Where, S is standard deviation of the replicated analysis with n-1 degree of freedom, t = 3.71 (T- test value for a 99% of confidence level for six degrees of freedom).
Method Quantification Limit (MQL)
Method quantification limit was obtained from analysis of seven reagents blanks which were digested in the same digestion procedure as actual samples. The method quantification limit was calculated by multiplying standard deviation of the reagent blank by ten plus the mean of the reagent blank signals [11]. It can be calculated by: Where, is the mean of blank, is standard deviation of the blank.
Method blank
The method blank accounts for contamination that may occur during sample preparation and analysis. These could arise from the reagents, the glassware or the laboratory environment [8]. Sucrose was used as matrix since there was no other plant and clear soil that can serve as the matrix for the corm and soil samples. The blank which was prepared from the sucrose and any reagents used for the digestion was taken through the entire measurement procedure to detect contamination from reagents, sample handling, and the entire measurement process [12].
Matrix spike
Matrix Spike (MS) is portion of a sample spiked with known concentration(s) of target analyte(s). The spiking occurs prior to sample preparation and analysis. The purpose of a matrix spike sample is to determine whether the sample matrix contributes bias to the analytical results [13]. In this study, Matrix spike was prepared for each sample item by spiking aliquots of 0.5g of each corm and soil samples with 2.5ml standards mixture solution giving concentrations of 1.0mgL-1 for K, Zn, Cd and Pb; 2.0mgL-1 for Na, Cu, Fe and Mn. They were all carried through the same digestion and analysis steps as an unspiked sample (Table 1). And the mean recovery values of Matrix Spikes were calculated by:
Transfer factor of metals from soil to Ensete Ventricosum
Transfer factor is the ratio of the concentration of metals in a plant to the concentration of metals in soil. Transfer factor for each metals was computed based on the method Harrison and Chirgawi (1989) as described by [14] according to the following formula.
Where, pm is metals concentration in plant and Sm is metals concentration in soil.
Statistical analysis
Analysis of variance (ANOVA) and F-test at p<0.05 are used to examine statically significant differences in the mean concentrations of metals among groups of soil and corm of E. Ventricosum. A probability level of p<0.05 is considered statistically significant. All statistical analysis was done by Microsoft Office Excel 2007 was used for data analysis and SPSS Version 16.0 Software Window was used for Analysis of variance (ANOVA) and correlation between metals in corm and soil samples [11].
Results and discussion
Instrument detection, method detection and quantification limits
As indicated Table 2 the method detection values ranged from 0.08mgkg-1 (Cd in soil) to 2.441mg.kg-1(Zn in corm) and the MQL values lied in range from 0.3mg.kg-1(Pd in Corm) 4.21 mgkg-1(Mn in corm). The results revealed the both MDL and MQL values were greater than the IDL; hence, the results of the analysis could be reliable.
Calibration
The Calibration curves for the various concentrations were ranged between 0.9969 and 0.9999, which were all greater than the required limit (0.995) for trace element analysis. This showed that there was good correlation (relationship) between concentration and absorbance indicating good calibration of instrument (Table 2).
Method precision and accuracy
As it can be indicated Table 3, The mean percent recovery values ranged between 94.85% Fe in soil to 109% (Cu in corm), all lied in the acceptable range (80–120%) for metal analysis [12]. This showed that the analytical method provided results in the required level of accuracy. The RSD values of recovery was ranged between 0.05% (Mn in corm) to 12.22% (K in corm), all lied under the required limit ≤15% [15].
Laboratory control samples result
The percent recovery values of LCS measurements lied in the range 94.0% (Na in soil) to 113.6% (Zn in bulla) and their relative standard deviations 0.09 (Cu in soil) to 11.37 (Cd in soil), and all the values were found under standard control limits 80–120% for LCS recovery, and ≤15% for RSD [15]. This showed that the method used for the study has provided the required level of accuracy and precision throughout the analytical process.
Optimizations the digestion procedures of soil samples
Digestion of soil sample: 0.5g of soil with a mixture of 2ml of concentration of HNO3 , 1.5ml concentration of HClO4 and 1ml concentration of H2O2 digested at a temperature of 230 for 2:30 hours gave a clear colorless solution respectively. These optimum conditions were selected based on clarity of digest; minimum reagent consumption, minimum digestion time, and minimum temperature applied for compete digestion of samples. After digestion the samples were cooled and dilute to 50ml (Table 4).
Metal concentration in soil used for Enset (Ensete ventricosum) cultivated
The analyzed K concentration of the soil sample was ranged from 3403.33 to 3710mgkg-1. The highest K concentration was observed in the Dire Enchini and the lowest in the Jeldu, Which is found within the permissible level 1000 to 30000mgkg-1 of K in soil (EPA, 2002). The values of concentration of K in this study greater than with the one reported by Wodaje and Alemayehu [16], which was ranged from 1980 to 6065mgkg-1.
The analytical data of Ca and Mg concentration in soil sample used for cultivated of Enset were ranged from 2647.33 to 3949.12mgkg-1 and 2647.78 to 3934.67mgkg-1 respectively. The highest Mg concentration was observed in te Jeldu and the lowest in the Dire Enchini, Which is found within the permissible level 5000 to 30000 and 1000 to 15000mgkg-1 of Ca and Mg in soil respectively (EPA, 2002).When, have compared the concentration of Ca and Mg in this study areas soil samples similar with Ca concentration but greater than in case of Mg concentration with the one reported by Wodaje and Alemayehu [16], which was ranged from 23866 to 32262 and 1751 to 4288mgkg-1.
The levels of six trace metals were analyzed in soil samples the results of total concentration of all metals of interest in the soil samples. The mean concentrations of Fe, Mn, Zn, Cu, Pb and Cd were presented in this study 102.87 to 184.1, 83.1 to 99.87, 11.67 to 20.13, 2.5 to 3.07, 0.49mgkg-1 and not detect respectively in the soil samples. The concentrations of all metals in the analyzed soil samples of Enset environment were under the EPA maximum permissible limit of typical concentration in soil dry matter, Fe, Mn, Cu, Zn and Cd are 10–50, 20-30, 2–100, 10–200, 0.1–1mg kg-1 respectively (EPA, 2002) Table 5.
Optimizations the digestion procedures of corm sample
Digestion of corm of E. Ventricosum samples: 0.5g of corm with a mixture of 2ml of concentration of HNO3, 1.5ml concentration of HClO4 digested at a temperature of 210 for 1:45 gave a clear colorless solution. The corm sample digestion were a reagent mixture of 2ml HNO3 and 1.5ml of HClO4 1:45 hour and temperature of 210 Corm Sample of E. Ventricosum (Table 6).
Metal levels in corm of E. Ventricosum
The mean concentrations K was the second most accumulated corm next to calcium. The values of K concentration in corm sample was studied areas ranged from 12050.00 to 15013.33mgkg-1. The highest K concentration was observed in the Jibat corm and lowest in the Jeldu corm, which is below the permissible limit of K concentration in plant dry matter was ranged from 15% [18]. While, as have compared the concentration of K in corm in this study less than in corm with the one reported by Ayalew, et al. [6] around welkete, which was ranged from 14100 to 32200 mg.kg-1.
The analyzed sodium concentration of Corm was ranged from 1023.33 to 1326.67mgkg-1. The lowest Na concentration was observed in Jeldu and highest in Jibat, This concentration was below the WHO recommendation on sodium maximum consumption for adults, which is 2 g sodium/day (WHO, 2012).
The analytical data of Mg concentration in corm of Enset was ranged from 1268.67 to 1370 mgkg-1. The highest Mg concentration was observed in the Jibat and lowest in the Jeldu, which is below the maximum allowed concentration of Mg 0.1 to 0.4% in dry matter of plant FAO [18]. The mean concentration of Mg in corm of Enset in this study less than in corm with the one reported by Ayalew, et al. [6] around welkete, which was ranged from 24900 to 26900 mgkg-1.
The average concentration of Calcium in corm was studied areas ranged from 15912.22 to 16875.78mgkg-1. When compared the concentration of Ca, was observed highest in the Jibat lowest in the Jeldu. This concentration found within the range of the maximum permissible limit of Ca in plant dry matter was ranged from 15% [18]. While, as have compared the amount of Ca in corm of Enset in this study within the same range with the one reported by Ayalew, et al. [6] around welkete, which was ranged from 36100 to 39100 mg.kg-1.
The concentration of iron was analyzed in corm of Enset sample was ranged from 53.67 to 62.0mgkg-1. The lowest Fe concentration was observed in the Jibat Corm whereas highest in the Jeldu Corm. This concentration is found within the maximum permissible limit of iron ranged between 50 to 250mg.kg-1 in plant dry matter [18]. When, as have compared concentration of Fe in this study almost similar concentration in corm with the one reported by Ayalew, et al. [6], which was ranged from 18.2 to 54.4mgkg-1.
The Concentration of Manganese in Corm of Enset was found between 1.13 to 2.40mg.k-1, which was found below the FAO [18] maximum permissible limit of 20 to 300mg.kg-1. When as compared the manganese concentration in Corm from the three sites the lowest Mn concentration was observed in the Jeldu corm but, the highest in the Dire Enchini Corm and the concentration of Mn in Corm of Enset in this study slightly less than with the one reported by Ayalew, et al. [6], around welkete,which was ranged from ND to 5.61μgg-1 and 2.0 to 5.0μg-1.
The Concentration of Zn accumulated in corm next to iron among of micro nutrients from the study areas, which was ranged from 15.43 to 17.43mgkg-1. While, the lowest Zn concentration is observed in the Jibat and the highest in the Dire Enchini, Which was found below the maximum permissible limit of Zn set FAO [18], was ranged from 50 to 250mgkg-1 in plant dry matter. The concentration of Zn in this study with the one reported by Ayalew, et al. [6], which was ranged from 2 11.9 to 42.3mgkg-1.
The mean concentrations of Cadmium and lead were not detected in corm sample ,those were below the acceptable concentration for food stuff which is around 1ppm [19-22], indicating no exposure risk to Cd and pb. The lowest level of Cd which can cause yield reduction is 5–30pm [19-22] Table 7.
Transfer factor of metals from soil to E. ventricosum
The transfer factor of metals from soil to E. ventricosum, Ca, K, Na, Mg and Cu concentration were more accumulated. When as compared the transfer factor among the different metals, Ca, K, Cu, Na and Zn concentration showed the maximum transfer factor value (Table 8), which ranged from 1.0 Mg in the Jibat to 6.0 Ca in the Dire Enchini and Mn and Fe concentration were minimum value which, ranging from 0.01 in the Jeldu to 0.6 in the Jeldu. This is indicated that corm is rich in essential metals. The high level of these metals in Ensete ventricosumm high due to direct deposition a foliar absorption more than the translocation upper part of the plant to the root of the plants. This can be attributed to the redistribution of elements within the soil profile (Zubillaga, et al. 2008).
Conclusion
The level of essential and non-essential elements in corm of Enset and soil sample was determined by flame atomic absorption spectrometry, flame photometry and complexometric titration with EDTA. The distribution of the selected essential and non-essential metals over corm sample of E. Vectricosum and soil were observed, they were found to vary in the order of decreasing Ca > K> Mg>Na > Fe > Zn >Cu> Mn but, Cd and Pb could not be detected from Corm sample and K > Ca >Mg>Na >Fe >Mn >Zn >Cu >Cd >Pb(ND) in soil samples of the three areas. Based on the WHO recommended limit and FAO (2008) the maximum permissible limit for plant, K, Na, Mg, Ca, Fe, Mn, Cu, Zn and Pb, Cd were not found to cause any risk to the people by consuming the E. Vectricosum plants grown in the area where the E. Vectricosum is planted (studied areas). Statistical test of significance using ANOVA revealed that there were significant differences (P<0.05) between the values of metals in the corm and soil samples obtained from all the sampling sites except Zn concentration in corm and Ca concentration in soil.
Statistical test of significance using ANOVA revealed that there were significant differences (P<0.05) between the values of metals in the corm and soil samples obtained from all the sampling sites except Zn concentration for corm and Ca concentration in soil) is not found.
Acknowledgement
The authors would like thank to the Nono Woreda administration office for sponsoring his study and the Department of Chemistry, Ambo University, Ethiopia, for providing the laboratory facilities and the necessary supports.
- Addis T (2005) Biology of Enset toot mealybug (Cataenococcus Ensete) Williams and Matile Ferrero (Homoptera: Pseudococcidae) and its geographical distributionin. Southern Ethiopia. MSc. Thesis, Alemaya University, Ethiopia 1-96.
- Spring A, Haile B, Tesfaye S, Abebe Y, Amaldegen A, et al. (1996) Enset farming in Southern Region, Ethiopia: Report on a Rapid Rural Appraisal in Gurage, Hadiya, and SidamaZones. Addis Ababa.
- Brandt S, Spring A, Hiebsch C, McCabe J, Endale T, et al. (1997) The “Tree against Hunger” Enset-based agricultural systems in Ethiopia, American Association for the advancement of Science. Washington, DC. 1-41. Link: https://bit.ly/2M6x0bV
- Genet B, Hilde N, Endashaw B (2004) Distniction between wild and cultivated E. ventricosum gene pools in Ethiopia using RAPD markers. Heriditas 140: 139- 148. Link: https://bit.ly/37viPVx
- Mohammed B, Gabel M, Karlsson L (2013) Nutritive values of the drought tolerant food and fodder crop Enset. Afr J Agr Rese 8: 2326-2333. Link: https://bit.ly/2ZzNSes
- Ayalew D, Chandravanshi BT, Wondimu T (2012) Metallic nutrients in Enset (E. ventricosum) Corm cultivated in Wolliso and Wolkite towns in Ethiopia. SINET Ethiopia J Sci 35: 71–80. Link: https://bit.ly/3ugPecc
- Endashaw B (2007) Study on actual situation of medicinal plants in Ethiopia. Japan, Association for International Collaboration of Agriculture and Forestry. Link: https://bit.ly/2OVt6DX
- Mitra S (2003) Sample preparation techniques in analytical chemistry, John Wiley and Sons, Hoboken, New Jersey 162: 6-244. Link: https://bit.ly/2ZyYSsA
- Javed I, William AC, Shelly LC, Chandra ST (2010) Metals determination in biodiesel (B100) by ICPOES with microwave assisted acid digestion. Open Anal Chem J 4: 18-26. Link: https://bit.ly/3scalu6
- USEPA (2007) Determination of metals and trace elements in water and waste by Inductively Cuopled Plasma Atomic Emission Spectrometry.
- Miller N, Miller J (2010) Statistics and chemo metrics for analytical chemistry 6th edition, Pearson Education limited, UK.
- USEPA (2008) National functional guidelines for superfund organic methods data Review USEPA-540-R08-01, Washington, DC.
- Marella A (2010) Laboratory quality assurance and quality control. Guidance reasonable Confidence Protocols, 79 Elm Street, Hartford. Link: https://bit.ly/3k4rfbk
- Uwah EI, Gimba M, Gwqski P (2012) Determination of Zn, Mn, Fe, and Cu Spinach and Lettuce Cultivated in Potiskum, Yobe State, Nigeria. J Agr Econ Dev 1: 69-74. Link: https://bit.ly/3dvXCyk
- Csuros M, Csuros C (2002) Environmental sampling and analysis for metals Lewis publishers, A CRC Press Company Boca Raton London, New York, Washington, DC. Link: https://bit.ly/2OWIkZp
- Wodaje A, Alemayehu A (2014) Analysis of selected physicochemical parameters of soils used for cultivation of garlic (Allium sativum L.), Sci Tech Arts Rese J 3: 29-35.
- FAO/WHO (Codex Alimentarium commission) (2001) Food additive and contaminants Join FAO/WHO standard programme ALINORM 01/12A: 1-289. Link: https://bit.ly/3ue6rTr
- FAO (2008) Guide to Laboratory Establishment for Plant Nutrient Analysis by Roy RN., M.R. Motsara, Rome. FOA fertilizer and plant nutrition bulletin 19: 1-219. Link: https://bit.ly/3ua1VVY
- Khan SA, Khan L, Hussain I, Marwat K, Akhtar N (2008) Profile of heavy metals in selected medicinal plants. Pak J Weed Sci Res 14: 101–110. Link: https://bit.ly/3k4yn7H
- Ararso N, Alemayehu A (2013) Analysis on selected essential and non-essential metals in the stem, leaf and soil Environment of Rhamnus Prinoides (Gesho): in the Case of Lowland and Medium Altitude Areas of Bako Tibe District, Oromia, Ethiopia. J Sci Tech Arts Res 2: 20-26.
- Menegesha T, Vegi M, Gezahegn F (2014) Determination of essential and non- essential metals concentration in papaya (carica papaya) Seeds, Leaves and supporting Soil of Odo- Shakiso district in South east Oromia region, Ethiopia. Inter J Rese Pharmacy Che 4: 202-216. Link: https://bit.ly/3aCN3YI
- WHO (2008) Permissible limits of heavy metals in soil and plants, (Genava: World Health Organization), Switzerland.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
