International Journal of Agricultural Science and Food Technology
Seed Quality Analysis of Field Pea (Pisum Sativum L.) from Formal and Informal Sources in Enarj Enawuga and Yilmana Densa Districts, West Amhara Region, Ethiopia
Yenus Ali1*, Firew Mekibib2 and Zewdie Bishaw3
2School of Plant Sciences, College of Agriculture and Environmental Science, Haramaya University, Ethiopia
3Seed Section, International Center for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria
Cite this as
Ali Y, Mekibib F, Bishaw Z (2021) Seed Quality Analysis of Field Pea (Pisum Sativum L.) from Formal and Informal Sources in Enarj Enawuga and Yilmana Densa Districts, West Amhara Region, Ethiopia. J Agric Sc Food Technol 7(1): 001-013. DOI: 10.17352/2455-815X.000081The production and productivity of field pea crop in Amhara region depends mainly on the un availability of quality seed supply system for a number of improved varieties. Therefore, this study was conducted to assess the quality and seed management practices of field pea seeds from the informal and formal seed systems in Enarj Enawuga and Yilmana Densa Districts during 2016/17 cropping season. Seed samples of two field pea varieties were collected from both formal and informal sectors and tested for quality in Complete Randomized Design (CRD) with four replications. The quality of seed samples had significant difference for physical purity, germination and vigor indices among seed samples. Except for other crop seeds, all the seed samples from informal seed sector maintained the physical /analytical/ purity of seed quality components (above the standards). Most of the seed samples except four samples (seed samples collected from farmer two and twelve of Enarj Enawuga districts who grow Tegegnech variety (F2 and F12), farmer 18 and 22 of Yilmana Densa districts who grow Tegegnech variety (F18 and F22) had registered germination capacity above the standards (75%). The speed of germination was better to predict field emergence of the seed lot than the standard germination. Seedborne fungi such as Ascochyta pinodes, Ascochyta pisi, Fusarium sp, Phoma sp, Septoria pisi, Colletotrichum sp were found associated with the field pea seed. Among those fungi Ascochyta pinodes was the dominant. Most farmer seed management practices enable to maintain and improve their field pea seed quality in both districts. Extension should play a crucial role in training farmers in on-farm quality seeds of the field pea crop production and is therefore a prerequisite for the improvement of the informal seed system in both districts.
Introduction
Field pea is a cool season legume that is grown on 7,881,943 ha with production of 12.5 million tons worldwide. Ethiopia ranked first in Africa and sixth in the world in field pea production with an area of 0.23 million ha and 0.34 million tons [1]. Field pea is an important source of food and feed in developing and developed countries, respectively. Field pea is the major food legume with a valuable and cheap source of protein having essential amino acids (23-25%) that have high nutritional values for resource poor households [2]. The crop has important ecological and economic advantage in the highlands of Ethiopia, as it plays a significant role in soil fertility restoration being used as a break crop to minimize the negative impacts of cereal based mono-cropping [3]. It is also used as a source of income for the farmers and foriegn currency for the country [4]. In Ethiopia, the pulse crops showed a slow growth in productivity for the last ten years. The average yield of field pea was 1.461 t ha-1 in 2015/16 cropping season [5] and it was one of the crop where more work is expected to enhance its productivity [6].
According to [5], the Amhara Regional State produced 1,150,035.6 tons (35.57% of the country) on 86,792.1 ha and it was the second major producing region of field pea in Ethiopia next to Oromia Regional state. However, the average yield of the crop was 1.325 t ha-1, which was less than the national average yield of field pea. Enarj Enawuga and Yilmana Densa are potential districts for field pea production in Amhara Regional state but the productivity of field pea is declining. The total area and production of field pea were decreased by 40% and 25.9% respectively from 2008/09 to 2016/17 [7,8]. The limited used of new technologies of field pea production as one of the low productivity in the region and districts mentioned.
Seed is a crucial input for agricultural production and the most affordable external input for farmers (Kumar, et al. 2014). Seed availability and accessibility by farmers are determined by many factors including the crop breeding systems, institutional/organizational arrangements and socio-economic conditions of farmers [9]. It is noteworthy that an effective seed system is relevant to increased productivity and overall agricultural production [10].
The formal and the informal systems are in place in Ethiopia. There is also a system that combines the two referred to as an integrated seed system [11]. Formal Seed System Characterized by a clear chain of activities. It usually starts with plant breeding and promotes materials for formal variety release and maintenance. Regulations exist in this system to maintain variety identity and purity as well as to guarantee physical, physiological and sanitary quality. Seed marketing takes place through officially recognized seed outlets, and by way of national agricultural research systems and even through relief seed programs. The major actors of the formal system in Ethiopia are the Federal and Regional National Agricultural Research Systems (NARS), Ministry of Agriculture and Natural Resources (MoANR), Federal (Ethiopian Seed Enterprise (ESE), and Regional Seed Enterprises (Oromia-OSE, Amhara-ASE, Southern-SSE, Somali-SoSE) and private seed companies [11].
Informal seed systems are about the knowledge, skills and practices of seed selection, production, and exchange by farmers. Farmers obtain seed and varieties through informal networks based on exchange (bartering) or gifts from relatives, neighbors, and other farmers or cash purchases from other farmers or local markets [9].
A number of improved varieties of field pea are released by the federal and regional agricultural research institutes but still the quality seed supply is negligible and unable to satisfy the seed demand of end users. Moreover, there is little information on field pea seed quality and local knowledge in seed selection, maintenance and management practices. Therefore, baseline information on the current field pea seed quality used for production is important to identify the field pea quality seed supply problem and suggest the establishment/strengthening of field pea seed system. The objectives of this study were to asses: the quality and seed management practices of field pea seeds from informal and formal seed systems in Enarj Enawuga and Yilmana Densa Districts.
Materials and methods
Description of the study area
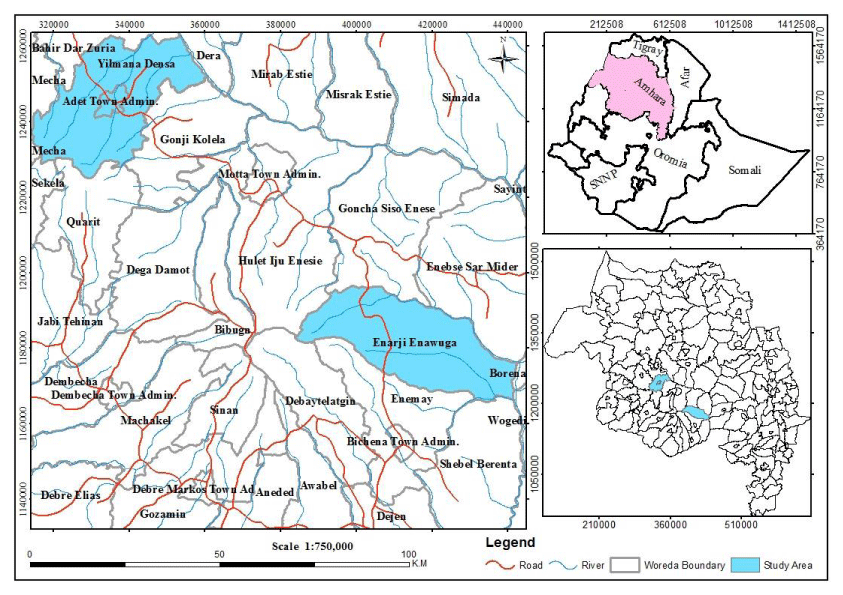
The field pea seed system study was carried out in Enarj Enawuga district in East Gojjam Zone and Yimana Densa district in West Gojjam Zone of Amhara Regional States ().
Enarj Enawuga has an altitude ranges from 1100 to 3200 masl where 30%, 50% and 20% of the total land area lies in Dega, Weynadega and Kolla, respectively. The area receives a mean annual rainfall of 1228 mm with a mean maximum temperature of 25 0C and a mean minimum temperature of 22 0C. From the total area of 96,095 hectares about 45,053 hectares (46%) is cultivated land and the major crops grown in the district are teff, wheat, barely, maize, faba bean, grass pea, field pea and potato. The Enarj Enawuga district consists of 25 rural and three urban (towns) kebele administrations with 165,415 farm households and a total population of 185,124; and over 98% of the population is involved in agriculture [7].
Yilmana Densa district has an altitude in the range between 1552 to 3535 masl, and average annual rainfall of 1270 mm with the main rainy season from May to October. The district is classified into three traditional agro climatic zones of which 24%, 57% and 19% of the total area lies in Dega, Weynadega and Kolla, respectively. The Yilmana Densa district consists of 33 rural kebele administrations with a total human population of 217,356. The total area is 99,180 hectares, and about 54,508 hectares (55%) is covered with annual crops. The mixed crop and livestock farming is predominant, since the area is suitable for both rearing of livestock and cultivating crop. The majority of the farmers depend on growing teff, maize, wheat, barley, faba bean, field pea, haricot bean, and chickpea as major source of cash income and household consumption [8].
Formal field survey
The study involved both multi stage purposive and random sampling techniques to select sample farmers. The two study zones, two districts and six Kebele Administrations (KAs) were selected purposively for being a major field pea growing areas. First, two major field pea growing districts (Enarj Enawuga and Yilmana Densa) were selected where some interventions have been made in the dissemination of improved field pea production technologies. Second, three KAs with highest field pea growing areas from each district were selected in consultation with the experts from the Agricultural Offices of the respective districts. Third, a total of 200 sample farmers, of which 120 from Enarj Enawuga and the remaining 80 from Yilmana Densa district were randomly selected for the survey.
The questionnaire was designed and pre-tested in randomly selected farm households before the beginning of the survey and a checklist was prepared for the survey. During the survey, data was collected with the help of trained enumerators. Enumerators were trained on seed management practices, capacity to innovate performed by a seed system to avail high quality seed of varieties preferred by farmers. Group discussions with key informants were also employed in order to acquire supplementary information.
Secondary data collection
Secondary data was obtained from various sources such as reports, agricultural research centers, Central Statistical Agency (CSA), Amhara Seed Enterprise (ASE), Amhara Bureau of Agriculture (BoA), district agricultural offices, pervious findings, internet and other published and unpublished materials.
Seed quality analysis
Seed samples of two improved field pea varieties, Tegegech and Hassabe, which are grown by most farmers, were selected and seed samples were collected for laboratory seed quality test. In each KA, two seed samples from each field pea variety were collected from farmers i.e. 4 seed samples per KA. In total 12 seed samples each of two varieties (total 24 seed samples) were collected during the survey. In addition, one sample of Tegegnech from ASE and one of Hassabe from Adet Agricultural Research Center representing the formal seed source was included making a total of 26 seed samples to carry out laboratory seed quality and seedling emergence tests.
A sample of 1 kg seed was drawn from the farmers’ seed harvested in 2016/17 cropping season for laboratory seed quality analysis including physical purity, thousand seed weight germination, vigor and seed-health tests. All tests were conducted according to ISTA rules [12] at Bahir Dar Seed Testing Laboratory and Bahir Dar Plant Health Clinic (for seed health). Field emergence tests were conducted at the green house of Amhara Regional Agricultural Research Institute, Bahir Dar.
Moisture content
For moisture test about 5g seed sample were taken and distributed on the surface of the container incubating in an oven at a constant high temperature of 130 oC ±1 and dried for about 1 hour with coarse grinding [12]. After drying, the sample were covered and allowed to cool for 30 minutes in desiccators and weighed again. The moisture content was calculated using the formula given below.
Where:
M1=the weight in grams of empty container and its cover
M2=the weight in grams of the container its cover and its content before drying and
M3= the weight in grams of the container and contents after drying
Physical purity
According to [12], the weight of submitted and working sample of field pea is 1000 g and 900 g, respectively. For each seed sample about 900 g seed sample was used for purity analysis. Each seed sample was divided into four replicates and separated in to three components: (i) pure seed, (ii) other crop seed and (iii) inert matter [12]. The components were weighed on precision balance to the nearest two decimal places and the percentages of each component were calculated as follows:
Thousand Seed Weight (TSW): Thousand seed weight was determined by counting from pure seed fraction and weighing eight replicates of 100 seeds and there mean weight has taken and multiplied by 10 [12].
Standard germination (StG) test
Standard seed germination test was carried for all seed samples in a completely randomized design (CRD). Four hundred seeds from the pure seeds component were taken and divided into four replicates of 100 seeds each and planted in sand media at a temperature of 20 oC. The first count were done 5 days after planting, and the final count 8 days after planting as specified by [12]. The result of the germination test was calculated as the average of four replicates. It was expressed as the percentage of normal seedlings. The percentages of abnormal seedlings, hard, fresh and dead seed were calculated in the same way.
Seed vigor test
Speed of germination: Hundred seeds were counted and taken from each source. The seeds were divided into four replicates of 25 seed each then after seeds were planted in between germination paper at 20 oC for 10 days in a dark room. The speed of germination (SG) was calculated according to [13].
The number of germinated seeds was counted every day from the first day of seed germination and the cumulative index was made by the formula:
Where:
n1…..nx is the number of speed seed germinated on day 1 to day x
1……x is the number of days
Seedling dry weight: The seedling dry weights were measured after the final count of the Standard Germination (StG) test. Ten randomly selected seedlings excluding cotyledon from each replicate were taken, and placed in envelopes and dried in an oven at 80 o c for 24 hours then the dried seedlings were weighed to the nearest milligram (three decimal) using sensitive balance and the average seedling dry weight was calculated [14].
Seedlings shoot and root length: Assessment of the seedling shoot and root were taken after the final count in the standard germination test. After 8th day of sowing, ten normal seedlings were randomly taken from each replicate. The shoot lengths were measured from the point of attachment to the cotyledon to the tip of the seedling. Similarly, the root lengths were measured from the point of attachment of the cotyledon to the tip of the root. The average shoot and root lengths were computed by dividing the total shoot or root lengths by the total number of normal seedlings measured [15].
Vigor index-I and Vigor index-II: For each sample, vigor indices were calculated. Seedling vigor index-I was calculated by multiplying the standard germination with the average sum of shoot and root length after the final days of germination:
Vigor index I = Germination % × [Root length + Shoot length (cm)] [15].
Vigor Index II= (Seedling dry weight X Germination (%))/ 100 [16].
Field emergence index
Pot experiments were conducted for field emergence index using well mixed soil. Twenty five seeds of four replications were planted from each seed source using complete randomized design (CRD). The number of seedlings emerged out of sown seed were counted till no more seedlings were emerged. The field emergence index was determined by following formula [13].
Field emergence (%): the total seedling emerged from the soil was summed up at the end of field emergence index experiment and it was calculated as field emergence in percent.
Seed health testing
Agar plate was used which is the most common method used for identification of seed borne fungi [17]. One hundred (100) undamaged seeds per sample were surface sterilized quickly by rinsing in 70% ethanol alcohol followed by soaking in 1% NaOCl for two minutes and then washing by distilled water. The seeds then plated on general purpose agar medium (five seeds per plate) and were incubated for two weeks. Petri dishes were incubated at 28±1oc with 12 hours alternating cycles of day and darkness and fruiting bodies of pathogens were examined under compound microscope [18]. During the period the fungi were identified and recorded and the percentage seed infection were calculated. At the end of the incubation period, fungi growing out from seeds on the medium are examined under compound microscope and fungi identified. Identification is based on colony characters and morphology; fruiting structures, size, shape, color and septations (cross walls) of the conidiophores and conidia (spores) and compare these characteristics with reference culture or descriptions in identification manuals. Identification of fungi and appropriate reference materials were used and finally the occurrence of each fungal pathogen was recorded. According to [19], the frequency and percent of infection were determined by the following formulae given below.
Data analysis
Information collected from the field survey was coded, tabulated and analyzed by using Statistical Package of Social Science (SPSS) version 23. Simple descriptive statistics was used to separate the mean, percentage and standard deviation to describe the socio-economic characteristics of the respondents.
Data collected data from laboratory and pot experiments were subjected to analysis of variance for Completely Randomized (CRD) using SAS (9.0). Treatment means were separated using Least Significance Difference (LSD) test. Pearson’s correlation coefficient was used for correlating the data from seed quality and the field emergence index.
Results and discussion
Farmers seed management
Farmers field pea seed management practices such as seed selection, seed cleaning, seed storage and protection practices in both Enarje Enawuga and and Yilmana Densa districts were discussed.
Seed selection
Farmers used different selecting criteria and method to select their seed at different stages. Response from most informants revealed that farmers’ selection criteria and practice were focused on observable attributes such as seed size and yield performance during harvesting, threshing and after threshing. Most of the selection practices were selected grain and very few based their selection on plants or pod. This indicates that farmers are interested in different traits of a variety.
Farmers select seed during harvesting and threshing (51.5%), just after threshing (37%) or just before planting (11.5%). Men (56.5%), women (21.5%), both men and women (7.5%) or all household members (14.5%) were responsible for selection as shown in Table 1. However female farmers are important in Enarj Enawuga (28%) as male in Yilmana Densa (58%). Some farmers selected; collected; threshed and stored seed separately for sowing and evaluated the crop the next season.
Most farmers select seed before and/or after harvesting the crop. Some farmers designate part of the field and use the harvest as a seed. Field and plant selections are based on a set of criteria, which vary from place to place, crop to crop and farmer to farmer [20]. Similarly [21], also found that among farmers practicing selection, 53% selected plants before harvest in the field, 15% at harvest just before threshing, and 7% just after threshing while 18% just before sowing of common bean in southern Ethiopia [22]. Reported that women played a significant and key role in on-farm seed management in Nepal.
Seed cleaning
The majority (62.5%) of the respondents’ clean by winnowing just before planting using homemade equipment known was Sefed, and this activity separate seeds by using differences in size and weight between seed and impurities. 37.5% of them by hand picking of large particles such as large soil clods or other inert matters to improve seed quality/ remove inert matter, remove small/broken damaged seeds and to remove weeds and other crops. Main cleaning time is just before planting. Men do the winnowing after threshing and women clean the seed at planting time.
About 57.5 % of the respondent farmers clean their seed to remove small or broken/damaged seeds, 30% of them were to remove weeds and other crops, and 12.5% to increase quality by removing inert matter (Table 2).
For most crops, cleaning of seed follows similar principles as for food grains using local practices and may include winnowing to remove light particles like straw and dust; sieving to select the seed by shape and size and hand-picking to remove damaged, diseased or discolored seeds [23]. Also reported that about 52% and 17% of the farmers cleaned their seed by hand-winnowing or hand-sieving, respectively, at planting time using handmade tools to increase purity, reduce weed contamination or even remove insect damaged seed grains. According to [24] all seed infested by insects must be destroyed to effectively remove sources of future infestation or contamination.
Seed storage and protection
Majority of the farmers (81%) used sacks and stored the seed in the house and 19% stored in local structures such as gota/gotera especially in the highland areas (Table 3). The average quantity of seed stored was 30 kg with range from the 15kg to 50 kg for field pea. They re-used fertilizer sacks without plastic linings to protect the seed from moisture. A study in Kenya shows that farmers stored beans in their houses (98%) or used some raised platforms near their houses (2%) [25]. During storage, the majority of the respondents (64%) used chemicals (phostoxin or actellic 2%) for storage pest control and the remaining 36% did not take any control measures. Farmers who take no control measure especially in Yilmana Densa distric believe that as bruchides are inside the seed treating the seed does not control the storage pest. None of the respondents use cultural methods to protect the seed from storage pest assuming that cultural measures do not have immediate result.
Advantages for farmers in storing their own seed include cash outlays are reduced, seed is available on time and nearby, and knowledge of varieties and management requirements [26]. The location, storage structures and the material used to construct the structures play an important role in enhancing the shelf-life and viability of the stored grain/ seed [25,27] found that the majority (63%) of farmers surveyed relied on chemical insecticides (actellic dust or phostoxin) and lack knowledge of cultural practices to control pea weevil in North and North West Ethiopia.
Seed quality of field Pea
Field pea seed samples of two improved varieties, Tegegnech and Hassabe, were collected from informal (farmers) and formal (ASE and AARC) sources were analyzed for the main quality parameters moisture content, physical seed purity, thousand seed weight, physiological quality (germination and vigor) and seed health presented below.
Physical purity
There was highly significance difference in all physical purity parameters among seed samples collected from formal and informal seed source (Table 4). The physical purity ranged from 98.47% to 98.87% with a mean value of 98.66%. The highest physical purity was observed from sample F17 (98.87%) of Hassabe variety in Yilmana Densa followed by F12 of Hassabe variety from Enarj Enawuga district where as the lowest was from samples F19 (98.47%) and F15 (98.47%) of Tegegnech variety all from Yilmana Densa district informal seed source (Table 4). The highest contamination by other crop seeds was observed in seeds obtained from sample F23 (0.36%) of Tegegnech variety whereas the lowest was from sample F18 (0.31%) of Hassabe variety both from Yilmana Densa district informal seed source. On the other hand, contamination of field pea seed by inert mater was highest in seeds obtained from F19 (1.20%) and the lowest from F17 (0.77%) both of Hassabe variety from Yilmana Densa district informal seed source.
The mean separation showed no observed differences (except other crop seed) in physical purity and inert matter between formal and informal seed sources, varieties and districts (Table 4). Crop seed admixtures could occur at the time of sowing, harvesting (poor threshing floors) and post-harvest activities (threshing, seed cleaning or storage) that would result in increased percentage of other crop seed in the informal seed source of the study area. Crop seed admixtures could occur at the time of sowing, harvesting (poor threshing floors) and post-harvest activities (threshing, seed cleaning or storage) that would result in increased percentage of other crop seed in the informal seed source of the study area. The result is in agreement with the reports of [28] and [29] who found no significant differences in physical purity and inert matter between seed sources except for other crop seed in teff and barley crops, respectively.
According to the national field pea certified seed standard [30], the percentage of pure seed, inert matter and other crop seeds should be 97%, 0.2% and 1%, respectively. Except for other crop seeds, all the seed samples maintained the physical purity above the certification standards. Similarly [31] reported that most of the samples collected from farmers satisfied the physical purity standards set for wheat seed production in Ethiopia.
Thousand seed weight
Highly significant differences in thousand seed weight were observed among treatments. The average thousand seed weight of the entire seed sample collected was 170.81g ranging from the lowest of F14 (143.72 g) and F16 (145.03 g) of Hassabe variety from Yilmana Densa district to the highest from ASE (197.966 g) from formal seed sector and F1 (197.04 g) of Tegegnech variety from Enarj Enawuga of informal seed source. Tegegnech variety showed higher thousand seed weight than Hassabe variety could be due to differences in seed size (Table 4).
Thousand grain weights is one of important traits of seed quality. It depends to embryo size and seed storage for germination and emergence [29,31,32]. Found significant difference in thousand seed weight in barley and wheat varieties, respectively.
Physiological seed quality
Standard germination: The overall average mean germination percentage was 84.27% with the range from 72.75% to 95.5%. The highest value was recorded for Tegegnech variety from seed sample F23 (95.5%) in Yilmana Densa district informal seed source and Amhara Seed Enterprise (94.5%) formal seed source. Seed samples of Tegegnech variety obtained from F19 (94.5%), F17 (92.75%), F13 (92.25%) from Yilmana Densa and F7 (92%) from Enarj Enawuga districts informal seed source showed better normal seedling percentage. Seed samples of Hassabe variety obtained from F12 (72.75%) in Enarj Enawuga district of the informal seed source showed lowest normal seedling percentage. The lowest abnormal seedlings were observed from seed samples F21 (0.00%) in Yilmana Densa and F11 (0.00%) Enarj Enawuga districts. It is followed by F23 (1.00%) all of Tegegnech variety from Yilmana Densa district informal seed source and Amhara Seed Enterprise (1.00%) of formal seed source. The highest abnormal seedlings were observed from F12 (12.00%) followed by F2 (8.00%), F4 (8.25%) from Enarj Enawuga and F18 (7.75%), F22 (8.25%) from Yilmana Densa districs. All of which were from Hassabe variety of informal seed source. The dead seedlings showed lower from seed samples F13 (3.00%), F19 (2.5%) and F23 (3.5%) of Tegegnech variety from Yilmana Densa district informal seed source while the highest were from F18 (18.75%) of Hassabe variety from Yilmana Densa district informal seed source (Table 5).
Most of the seed samples except four (samples 2 and 12 of Hassabe variety in Enarj Enawuga while 18 and 22 of Hasssabe variety from Yilmana Densa districts) from the informal seed sources had registered germination capacity above certified seed of EQSA (75%) (Table 5).
The mean separation showed observed difference in normal and abnormal seedlings between the seed sources and varieties but no observed difference in normal and abnormal seedlings and dead seeds between the districts. The dead seeds only showed difference between varieties (Table 5). The difference in production management, timely harvest and post-harvest activities are all important factors that would affect germination. Tegegnech variety has larger seed size with higher germination and vigor than Hassabe with lower seed size. Large seed size having more food storages and utilized it at a faster rate to have greater rate of stem elongation and accumulation of root and shoot dry weight than small seed sizes. The result is in agreement with the studies of [33] in pea [29,31]. Also found that there were highly significant (p < 0.001) differences in germination between different seed sources of wheat and teff respectively.
Vigor tests
Highly significant differences of vigor indices were observed among treatments (Table 6). The difference genetic, environmental condition during seed development, seed size and density, mechanical damage, seed aging and deterioration observed between seed source, variety and location could affect vigor differences among treatments.
Speed of germination
The average speed of germination was 25.34 (Table 6). The highest speed of germination F5 (28.41) of Tegegnech variety from while the lowest were from F4 (22.58) of Hassabe variety both from Enarj Enawuga informal seed source. The mean separation test has showed speed of germination was the same for formal and informal seed sources for both locations. There exist differences in speed of germination related to variety. The result is in agreement with [29,34] for soybean and barely, respectively. Both found difference speed of germination only for variety.
Root and shoot length
The highest and lowest shoot length was F6 (13.85) of Hassabe variety and F9 (11.62) cm Tegegnech variety both from Enarj Enawuga informal seed source, respectively and the root length was range from Ase (15.8) of Tgegnech variety representing the formal seed source to F22 (9.77) cm of Hassabe variety from Yilmana Densa district informal seed source (Table 6). It is assumed that seedlings with well-developed shoot and root systems would withstand any adverse conditions and provide better seedling emergence [23].
The mean separation showed observed difference among vigor indices (except for shoot length) between formal and informal seed sources and varieties but not between districts (Table 6). This could be due to the difference in pre and post-harvest management between seed sources and seed size between two varieties examined [29]. Found significant difference between varieties and seed sources for vigor one and speed of germination whereas [28,31] found the difference only for root length and vigor index one between different seed sources. Previous research indicated that under less suitable weather conditions during seed development, seed vigor was affected to a larger extent by the cultivar than by the location [35].
Field emergence
The mean filed field emergence percentage of collected sample was 81.45% and it ranges from the highest ASE (88.75%) of Tegegnech variety to the lowest F10 (71.75%) of Hassabe variety from Enarj Enawuga district informal seed source. The seed samples F4 (84.75%), F6 (85.00%) from Enarj Enawuga and F18 (85.00%) from Yilmana Densa district of Hassabe variety as well as F5 (86.25%) from Enarj Enawuga and F23 (86.75%) from Yilama Densa district of Tegegnech variety informal seed source also showed higher field emergence percentage.
The time and rate of seedling emergence are affected by an array of interacting factors including genetic constitution, seed dormancy, seed vigor, depth of planting, soil impedance and aeration, temperature and water supply [36,37].
Although many seeds germinate satisfactorily under ideal laboratory conditions, they may fail to emerge successfully in the field [38,39]. Simple correlation between germination and vigor tests with field emergence were done (Table 7). Standard germination showed an intermediate and non-significant correlation (r=0.25) with field emergence. The result showed that the standard germination test was not a good indicator for field emergence [40]. had also found no significant correlation between the standard seed germination and the field emergence [28]. However found that a positive and highly significant correlation between germination and seedling emergence of teff seed.
Speed of germination is the only vigor test that showed intermediate significant correlation (r=0.47) with field emergence. The speed of germination was better to predict field emergence of the seed lot than the standard germination. Speed of germination measures the rate at which the seeds germinate and where those seedlings with the higher index were expected to show rapid germination and seedling emergence and escape adverse field conditions. Speed of germination is an important measure of vigor. It depends on the time taken to reach 50% germination at constant temperature. Seeds with low vigor take longer time to germinate. Many researchers have examined speed of germination [13,38,39] had found a significant correlation with field seed emergence.
Seed health test
The seed health quality of field pea seed samples obtained from different sources was checked for the presence of seed-borne fungal pathogen. The fungi found associated with field pea seed were Ascochyta pinodes, Phoma sp, Fusarium sp, Septoria pisi, Ascochyta pisi and Colletotrichum sp. From the total field pea seed samples collected from informal and formal sources, 57.6% of the samples were infected with Ascochyta pinodes, 50% by Phoma sp, and 26.9% by Fusarium sp, 15.3% by Septoria pisi, 7.7% by Aschochyta pisi and 7.6% by Colletotrichum sp. (Table 8). The result is in agreement with [41] who found that out of 94 field pea samples tested for seed health, 57.4% were infected with Ascochyta with infection levels as high as 23%.
Since the percentage of seed infection is higher than 0.2%, all the seed samples did not meet the national seed standard of maximum infection level for certified seed in Ethiopia [42]. found that pre-basic seed of Adi and Tegegnech varieties produced at HARC had infection levels of 4.0% and 3.0%, respectively which are higher than the seed health standards in Ethiopia.
Summary and conclusion
Field pea can play an important role for household food security and sustainable farming systems. Availability of quality seed of improved field pea varieties at suffient quantity is one of the major constraints to increase productivity. This study was conducted to assess the seed quality of field pea seed grown in Enarj Enawuga and Yilmana Densa districts during the 2016/2017 cropping season.
Two varieties of field pea were collected from both formal and informal seed sources and were laid out in complete randomized design (CRD) with four replication in laboratory and seedling emergence tests in green house.
The existing seed system of field pea in both districts is dominated by the informal seed system and a very limited supply of seeds of field pea was available from limited activity of the formal seed system. Low efficiency of the current field pea formal system and due to poor support to strengthening the in formal seed system was one of the major reasons that most of the farmers in both districts to produce the crop from farm saved seeds of farmers’ cultivars.
It is well known that the formal sector is more regulated from variety development to seed marketing and distribution components in the national seed system. The seed from the formal seed sector should pass through the application of formal seed certification process in the country. Well organized seed management practices in the formal seed sector contributes to the seed quality which results better production and productivity of field pea crop in both districts.
The seed quality and seed emergence test indicated the presence of observed difference among seed samples of two varieties (Tegegnech and Hassabe) obtained from formal and informal seed system in the two districts (Enarj Enawuga and Yilmana Densa). The seed samples collected from some farmers also exhibited in seed quality test results as equal to the seed samples from formal seed system with the same parameters. There are also samples even better than seed sample from formal for some quality parameters. This may be due to farmers seed management practices (seed selection, cleaning and separate storage) enabled them to keep varieties seed quality comparable with the national standard. Most farmers, who save seed, however select seed at harvesting, threshing, and/or planting time. Farmers stored seed separately from grain either in sacks or in gotta/gottera, and the majority of farmers cleaned seed before planting to improve seed quality. However none of the seed samples from different sources met the seed health standards of EQSA. It could be concluded that high quality field pea seed from the informal sector could be produced under farmers’ condition by adopting better management practices. .
Some of the farmers in the study districts experienced some good practices to keep the field pea seed quality to the national standard for the crop. The seed management practice differs from farmers to farmers. This could be strengthened through good agricultural extension service to become as a source of quality seed supply.
- FAOSTAT (Food and Agriculture Organization Statistics) (2015) Statistical databases and data-sets of the Food and Agriculture Organization of the United Nations. Link: http://bit.ly/3nIJK5u
- Nawab N, Subhani G, Mahmood K, Shakil Q, Saeed A (2009) Genetic variability, correlation and path analysis studies in garden pea (Pisum Sativum L.). Journal of Agricultural Research 46: 333-340. Link: https://bit.ly/3nIJGTi
- Tsegie A, Tekleab A (1994) Fertilizer response Trials on Highlands Food Legumes. In: Cool-season food legumes of Ethiopia. Asfaw, T. (Ed.), ICARDA, Alepo, Syria.
- Balcha G (2003) The state of grain marketing in Ethiopia. Proceedings of the EDRI/IFPRI 2020 Network policy forum on toward sustainable food security in Ethiopia: Integrating the Agri-Food Chain, May 15-16.
- CSA (Central Statistical Agency) (2016) Agricultural Sample Survey for 2015/16: Report on Area and Production of Major Crops for Private Peasant Holdings, Meher Season. Central Statistical Agency of Ethiopia, Addis Ababa, Ethiopia.
- CSA (Central Statistical Agency) (2015) Agricultural Sample Survey 2014 / 2115 (2007 E.C.): Report On Farm Management Practices. (Private Peasant Holdings, Meher Season) Statistical Bulletin No. 578. Central Statistical Agency Of Ethiopia, Addis Ababa, Ethiopia.
- EEDOA (Enarji Enawga District Office of Agriculture) (2016) Annual report of Agricultural activity, East Gojjam Zone, Debre Markos.
- YDDOA (Yilmana Densa District Office of Agriculture) (2016) Annual report of Agricultural activity, West Gojjam Zone, Adet.
- Bishaw Z, Gastel VJ (2008) ICARDA Seed-Delivery Approach in Less Favorable Areas through Village Based Seed Enterprises: Conceptual and Organization. Journal of New Seeds 9: 68-88. Link: https://bit.ly/3ifJd9Y
- Oyekale KO (2014) Growing an Effective Seed Management System: A Case Study of Nigeria. Journal of Agriculture and Environmental Sciences 3: 345-354. Link: https://bit.ly/2XJvDlP
- Atilaw A, Korbu L (2011) Recent Development of Seed Systems of Ethiopia. In Improving Farmers' Access to Seed, eds Dawit Alemu, Kiyoshi, S. and Abebe Kirub, 13-30. Addis Ababa, Ethiopia.
- ISTA (International Seed Testing Association) (2008) International rules for seed testing. Published by International Rules for Seed Testing Association, Zurich,
- Maguire JD (1962) Speed of germination. Aid in selection and evaluation of seedling emergence and vigor. Journal of Crop Science 2: 176-177. Link: https://bit.ly/2XId3uo
- Woodstock LW (1976) Progress report on the seed vigor testing. AOSA Newsletter 59: 1-78.
- Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria. Crop Science 13: 630-633. Link: https://bit.ly/3qzk511
- Kharb RP, Lather BP, Deswal DP (1994) Prediction of Field Emergence through Heritability and Genetic Advance of Vigor Parameters. Seed Science and Technology 22: 461-466. Link: http://bit.ly/2XDDeCq
- Rao NK, Bramel PJ (2000) Manual of gene bank operations and procedures. Technical Manual no. 6. ICRISAT, Patancheru, India. Link: https://bit.ly/2Lzo5zx
- Agrios GN (2005) Plant pathology, 5th edition. Department of Plant Pathology, University of Florida, 948. Link: http://bit.ly/35HRMFt
- Bishaw Z, Struik PC, Gastel V (2013) Farmer’s seed sources and seed quality: 2. seed health. International Journal of Plant Production 7: 637-658. Link: https://bit.ly/3nTlwWl
- Baniya B, Subedi A, Rana R, Tiwari R, Chaudhary P, et al. (2003) What are the processes used to maintain genetic diversity on-farm? In: Agro biodiversity conservation on-farm: Nepal’s contribution to a scientific basis for national policy recommendations (Gauchan, D., Sthapit, B. and Jarvis, B. eds.). IPGRI, Rome, Italy 20-23.
- Asfaw A, Almekinders M, Struik C, Blair W (2013) Farmers’ common bean variety and seed management in the face of drought and climate instability in southern Ethiopia. Scientific Research and Essays 10: 1022-1037. Link: http://bit.ly/38Lwxo9
- Bajracharya B (1994) Gender issues in Nepali agriculture: A review. Research Report No. 356.
- Bishaw Z (2004) Wheat and barley seed systems in Ethiopia and Syria. PhD thesis. Wageningen, Wageningen University. Link: https://bit.ly/35DTBDi
- FAO (Food for Agricultural Organization) (2014) Appropriate Seed Varieties for Small-scale Farmers: Key Practices for DRR Implementers. Link: http://bit.ly/35KeVqG
- Odhiambo W, Ngigi M, Lagat J, Binswanger H, Rubyogo J (2016) Analysis of Quality Control in the Informal Seed Sector: Case of Smallholder Bean Farmers in Bondo Sub-County, Kenya. Journal of Economics and Sustainable Development 7: 1-21. Link: https://bit.ly/38KeleL
- Walsh S, Baributsa D, Remington T, Sperling L (2014) Seed storage brief No.2: Hermetic seed storage technology: Principles, use, and economics a practitioner`s guide. Nairobi: Catholic Relief Services.
- Mendesila E, Shumetab Z, Peter A, Birgitta R (2016) Smallholder farmers' knowledge, perceptions and management of pea weevil in north and north western Ethiopia. Journal of Crop protection 81: 30-37. Link: http://bit.ly/35JH4OR
- Anteneh M (2015) TEF [Eragrostis TEF (ZUCC.) Trotter] Seed Quality Variation in East Gojjam Zone, Ethiopia. Malaysian Journal of Medical and Biological Research 2: 54-62. Link: http://bit.ly/3nKxK3l
- Megersa G, Mekibib F, Lakew B (2015) Performance of farmers` and improved varieties of barely for yield components and seed quality. Journals of Plant Breeding and Crop Science 7: 107-124. Link: https://bit.ly/35Fuf82
- EQSA (Ethiopia Quality Standards Authority) (2012) Field pea Specification. Addis.
- Bishaw Z, Struik P, Gastel VA (2012) Farmers' Seed Sources and Seed Quality: 1. Physical and Physiological Quality. Journal of Crop Improvement 26: 655-692. Link: https://bit.ly/3iglruv
- Cordazzo CV (2002) Effect of seed mass on germination and growth of three dominant species in Southern Brazilian coastal dunes. Braz J Biol 62: 427-435. Link: http://bit.ly/3qszL69
- Peksen E, Peksen A, Bozoglu H, Gulumser A (2004) Some seed traits and their relationships to seed germination and field emergence in pea (Pisum Sativum L.). Journal of Agronomy 3: 243-246. Link: https://bit.ly/3iglw1h
- Khaliliaqdam N, Soltani A, Latifi N, Ghaderi F (2013) Laboratory tests for predicting emergence of soybean cultivars. Plant Knowledge Journal 2: 89-93.
- Chloupek O, Hrstková P, Jurečka D, Graner A (2003) Tolerance of barley seed germination to cold- and drought- stress expressed as seed vigor. Plant Breeding 122: 199-203. Link: https://bit.ly/38HWVPO
- Forcella F, Arnold LBR, Sanchez R, Ghersa CM (2000) Modeling Seed Emergence. Field Crops Research 67: 123-139. Link: http://bit.ly/2XGlAxR
- Samarah N, Al-Kofahi S (2008) Relationship of seed quality tests to field emergence of artificial aged barley seeds in the semiarid Mediterranean region. Jordan Journal Agricultural Science 4: 311-317. Link: https://bit.ly/3oKSwRP
- Kulik MM, Yaklich RW (1982) Evaluation of vigor tests in soybean seeds: Relationship of accelerated aging, cold, sand bench and speed of germination tests to field speed performance. Journal of Crop Science 22: 766-770. Link: https://bit.ly/39vk3Ak
- Wang YR, Yu L, Nan ZB, Liu YL (2004) Vigor tests used to rank seed lot quality and predict field emergence in four forage species. Crop Science 44: 535-541. Link: https://bit.ly/35COGCH
- Aliloo AA, Shokati B (2011) Correlation between Seed Tests and Field Emergence of Two Maize Hybrids (SC704 and SC500). Online Journal of Animal and Feed Research 1: 249-254. Link: https://bit.ly/3bHHc58
- Gorfu D, Sangchote S (2008) Prevalence of Ascochyta pinodes on field pea seed produced in central Ethiopia and its relation to seedling infection. Pest Management Journal of Ethiopia 12: 9-17.
- Gorfu D, Ayalew A, Mekbib F, Sahlu Y (2011) Keynote Address. 169-180, International Conference on Sustainable Seed Systems in Ethiopia: Challenges and Opportunities, 1-3 June 2011. Addis Ababa, Ethiopia: Ethiopian Institute of Agriculture Research.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
