International Journal of Agricultural Science and Food Technology
A Review of Ochratoxin A Occurrence, Condition for the Formation and Analytical Methods
Salasib Atumo*
Cite this as
Atumo S (2020) A Review of Ochratoxin A Occurrence, Condition for the Formation and Analytical Methods. Int J Agric Sc Food Technol 6(2): 180-185. DOI: 10.17352/2455-815X.000071Ochratoxin A (OTA) is a mycotoxin produced by several fungal species including Aspergillus ochraceus, A. carbonarius, A. niger and Penicillium verrucosum. Various studies report shown that Ochratoxin A can be leads several health problems for both animal and human health through the consumption of Ochratoxin A contaminated plant and animal origin foods. For instance, Ochratoxin A has been shown to be nephrotoxic, teratogenic, immunotoxic, and carcinogenic in human health. Therefore, the main aim of this review focused on the occurrence, analytical methods and the condition for the formation of Ochratoxin A. Number of studies finding report indicate that Ochratoxin A was existed in several processed and unprocessed food stuffs, species and different alcoholic beverage. Primarily, cereals and cereals contained food products have highly vulnerable for Ochratoxin A due the presence of high moisture contents. On the other hand, several environmental conditions are playing an important for the formation of Ochratoxin A in different food stuffs. For example, most important abiotic factors which influence the growth and Ochratoxin A production by such Spoilage fungi include water availability, temperature and gas composition. Finally, several analytical methods are used for detection of Ochratoxin A from different plant and animal origin foods such as Thin layer chromatography, Enzyme linked immunosorbent assay and High performance liquid chromatography. However, based on the sensitivity, resolution and efficiency currently high performance liquid chromatography techniques are more popular and advanced analytical techniques for different mycotoxins groups particularly, Ochratoxin A and Aflatoxins detection.
Introduction
Mycotoxins are secondary metabolites produced by a wide variety of filamentous fungi, including species from the genera Aspergillus, Fusarium, Penicillium, Alter aria and Clavicles that grow under different climatic conditions on agricultural commodities [1]. The Food and Agricultural Organization (FAO) of the United Nations has estimated that up to one quarter (25%) of the world’s food crops are significantly contaminated with secondary metabolites of different toxigenic moulds (FAO 2004). Ochratoxins belong to a family of structurally related, secondary fungal metabolites produced by various Penicillium and Aspergillus strains [2]. Based on their structure and chemical compounds Ochratoxin can be categorized into three. Among them, Ochratoxin A (OTA) is the most commonly occurring metabolite and the most hazardous, since it is a nephrotoxic and carcinogenic agent [2]. Additionally, exposure of the human being for a long period of time with Ochratoxin A contaminated diets it leads several health problems such as, kidney, liver cancer and weakening of the immunity system [3], and has been classified by the International Agency for Research on Cancer (IARC) as a possible carcinogen to humans [4]. Likewise, Ochratoxin A it can be leads serious health problem to animal during the feeds of contamination agricultural products by these mycotoxin types. For example, hepatotoxic, carcinogenic, genotoxic, Immunotoxic, teratogenic, and neurotoxic health impacts on animals [5]. Furthermore, Ochratoxin A is toxic metabolites of Aspergillus fungi that can contaminate various foods and feed products.
One of the recent research finding result shows that the presence of Ochratoxin A in wheat bread and maize bread is (12.9 and 70)µg/kg respectively[6]. And the result showed that the concentration level of maize breads has high Ochratoxin A contents and maize and maize contain food stuffs is a worldwide problem of Ochratoxin A. Especially, maize kernels are a good substrate for mould infection and production of mycotoxins harmful to both humans and animals. However, these products (maize) play an important role in developing countries particularly the diets of Ethiopian people. The infection of these crops by Ochratoxin A fungi and hence contamination with Ochratoxin A is generally higher [7]. On the other hand, majority of Ethiopian people depend their own life by agricultural product especially cereal products occupy the primary position. These indicate that the damage caused by Ochratoxin A in Ethiopia is increasing this may be due to lack of well documented information, poor management and lack of awareness on occurrence Ochratoxin A from agricultural product. Consumption of Ochratoxin A in higher amount through feed and foodstuffs may impact animal and human health. The effects of Ochratoxin A not only human and animal health impact but also play a significant role for economic loss and boundary less distribution. Therefore this review aims to address the occurrence, condition for the formation and analytical methods for Ochratoxin A. In addition, they are beneficial to the society, scientific community and research institution.
Material and methods
A systematic analysis and review of research literature related to Ochratoxin A occurrence, the condition for the formation and detection mechanisms of Ochratoxin A from different plant and animal origin food. To compile this review article the reviewer used a web based systematic research literature search was employed. The occurrence, contamination, environmental and climatic condition for the formation and analytical technique were gathered by different search approach, including search published different journal related with present title by using international scientific data bases such as PubMed, Google search, Google scholar and the like. For instance, literature search was performed using the following key terms: Ochratoxin A, the effects of Ochratoxin A for both animal and human health and analysis of Ochratoxin A from different food contained sample. Finally, the idea that gathered from each journal article was paraphrased and some report was expressed in the form table and figure.
Ochratoxin A
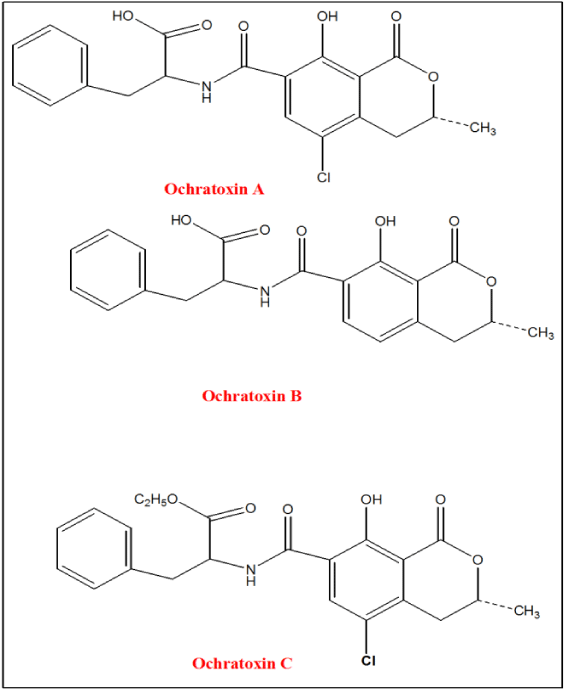
Ochratoxin are a group of mycotoxins produced as secondary metabolites by several fungi of the aspergillus and penicillium families. Early studies investigation data indicate that Ochratoxin A (OTA) was first identified and characterized from fungal cultures in South Africa chemist in 1965 and have three derivatives such as Ochratoxin A, Ochratoxin B and Ochratoxin C [8]. These three types of Ochratoxins are closely related molecular structure. However, which differ in that Ochratoxin B (OTB) is a non-chlorinated form of Ochratoxin A (OTA) and Ochratoxin C (OTC) is an ethyl ester of OTA as described in Figure 1 [9]. Among them Ochratoxin A is the most toxic member of the Ochratoxin which is structurally similar to the amino acid phenylalanine. Chemical name for Ochratoxin A contains 7-carboxy-5-chloro-8-hydroxy-4,4-dihydro-3(R)-methyl isocoumarin (Ochratoxin α; OTα) that is linked through the 7-carboxy group to l-β-phenylalanine by a peptide bond. Briefly, the chemical abstract number, molecular formula and molecular mass of each Ochratoxins type is described in Table 1 [10]. On the other hand in acidic and alkaline solution Ochratoxin A shows different properties. For instance, in case of acidic and neutral pH, Ochratoxin A is easily soluble in polar organic solvents such as alcohol, ketone and chloroform. While in case of alkaline solution Ochratoxin A molecule is soluble in aqueous sodium bicarbonate and in all alkaline solution in general. However, OTA molecule in water, petroleum ether and saturated hydrocarbons are slightly soluble [11].
Occurrence of Ochratoxin A in food and feeds
Ochratoxin A is one of the secondary metabolite of mycotoxin group and can be existed in several agricultural products and in animal feeds [10]. Number of studies finding report indicate that Ochratoxin A was existed in several processed and unprocessed food stuffs, species and different alcoholic beverage. For instance, in cereal, milk, meat and species [12-15] in alcoholic beverage such as beer and wine [16,17]. Likewise, some dried fruit and coffee was infected by Ochratoxin A in [17,18]. But cereals occupy the first position of the total exposure to Ochratoxin A with 60% because of cereal have high moisture contents some times more than 20% [19]. Hence, to protect consumer from different health risks different International Organization was set the maximum permissible limits for Ochratoxin A in different food stuffs and alcoholic beverage. Then, the maximum limits of 5.0 ng/g Ochratoxin A in raw cereal grains whereas 3.0 ng/g in cereal-processed. Similarly, coffee and dried fruit 10 ng/g wines and cereal based baby food 2 µg/L and 0.5 ng/g respectively set by European commission [20]. Likewise, Ochratoxin A has also been determined in foods of animal origin such as pork blood products, pork kidney, pork liver, or pork meat. The reason the pigs are predominantly exposed to Ochratoxin A through their contaminated feed [18]. In short, Ochratoxin A is a secondary metabolite produced either by penicillin in cereal and cereal proceeds food or Aspergilli in wine, grapes, coffee and cocoa [21]. According to this in recent time several scientific communities has received increased primary attention towards the effects of Ochratoxin A because of its hazard to human and animal health [22]. In general, animals are directly exposed to mycotoxins through the consumption of mould feedstuff Whereas, human exposure can be via one of two routes; direct exposure due to the consumption of mould plant products, or indirect exposure through the consumption of contaminated animal products [23].
Food and Agricultural Organization (FAO, 1999) report estimated that around 12% of total Ochratoxin A intake comes from consumption of coffee. Through these contaminated foods and animal product consumption it leads several health problem to human beings. For instance, Ochratoxin A has been shown to be nephrotoxic, teratogenic, immunotoxic, and carcinogenic in human health [20]. The effect of Ochratoxin A is not only for human and animal health but also negative impacts on country economy and trading system. According to many experimental studies data indicate that the effect of mycotoxins leads serious problem to developing country marketing system. For example, one of the early studies report shown that around 2.3 million bags of maize was rejected totally from marketing and consumption during the presence of aflatoxin in Kenya from 2004 up to 2006 [24].
Moreover, recent investigation on similar country different agricultural product especially the price of maize was dropped by half from 1800 to 900 Kenya shillings due to the effects mycotoxin [25]. Furthermore, coffee is one of the most extensively consumed beverages all over the world and important for foreign exchange in developing and developed country. However, the occurrence of Ochratoxin A in coffee has gained high attention in the International coffee trade market [26].
Effects of Ochratoxin A on human health
Ochratoxin A (OTA) is a major secondary metabolite of mycotoxins present in several agricultural commodity and leads several health problem for both animal and human beings. Primarily, human exposure directly from Ochratoxin A cause through the consumption of Ochratoxin A contaminated plant origin foods (cereal) and indirectly from animal product when the animals are feeds Ochratoxin A contaminated feeds. Once, the humans are exposure to Ochratoxin A, Ochratoxin A has several toxigenic effects to human health such as, teratogenic, hepatotoxic, nephrotoxic, carcinogenic, and immunosuppress [27]. In addition, the human health effects or disorders due to consumption of mycotoxin contaminated foods include diarrhea, reproductive disorder, cancer, growth impairment and immunomodulation [28]. In short, the impacts of Ochratoxin A on human health can be sever especially in the developing countries due to the combination of social, agricultural, economical and storage system can be contributed for exposure [2931].
Effects of Ochratoxin A on animal health
Similar to that of human being, animals are exposed to Ochratoxin A causes when the feeds have been known to exhibit the form of intoxication which can leads to death through consumptions [30]. Once the animals are exposed to Ochratoxin shows different clinical signs which leads to liver damage, reduced weight gain, and decline of products (like egg, meat, milk and milk products) [313]. As, a result the consequence becomes economic losses to both society and industry. A few scientists believed that Ochratoxin A contamination was linked to micronutrient deficiencies in animals whereas a few of them are now found to have reported that there is no relationship between ochratoxin-albumin. In addition, clinical manifestations poisoning include low reproductive capacity, gastrointestinal dysfunction and decline in feed utilization [32]. However, animal susceptibility to carcinogenesis by Ochratoxin A are varies with their sex, age, species, hormonal and nutritional status of the animal [33].
Condition formation of Ochratoxin A in different commodities
The most important abiotic factors which influence the growth and Ochratoxin A production by such Spoilage fungi include water availability, temperature and gas composition [34]. Water activity is perhaps the most critical factor influences the germination, growth and establishment of molds on nutrient worthy substrates. The previous studies shown, that Ochratoxin A contamination was produced by P. verrucosum at water activity of 0.95 and a temperature of 250C [35]. Similar to water activity, temperature is also second factor and highly contribution for the production of Ochratoxin A in different agricultural commodities. Number of investigation mentioned as the optimal temperature for Ochratoxin A production is between 25-30°C for A. ochraceus, 10-20°C for A. carbonarius and 20-25°C for A. niger aggregate [36-38]. Not only water and temperature factors but also some of the countries are creating favorable conditions for the formation of Ochratoxin A in different food and feeds.
For instance, in countries of South America, South Asia, and Africa where climate is hot and dry hence aspergillus species are the major Ochratoxin A producers [39]. Similarly, in temperate countries such as the United States, Canada, and Europe, where temperatures are moderate and can be contributed for the production of penicillium genus is the major Ochratoxin A producer [40]. Grain moisture contents are their own important factors for the growing of fungi and production of Ochratoxin A. Several literature data indicate that Penicillium verrucosum is found mostly in grains with moisture contents are higher than about 14.5% [41]. In addition, the development P. verrucosum and formation of Ochratoxin A on different grain (wheat) when the moisture content ranging from 10–30% and 18-22% [42].
However, when the moisture content was detected below 17% the probability for the growth of P. verrucosum and production of Ochratoxin A is very low [34]. Similar to temperature and moisture contents, gas compositions are other factors for the growth of fungi and production of Ochratoxin A in different food stuffs, dried fruit, alcoholic beverage and coffee. According to [41] the effect of gas composition is highly contributed for the developments of p.verrucosum and Ochratoxin A formation in inoculated wheat grains for 28 days at 25°C. The effect Ochratoxin A was produced in a commodity largely depends on one or more fungal species is contaminating the commodity due to the presence favorable climatic factors, environmental condition and the way of handling practices such as, drying, storage, processing and transportation) and agricultural methods are play an important role for the growth of fungi and production of Ochratoxin A [43,44]. In generally, all the commodities including of processed and unprocessed food stuffs which are stored in improper manner and exposure for temperature and humidity for a long period of time create favorable conditions for mould growth and can be cause to mycotoxin contamination [42].
Analytical methods for Ochratoxin A
Several methods have been developed for the determination of Ochratoxin A in a variety of food commodities including cereals (barley, corn, wheat bran, and flour), coffee, cocoa, wine, beer, and dried fruits. In the present review some of the analytical methods that used for determination, separation and quantification of Ochratoxin A from different food stuffs, feeds and alcoholic beverage were discuss as follow.
Thin layer chromatography
Thin Layer Chromatography is a technique used to isolate non-volatile mixtures. During conducting the experiments, sheet of aluminum foil, plastic, or glass which is coated with a thin layer of adsorbent materials and materials usually including aluminum oxide, cellulose or silica gel [45]. Similar to the other chromatography methods, thin layer chromatography is depends on the separation principles. The separation relies on the relative affinity of compounds towards both the phases. The mobile phase move over the surface of the stationary phase [45]. On the other hand, thin layer chromatography the most widely used and established separation and detection technique for aflatoxin since its developments in the 1960s [46]. Not only for aflatoxin analysis but also used for determination of Ochratoxin A from different agricultural crops product. For instance, detection of 2.4 – 4 mg/Kg of Ochratoxin A, and 10 mg/Kg of Ochratoxin A from rice and wheat respectively [47]. The use of thin layer chromatography analysis for mycotoxins is still popular for both quantitative and semi-quantitative purposes. The main reason is due to its high throughput of samples, low operating cost and ease of identification of target compounds, using UV–vis spectral analysis especially in developing country [48]. However, when compared with other chromatographic techniques, thin layer chromatography detection limit is high, length of separation is limited and lack of automation [49].
Enzyme-Linked Immunosorbent Assay (ELISA)
Enzyme linked immunosorbent assay is a plate based assay technique which is used for detecting and quantifying substances such as peptides, proteins, antibodies and hormones. Similar to the previously discussed analytical methods, also enzyme linked immunosorbent assay methods have their own advantages and limitations during the conducting of analysis. Enzyme linked immunosorbent assay methods have advantages due to their simplicity, and number of samples that can be analyzed at the same time. In addition, it requires low volume samples, quicker sample cleanup, simple and specific [45]. However, enzyme linked immunosorbent assay is less accurate and relatively low sensitivity and low efficiency compared with other chromatography techniques [50]. In addition, false positive or negative results are observed because of cross-reactions among molecules or interferences. Therefore, enzyme linked immunosorbent assay kits should not be used as a quantitative method and should only be used with foods for which they have been extensively tested and demonstrated to work [51]. Moreover, one of the documented reports showed that during the uses of enzyme linked immunosorbent assay methods sufficient controls must be employed for each test, to ensure the validity of the quantification unless difficult to obtain accurate result [52].
High Performance Liquid Chromatography (HPLC)
In fact, more advanced and sensitive analytical methods for determining Ochratoxin A and Ochratoxins in biological materials are being developed consecutively toward the sophisticated development of instrumentation and analytical techniques. Like other forms of chromatography, HPLC allows the separation of chemical constituents through the use of a mobile phase and a stationary phase. The mobile and stationary phase is liquid and solid respectively. In the recent time high performance liquid chromatograph techniques are more popular and preferable analytical methods for mycotoxins analysis compared with other chromatographic methods [53]. For example, compared with thin layer chromatography methods HPLC is extremely quick and efficient [54]. The reason that it uses a pump, rather than gravity, to force a liquid solvent through a solid adsorbent material, with different chemical components separating out as they move at different speeds[49]. Moreover, the process can be completed in roughly 10 to 30 minutes, and it delivers high resolution. As the above mentioned early a number of methods are used for determination of Ochratoxin A from different plant and animal origin food stuffs. However, high performance liquid chromatography with fluorescence light detector (HPLC-FLD) and Immunoaffinity column cleanup techniques are primarily used for quantification of Ochratoxin A in large number of sorts of food stuffs both animal and plant origins [55]. The recent study have documented the use of Immunoaffinity column in clean up steps during the analysis of mycotoxins has a numerous advantages. For instance, clean the extracted sample, precision and accuracy, rapidity and finally to reduce some interference from the analyte [56]. In addition, basic advantages of these clean up techniques are the specific binding of Ochratoxin A on to the antibody and the near –complete removal of the matrix interference [57]. Nevertheless, in the case of Ochratoxin A, underestimation can be observed if extraction is done in an alkaline condition, because Ochratoxin A is converted into open-ring Ochratoxin A (OP-OA) and no longer recognized by antibodies [58].
Conclusion
Ochratoxin A is belonging to a family of structurally related, secondary fungal metabolites produced by various Penicillium and Aspergillus strains. This secondary metabolite mycotoxin groups are leads sever heath problem for human and animal health during the consumption of contaminated food and feeds. For instance, the human being exposure for a long period with Ochratoxin A contaminated diets it leads several health problems such as, kidney, liver cancer and weakening of the immunity system. Likewise, hepatotoxic, carcinogenic, genotoxic, Immunotoxic, teratogenic, and neurotoxic type of disease are occurred on animal health. Several environmental and climatic conditions are playing an important role for the formation of Ochratoxin A in different agricultural commodity. For example, optimal temperature between 25-30°C and moisture contents are higher than about 14.5% of the food contained food stuffs are easily exposure for mould development and formation of Ochratoxin A. In addition, the effect of gas composition is highly contributed for the developments of p.verrucosum and Ochratoxin A formation when incubated and storage of agricultural commodity for a long period of time. Finally, different analytical techniques are widely used for determination and quantification of Ochratoxin A concentration from plant and animal origin sample such as, thin layer chromatography, enzyme linked immunosorbent assay and high performance liquid chromatography. However, majority of the present study report indicate that Ochratoxin A widely detected and quantify from the different food and food contained sample by using high performance liquid chromatography analytical techniques. The main reasons for this assay the instrument is highly sensitivity, high resolution and high efficiency compared with other analytical techniques.
- Angelina P, Celeste L (2010) Mycotoxin food and feed regulation and specific case of Ochratoxin A: A review of the worldwide status. Journal of Food Additives and Contaminations 27: 1-11. Link: https://bit.ly/35IlucL
- Rosa V, Antonella A, Carlo G, Francesco P (2008) Determination of Ochratoxin A in green coffee beans by solid phase micro extraction and liquid chromatography with fluorescence detection. Journal of Chromatography A 1187: 145-150. Link: https://bit.ly/2HzBJRp
- Stoev D, Vet H (2010) Toxicol. Journal of food science 6: 40-46.
- IARC (1993) Monography on the evaluation of carcinogenic risks to humans some naturally occurring substance. Journal of International agency for research on cancer 56: 489-496.
- Lucchetta. G, Bazzo G Dal C, Bellotto M (2010) Occurrence of black aspergilli and Ochratoxin A on grapes in Italy. Journal of Toxins 2: 840–855. Link: https://bit.ly/35NdtTG
- Ali A, Shehzad A, Muhammad R, Muhammad S (2013) Ochratoxin A in Cereal Products, Potential Hazards and Prevention strategies: A Review. Journal of Food Sciences 23: 52-61. Link: https://bit.ly/3oApioU
- Molhannad R, Ali A, Mohammad S, Shehzad A (2013) Ochratoxin A in cereal products potential hazards and prevention strategies: A review Journal of Food Science 23: 52–61. Link: https://bit.ly/3kEtrpy
- Temesgen A, Teshome G (2018) Major mycotoxin, occurrence, prevention and control approach. Journal of Biotechnology 12: 1-11. Link: https://bit.ly/2HN5mhU
- Sofia C Angelina P, Celeste M (2011) Human Ochratoxin A biomarkers from exposure to effect. Crit Rev Toxicol 41: 187-212. Link: https://bit.ly/2HHpAt9
- Gumus. R, Ercan N, Imik H (2018) Determination of Ochratoxin A levels in mixed feed and feed stuffs used in some laying hens and ruminants enterprises of Sivas city. Journal of Poultry Science 20: 85-90. Link: https://bit.ly/31TbHiP
- Andre K, Ali A (2010) Ochratoxin A: General overview and actual molecular status. Journal of Toxins 2: 461-493. Link: https://bit.ly/37Lh9rQ
- Frantisek M, Vladimir O, Annie P, Jakub T (2016) Ochratoxin A: 50 years of research of toxins. Journal of Toxins 87: 191. Link: https://bit.ly/35HMf0N
- Pittet A (1998) Natural occurrence of mycotoxin in foods and feeds. Journal of Rev Med Vet 6: 479-492.
- Zhao Y, Yuan Y, Bai X, Liao X (2020) Multi mycotoxins analysis in liquid milk by UHPLC after magnetic solid phase extraction based on PEGYLATED multi walled carbon nano tube. Food Chemistry 305: 125429. Link: https://bit.ly/3jHVB1n
- Meftah S, Abid S, Dias T (2015) Mechanisms underlying the effect of commercial starter culture and a native yeast on Ochratoxin A production in meat product. Journal of Risk Assessment 33: 157-166.
- El D, Gambacorta L, Solfrizzo M (2019) Multi mycotoxin occurrence in species and herbs commercialized in Lebanon. Journal of Food Control 95: 63-70. Link: https://bit.ly/3oC3Csv
- Balendres M, Karlousky P, Cumagun C (2019) Mycotogenic fungi and mycotoxin agricultural crop commodities in Philippines. Journal of Food Science 8: 249. Link: https://bit.ly/2G8KjWt
- Peter K (2016) Assessment of the levels of Ochratoxin a in coffee bean from the coffee growing region of Kamba, Kenya. Journal of Food Science and Quality Management 53: 55-70. Link: https://bit.ly/2HHpmCj
- Eskola M, Kos G, Elliott T, Krska R (2019) Worldwide contamination of food crops with mycotoxins. Crit Rev Food Sci Nutr 59: 1-17. Link: https://bit.ly/34AhU54
- Durate S, Pena A, Lino C (2010) A review on Ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol 27: 187-198. Link: https://bit.ly/2HO62TR
- Maria P, Maria L, Juan M, Sofia C (2012) Control of Ochratoxin A production in grapes. Journal of Toxin 4: 364-372. Link: https://bit.ly/2HL8HxC
- Mantle P (2002) Risk assessment and the importance of Ochratoxins. International Biodeterioration & Biodegradation 50: 143-146. Link: https://bit.ly/3mvXmR7
- Scudamore K, MacDonald S (1998) A collaborative studies of an HPLC method for determination of Ochratoxin a in wheat using Immunoaffinity Column clean-up. Food Addit Contam 15: 401-410. Link: https://bit.ly/37XNFqy
- Marechera G (2015) Estimation of the potential adoption of Ala safe among smallholder maize farmers in lower eastern Kenya. African Journal of Agriculture and Resource Economics 10: 72-85. Link: https://bit.ly/37P8KUj
- Medina A, Akbar A, Baazeem A, Magan N (2017) Climate change, food security and mycotoxins. Journal of Food Science 31: 143-154. Link: https://bit.ly/37TUols
- Kulandaivelu V, Thirukonda N, Rajeev B, Periyasamy P (2011) Management of Aspergillus ochraceus and Ochratoxin-A contamination in coffee during on-farm processing through commercial yeast inoculation. Journal of Biological Control 57: 215-222. Link: https://bit.ly/31SfFsa
- Lalini R, Kanti B (2010) Ochratoxin food contaminants: impacts on human health. Journal on Human of Toxins 2: 771-779. Link: https://bit.ly/3myasxi
- Muzaffer D, Jose F (2010) Ochratoxins in feeds, a risk for animal and human health. Journal of Toxins 2: 1065-1077. Link: https://bit.ly/2TBcW1I
- Ferrao J, Bell V, Chabite T, Fernades T (2017) Mycotoxins, food and health. Journal of Health and Food Science 5: 1-10. Link: https://bit.ly/3e4H9zm
- Madhavan T, Gopalan C (1968) The effects of dietary protein on carcinogens of ochratoxins. Arch Pathol 80: 133–137. Link: https://bit.ly/2TyHzVl
- Patterson D, Allcroft R (1970) Metabolism of ochratoxins in susceptible and resistance animal species. Journal of Food and Toxicology 8: 43–53.
- Robens J, Richord J (1992) Contamination and Toxicological. Journal of Environmental Contamination 127: 69-85.
- Alim N, Naseem Z, Yasha N, Sajia H (2014) Aflatoxin: a potential threat to human health. Journal of Food Science 24: 256–271. Link: https://bit.ly/2Tw3ble
- Naresh M, David A (2005) Conditions of formation of Ochratoxin A in drying, transport and in different commodities. Food Addit Contam 1: 10-16. Link: https://bit.ly/2G7TQwS
- Bucheli P, Taniwaki M (2002) Research on the origin and the impact of postharvest handling and manufacturing on the presence of ochratoxin A in coffee – Review. Food Addit Contam 19: 655–665. Link: https://bit.ly/3mplrt0
- Ramos A, Labernia N, Marın S, Sanchis V, Magan N (1998) Effect of water activity and temperature on growth and ochratoxin production by three strains of Aspergillus ochraceus on a barley extract medium and on barley grains. Int J Food Microbiol 44: 133-140. Link: https://bit.ly/31RMUf6
- Bellí N, Mitchell D, Marín S, Alegre I (2005) Ochratoxin A-producing fungi in Spanish wine grapes and their relationship with meteorological conditions. Journal of Plant Pathology 113: 233-239. Link: https://bit.ly/3oxW2PP
- Esteban A, Abarca M, Bragulat R, Cabañes F (2006) Effect of water activity on ochratoxin A production by Aspergillus niger aggregate species. Int J Food Microbiol 108: 188-195. Link: https://bit.ly/3kDH3Bx
- Lee H, Magan N (2000) Impact of environment and interspecific interactions between spoilage fungi and Aspergillus ochraceus on growth and Ochratoxin production in maize grain. Int J Food Microbiol 61: 11-16. Link: https://bit.ly/3kyUJO1
- Kuruc. J, Hegstad H, Lee K, Wolf-Hall C (2015) Infestation and Quantification of Ochratoxigenic Fungi in Barley and Wheat Naturally Contaminated with Ochratoxin A. J Food Prot 78: 1350-1356. Link: https://bit.ly/2G7FFrC
- Cairns V, Aldred D, Magan N (2005) Water, temperature and gas composition interactions affect growth and Ochratoxin A production by isolates of Penicillium verrucosum on wheat grain. J Appl Microbiol 99: 1215-1221. Link: https://bit.ly/34Agzey
- Ahmad A, Jae H (2017) Occurrence, Toxicity, and Analysis of major Mycotoxins in Food. Occurrence, Toxicity, and Analysis of major Mycotoxins in Food 14: 632-638. Link: https://bit.ly/3oBc4bn
- Marta T, Bedaso K (2016) Occurrence, importance and control of mycotoxins: A review. Journal of Cogent Food & Agriculture 2: 1191-1203. Link: https://bit.ly/3jCXoVM
- Ogara M, Zarafi A, Alabi O, Banwo O, Krska R (2017) Mycotoxin patterns in ear rot infected maize: A review comprehensive case study in Nigeria. Food Control 73: 1159-1168. Link: https://bit.ly/34CIsmd
- Reich E, Schibli A (2007) High-performance thin-layer chromatography for the analysis of medicinal plants. Theme. Link: https://bit.ly/3kK77ek
- Santos E, Vargas A (2002) Immunoaffinity column clean up and thin layer chromatography for determination of OTA in green coffee. Food Addit Contam 19: 447-458. Link: https://bit.ly/3e4nYG9
- Pittet A, Royer D (2002) Rapid, low cost Thin Layer Chromatographic screening for the detection of OTA in green coffee. J Agric Food Chem 50: 243-247. Link: https://bit.ly/3kHmIv8
- Nicholas W, Turnera S, Subrah M, Sergey A (2009) Analytical methods for determination of mycotoxins: A review. Anal Chim Acta 632: 168–180. Link: https://bit.ly/3jKgw4g
- Jarmila SS, Frantisek M, Vladimir O, Tomas R (2013) Determination of Ochratoxin A in Food by High Performance Liquid Chromatography. Journal of Analytical Letters 46: 1495-1504. Link: https://bit.ly/2HJDtao
- Rašić D, Domijan M, Ivic D, Cvjetković B (2009) ELISA and HPLC analysis of ochratoxin A in red wines of Croatia. Journal of food control 20: 590–592. Link: https://bit.ly/34zU1KN
- Scott P (2002) Methods of analysis for OTA. Journal of mycotoxins and Food Safety 10:117-134.
- Sharaf S, Moawiya A, Haddad F, Parisi S (2020) Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) Techniques for Detection and Quantification of Aflatoxins in Different Food Samples. Foods 9: 661. Link: https://bit.ly/3jz9xL7
- Pruslin F, Winston R, Rodman TC (1991) Caveats and suggestions for the ELISA. J Immunol Methods 137: 27–35. Link: https://bit.ly/35Mjpwn
- Valenta H (1998) Chromatographic techniques. Journal of Chromatography 75: 211-242.
- Frantisek M, Annie L, Vladimir O, Jakub T (2016) Ochratoxin A: 50 Years of Research. Journal of Toxins 8: 191. Link: https://bit.ly/34D9f1N
- Scott P, Trucksess M (1997) Application of Immunoaffinity columns to mycotoxin analysis. J AOAC Int 80: 941–994. Link: https://bit.ly/2HN2bXw
- Castellari M, Fabbri S, Fabiani A, Amati A (2000) Comparison of different Immunoaffinity clean-up procedures for high-performance liquid chromatographic analysis of Ochratoxin A in wines. J Chromatogr A 888: 129–136. Link: https://bit.ly/3jz9fE1
- Castegnaro M, Tozlovanu M, Wild C, Molinié A, Pfoh L (2006) Advantages and drawbacks of Immunoaffinity columns in analysis of mycotoxins in food. Mol Nutr Food Res 50: 480-487. Link: https://bit.ly/3jFHFVP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
