International Journal of Agricultural Science and Food Technology
Factors Affecting Iron Absorption and Mitigation Mechanisms: A review
Fikiru Dasa1* and Tilahun Abera2
2Jimma University, Ethiopia
Cite this as
Dasa F, Abera T (2018) Factors Affecting Iron Absorption and Mitigation Mechanisms: A review. Int J Agric Sc Food Technol 4(1): 024-030. DOI: 10.17352/2455-815X.000033Iron has many special functions in the body. More than 65 percent of the body’s iron is in the blood in the form of hemoglobin, which is a protein in red blood cells that transports oxygen to tissues in the body. A compound that carries oxygen to the muscle cells called myoglobin, also requires iron. In addition, iron has a role in many chemical reactions within the body that generate energy. A human body can store excess iron as a reserve. The World Health Organization reported that iron deficiency anemia is one of the most widespread nutrient deficiencies in the globe. Diverse factors may affect its absorption like low dietary intake of iron, deprived iron absorption, or too much blood loss. Moreover, polyphenolic compounds widely found in coffee and tea such as chlorogenic acids, monomeric flavonoids, and polyphenol polymerization products also strongly inhibit dietary nonheme-iron absorption. Children, adolescents, pregnant women, women of child-bearing age, athletes, and older adults are groups at greater risk for iron deficiency. The objective of this review paper is to review factors influencing iron absorption.
Introduction
Minerals are indispensable part of a complete diet of animals. Minerals are inorganic elements that are found in all body tissues and fluids. Even though they yield no energy, they have vital roles in many activities in the body [1,2]. These compounds are necessary for the maintenance of certain physicochemical processes which are essential to life because they are chemical constituents used by the body in many ways. Every form of living matter requires these inorganic elements or minerals for their normal life processes [3,4].
The value of micro-minerals in human, animal and plant nutrition has been well recognized [5,6]. Deficiencies or disturbances in the nutrition of an animal cause a variety of diseases and can arise in several ways. Iron is the most abundant element on earth, yet only trace elements are present in living cells. Iron is essential to all cells of the human body. Iron is a micro-mineral that has a number of key functions. It’s a major part of hemoglobin in red blood cells; as it carries oxygen from the lungs to all parts of the body and facilitates oxygen use and storage in muscles. In addition, every cell in the body needs iron to produce energy [7].
Almost one-quarter of the worldwide population are affected by anemia, of which iron deficit is the primary cause [8,9]. Its deficiency is linked with impaired physical work ability, reduced mood and cognitive function, and poor pregnancy related outcomes [10,11]. An individual’s iron condition falls on a range (ranging from replete to depleted iron stores), iron deficiency and iron deficiency anemia. Therefore, individuals with iron deficiency are at increased risk of developing iron deficiency anemia [12]. In spite of advances in healthcare, iron deficiency remains a foremost public health fear in both developed and developing countries, with adolescent women being mainly susceptible. In industrialized countries, iron deficiency should be easily identified and treated, and yet it is often overlooked by medical practitioners or not recognized by women as a concern [13]. Thus, even in these countries the prevention and treatment of iron deficiency in innovative approaches are required [14].
The recommended nutrient intakes of an individual’s to meet the defined requirement vary, depending, among other factors, on the criterion used to define nutrient adequacy [15-17]. For many nutrients to scientifically support the definition of nutritional needs across age ranges, sex and physiologic states, the information base is limited. The use of nutrient balance to define requirements has been avoided whenever possible, since it is now generally recognized that balance can be reached over a wide range of nutrient intakes. However, requirement levels defined using nutrient balance have been used if no other suitable data are available. The dietary requirement for a micronutrient is defined as an intake level which meets specified criteria for adequacy, thereby minimizing risk of nutrient deficit or excess. Measures of nutrient stores or critical tissue pools may also be used to determine nutrient adequacy. Functional assays are presently the most relevant indices of subclinical conditions related to vitamin and mineral intakes. Anaemia, the defining marker of dietary iron deficiency, may also result from, among other things, deficiencies in folate, vitamin B12 or copper [18].
Individual dietary inhibitory and enhancing factors exert profound influences on iron absorption [19-21]. Polyphenol compounds are widely present in the human diet as components of fruits, vegetables, spices, pulses and cereals, and they are especially high in tea, coffee, red wine, cocoa and the different herb teas. The potent inhibitory effect of phytic acid on nonheme-iron absorption is well known [22-24]. Polyphenolic compounds such as chlorogenic acids, monomeric flavonoids, and polyphenol polymerization products widely present in coffee and tea also strongly inhibit dietary nonheme-iron absorption [25-27]. The effects of ascorbic acid (AA) in dramatically improving iron absorption have consistently been observed [28]. Heme iron found in animal foods is also an important iron source because of its high bioavailability. In addition, many studies have suggested that the enhancing effect of muscle tissue on iron absorption is due to cysteine-containing peptides [29].
The biochemistry of iron metabolism in human digestive system
Iron chemistry and physiology
Iron is found on the 26th element of the periodic table and has a molecular weight of 55.85. It has two ordinary aqueous oxidation states namely ferrous (Fe2+) and ferric (Fe3+). These states enable iron to take part in oxidation/reduction reactions that are crucial to energy metabolism by accepting or donating electrons. On the other hand, this property also enables free iron to catalyze oxidative reactions, ensuing in reactive and damaging free radicals. Hence, body iron required to be chemically bound to assist appropriate physiological role, transport, and storage, with minimal opportunity for free ionic iron to catalyze harmful oxidative reactions [30].
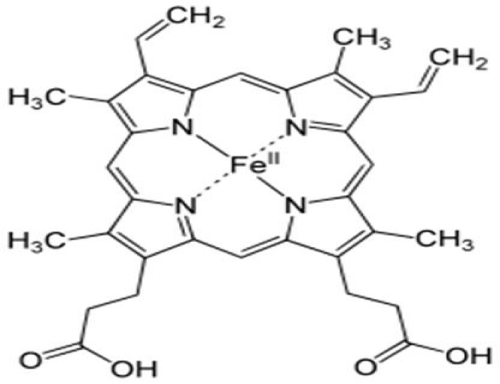
The majority of the body’s iron functions in heme protein complexes that transport oxygen as hemoglobin and myoglobin. Approximately two-thirds of the body iron is in hemoglobin, a 68,000MW structure containing four subunits of heme, a protoporphyrin ring with iron in the center, and four polypeptide chains (two chains each of α- and β-globin). For transport by hemoglobin, oxygen bonds directly to the iron atom, stabilized in a Fe2+ oxidation state surrounded by the protoporphyrin ring and histidine residues. Hemoglobin iron easily binds and releases oxygen, circulating in blood erythrocytes. Myoglobin, consisting of a single heme molecule and globin, enables oxygen transfer from erythrocytes to cellular mitochondria in muscle cytoplasm [30]. Lesser amounts of iron in the heme form function in mitrochondrial cytochromes involved with electron transfer, oxygen utilization, and the production of ATP. A small fraction of body iron functions in heme-containing hydrogen peroxidases such as catalase that protect against excessive hydrogen peroxide accumulation by catalyzing its conversion to hydrogen and oxygen [30].
In addition, iron also has functions in non-heme proteins that contain an iron–sulfur complex (a cubical structure of four iron and four sulfur atoms). It’s the principal form of iron in mitochondria that functioning in enzymes of energy metabolism such as aconitase, NADH dehydrogenase, and succinate dehydrogenase. Aconitase is sensitive to iron concentrations in mitochondria and cytosol. When iron is abundant, the aconitase enzyme assumes the full iron–sulfur cubic structure that is associated with carbohydrate metabolism. However, when iron concentrations are reduced, the protein loses aconitase activity and functions as an iron binding protein (IRP). IRPs interact with iron response elements (IREs) of the mRNA to regulate the synthesis of proteins involved with iron transport, storage, and use, in response to changes in cellular iron concentrations [30] (Figure 1).
Iron absorption and metabolism
The new born infant has a total of about 250mg in the body. The total body iron in an adult male is 3000 to 4000 mg. In contrast, the average adult woman has only 2000-3000 mg of iron in her body. This difference may be attributed to lesser iron reserves in women, lower concentration of hemoglobin and a smaller vascular volume than men. Of this approximately two-thirds are utilized as functional iron such as that in hemoglobin (60%), myoglobin (5%) and various heme and nonheme enzymes (5%). The remainder is found in storage as ferritin (20%) and hemisoderin (10%) [31].
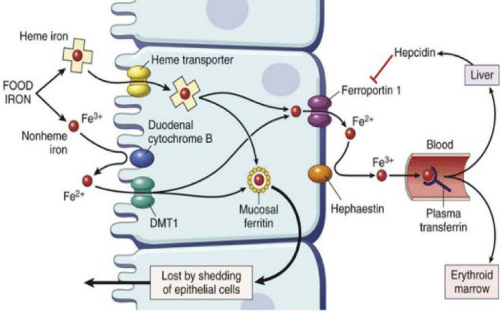
Control of iron uptake is undoubtedly of principal importance due to the lack of a regulated means of excreting iron. Once the food is consumed and digested, dietary iron is mainly absorbed in the duodenum and proximal jejunum. Reasonably, haem iron is absorbed more efficiently than non-haem iron, apparently by endocytosis of the intact iron–protoporphyrin complex at the enterocyte brush border. After the digestion iron from all dietary sources enters a common intracellular pool from which depending on the iron status of individuals it is either stored as ferritin in the enterocyte or exported from the enterocyte via the ferroportin transporter on the basal side of the cell. Ferroportin transports ferrous iron which is immediately oxidized to Fe3+ and picked up by transferrin to be transported to cells expressing transferrin receptors (Figure 2).
Iron status, iron deficiency and iron overload
Infants, children, teenagers, and women of childbearing age are commonly affected by iron deficiency; whereas healthy adult males are seldom deficient. Deficiency is caused by several factors, usually by a combinations of increased need (rapid growth in the young population, menstruation and pregnancy in fertile women) and insufficient uptake, which in turn may depend on other factors such as a decreased caloric intake and/or a larger fraction of calories derived from food ingredients which contains less absorbed iron [32].
The earliest stage of iron deficiency is characterized by loss of storage iron (indicated by ferritin) and is called iron depletion or prelatent iron deficiency. The concentrations of serum iron and the iron-carrying serum protein transferrin are normal at this stage. When iron stores are exhausted (serum ferritin <12μg/L), serum iron decreases and serum transferrin, which is usually measured as total iron-binding capacity (TIBC) increases. If the hemoglobin concentration is still normal, this stage is called iron deficiency without anemia or latent iron deficiency. When the iron stores are completely exhausted, there is not sufficient iron for hemoglobin synthesis. This final stage is called iron deficiency anemia or manifest iron deficiency [32].
Symptoms frequently associated with iron deficiency anemia include palor, weakness, fatigue, dyspnea, palpitations, sensitivity to cold, abnormalities in the oral cavity and gastrointestinal tract, and reduced capacity for work [33]. It further appears that even mid/prelatent iron deficiency may have significant health consequences which can be attributed to decreases in essential body iron and limitations in tissue oxidative capacity [34-36].
Iron overload is associated with increases in non-protein bound iron resulting from the physiologic iron-binding capacity being overwhelmed [37]. Disadvantages with overload are for example increased risk for bacterial infection and cardiomyopathy. Overload can result from inborn errors in metabolism leading to hyper-absorption of iron or inadequate synthesis of the iron-binding proteins. Overload can also result from excessive absorption of dietary iron due to various causes including chronic ingestion of greater than adequate amounts of dietary iron, especially heme iron. These observations concerning of iron overload have raised the question as to whether or not general fortification of food with inorganic iron is beneficial [37].
Dietary and non-dietary factors affecting iron absorption
Dietary factors contribute a significant role in the development of iron deficiency and then iron deficiency anemia. Iron absorption by the gut enterocytes controls iron balance but there is no route of controlled iron excretion. This means that iron absorption is regulated by dietary and systemic factors. Dietary iron is largely non-heme iron with about 5%–10% in the form of heme iron in diets containing meat. Even though heme iron constitutes a smaller part of dietary iron, it is highly bioavailable and 20%–30% of heme iron is absorbed. Contrary, the absorption of non-heme iron is much more variable and significantly affected by other components of the diet; with 1%–10% of non-heme iron absorbed [38].
Moreover, iron in the environment and the diet is primarily ferric iron (Fe3+) which is insoluble and so not bioavailable. Thus, before it can be absorbed the non-heme iron has to be reduced from ferric (Fe3+) to ferrous (Fe2+) iron by dietary reducing agents, such as ascorbic acid or by endogenous ferri-reductases, such as duodenal cytochrome B (dcytB) [39]. Ferrous iron is transported across the apical membrane of the duodenum by the divalent metal transporter 1 (DMT1), which is localized on the brush border membrane close to dcytB. As Wang & Pantopoulos, 2011 reported, the uptake of ferrous ions by dcytB is driven by proton co-transport, so an acidic duodenal pH facilitates iron uptake, and is competitively inhibited by other divalent cations.
Ascorbic acid is one of the most effective enhancer of non-heme iron absorption. Other dietary factors such as citric acid and other organic acids, alcohol and carotenes similarly enhance non-heme iron absorption [38]. Furthermore, animal based proteins such as meat, fish, and poultry, enhance iron absorption but the bioactive component of the “meat factor” has yet to be identified. Meat also promotes non-heme iron absorption by activating gastric acid production. Conversely, absorption of non-heme iron is inhibited by phytic acid (inositol hexaphosphate and inositol pentaphosphate) in grains and cereals and by polyphenols in some vegetables, coffee, tea, and wine. These inhibitors bonded to non-heme iron so it is not available for uptake. Dietary factors influencing iron absorption are outlined in Table 1.
Dietary factors inhibiting iron absorption
Calcium: Calcium does inhibit both non-heme iron and heme iron absorption. Calcium inhibits the absorption of both heme and nonheme iron in a comparable way and thus, it is likely that this inhibition by calcium occurs after the heme iron is freed from the porphyrin ring [40]. Calcium has been shown to inhibit iron absorption in both rats [41] and man [42-45], reported that giving 165 mg Ca as milk, cheese or calcium chloride reduced iron absorption by 50-60% in single-meal measurements with maximum inhibition of approximately 300 mg calcium in the meal. Further increasing the amount of dairy products above a basal level of 300 mg appears to have no further inhibiting effect on iron absorption (Galan et al. 1991). However, the duration of the inhibitory effect of calcium on iron absorption has been shown to be less than two hours [46]. On the other hand, ingestion of 1000 mg Ca as the carbonate daily with meals over a twelve-week period did not appear to be harmful to their iron status [47].
Phytate: During digestion, the phytate molecule can be negatively charged, indicating a potential for binding positively charged metal ions like iron [48]. The negative consequence of phytate in bran on iron absorption was first demonstrated by Sharpe et al. (1950)[49], using white bread and brown bran bread. This effect was earlier supposed to be because of its high content of phytate which has been demonstrated in a number of more recent studies [23,50]. Brune et al. (1989)[51] investigated the likelihood to long-term consumption of high bran containing phytate diet could induce changes in the intestine which would bring about an adaptation to the inhibitory effects of phytate on the intestinal iron absorption. They examined vegetarians and non-vegetarians and found that no adaptation of intestinal brush border to a high phytate intake and concluded that the satisfactory iron stores in the vegetarian group were due to a high consumption of organic acids like ascorbic acid.
Several methods of preparations of cereal grains including soaking, germination and fermentation have been shown to completely reduce the phytate content of cereals and vegetables under optimal conditions [52] and could thereby eliminate their effects on iron absorption. In addition, negative effects of phytate and fiber on iron absorption have been demonstrated in the rat [53], found a reduction in iron absorption when high fiber breads were fed to rats. However, the magnitude of this inhibition was unrelated to the amount of phytate phosphorus or dietary fiber present in the diet.
In contrary, results from experiments by [54], indicated higher absorption from FeSO4 than from the endogenous Fe present in bread, both expressed as mg Fe absorbed and fractional Fe absorption. Using balance and single meal radioisotope measures, she found no differences in Fe absorption among three different breads with fiber contents of 16.1, 38.1 and 72.4 g/kg but with the same phytate concentration (6.3-6.4 g/kg) [55], used the exact same bread in balance measures in humans during 3 x 24 days in 6 subjects and did not find any influence of fiber concentration on iron balances. This indicates indirectly that the inhibitory effect of fiber on iron absorption is probably due to the phytate in the fiber fraction and further supports the study of Morris and Ellis (1980)[56], who found that iron absorption in rats was higher from dephytinized bran.
Phenolic Compounds: During digestion, the phenolic compounds from the food or beverage released and can form complex with Fe in the intestinal lumen making it unavailable for absorption. Nearly all beverages reduced iron absorption depending on the amount of total polyphenols, with the inhibition of black tea the greatest at 79–94%. Few studies revealed that an amounts of only 20 mg polyphenols from black tea per meal reduced iron absorption by as much as 66%, possibly because of the higher content of galloyl esters in black tea and probably because the simple bread meal was devoid of any enhancers of iron absorption to counteract the polyphenols (Prashanth et al., 2008). Similarly, the consumption of black tea and coffee has been shown to strongly inhibit iron absorption from composite meals [16,25,26], with coffee having about half the inhibitory effect of tea.
Moreover, Prashanth et al. (2008) reported that adding of a tea drink to the reference meal resulted in a dramatic reduction in iron absorption in iron deficiency anemia (IDA) and controlects. According to this study, iron absorption from the reference meal consumed with 1 cup of tea was decreased by 59% (P<0.001) and 49% (P <0.05) in the IDA and control subjects, respectively. Consumption of 2 cups of tea with the reference meal decreased iron absorption by 67% (P < 0.001) and 66% (P < 0.01), respectively, in the same subjects. Other beverages such as red wine [57,58] and cocoa [59], as well as various vegetables with high polyphenol content [22,60], have also been reported to inhibit Fe absorption. However, polyphenols in red wine would appear to be less inhibitory than the phenolics of tea or coffee [58]. Similarly, red wine reduced iron absorption from a simple bread-roll meal, but had little effect on iron absorption from more complex composite meals [16]. In addition, a serving of yod kratin (leaves of the lead tree, Leucaena glauca), a vegetable that is high in phenolics, commonly and widely consumed in Thailand reduced iron absorption from a composite meal of rice, fish and vegetables by almost 90% [60]. Other vegetables rich in polyphenols, such as spinach and aubergine, has also been observed to be lower and a significant negative correlation has been reported between the total polyphenol content of vegetable foods and absorption of their Fe content in man [22].
On the other hand, addition of milk to tea or coffee had little or no effect on their inhibitory capacity. Gaur and Miller (2015)[61], reported that the percentage values for total dialyzable iron, total soluble iron and soluble iron. Results showed that, addition of milk to tea significantly reduced the concentration of dialyzable iron (α<0.05) compared to tea or milk alone. The percentage of dialyzability from treatment of tea+milk was 35.65% less than treatment of tea and 19.78% less than treatment of milk. Dialyzable iron and total soluble iron concentration were significantly lower in control compared to all other treatments. In general, all major types of food polyphenols can strongly inhibit dietary non-haem iron absorption. The inhibitory phenolic compounds would appear to include phenolic acids, such as chlorogenic acid from coffee, monomeric flavonoids such as found in herb teas, and the complex polymerization products found in black tea and cocoa (Richard et al. 1998).
Dietary factors enhancing iron absorption
Ascorbic acid: Ascorbic acid appears to be the factor which is most potent in enhancing of iron absorption particularly the non-heme iron in single-meal studies. This was reported by several authors. There are two mechanisms for this effect of nonheme iron on absorption. Firstly, it prevents the formation of insoluble and bonded iron compounds and secondly reduces ferric iron (Fe 3+) to ferrous (Fe2+) iron states [62].
In study by Prashanth et al, (2008) showed that when ascorbic acid added to the meal at a molar ratio to iron of 2:1, ascorbic acid increased iron absorption by 291% in the IDA group and by 270% in the control group (P < 0.001 for both groups). In addition, an increase in iron absorption was observed in both the groups with ascorbic acid added at a molar ratio to iron of 4:1 (350% and 343%) respectively. However, a comparison of iron absorption ratios between the different iron status groups showed no significant differences in the enhancing effect of ascorbic acid between IDA and controls, which indicated that the enhancing effect is likely to be independent of iron status (Prashanth et al., 2008).
On the other hand, the long-term-effect of ascorbic acid on iron status in the body is however less clear. Adding high amounts of ascorbic acid to daily diets for a longer period have in several studies failed to increase ferritin levels [63-65] although single meal absorption measures using radio iron have in the same subjects indicated that ascorbic acid enhances the iron absorption. When a glass of orange juice is included in a breakfast meat, iron absorption can be increased 2.5 times [66].
Meat: The absorption of iron particularly of heme iron is influenced very little by other food components in the diet with the exception of meat which enhances absorption and calcium which inhibits iron absorption [40]. Meat also increases the absorption of nonheme iron [67,68]. The mechanism behind this enhancing effect of meat on both heme and nonheme iron absorption is as yet unknown. It has however been reported by Hurrell et al. (2006)[69] that meat enhancement of iron absorption is by multiple mechanisms involving both gastric acidity effects and chelation. Initially, meat may enhance nonheme iron absorption by stimulating production of gastric acid, and thereby promoting iron solubilization within the stomach. Thereafter, a meat factor(s) may chelate the solubilized iron in the acidic (lower pH) environment of the stomach and thereby maintain iron solubility during intestinal digestion and absorption. Fish and poultry also have an enhancing effect on nonheme iron absorption [70].
Alcohol: Alcohol increases the absorption of nonheme iron slightly in man [16]. It has been shown that chronic alcohol abusers have increased serum ferritin concentrations and calculated total body iron compared with nondrinkers although it is controversial whether the alcohol affects the actual absorption process of iron.
Form of iron from various food sources
Both the source and chemical form of dietary iron can markedly affect its availability for absorption. For most of the world’s population, animal-derived foods are not available, and fortification iron is not yet widely distributed. In the vegetable category, the staples rice, maize, wheat, and beans have either moderate or poor iron availability [71]. There are two major chemical forms (Fe2+ Fe3+) of iron in a diet, and each is absorbed by a different mechanisms. Heme, containing iron in a porphyrin ring structure is found in hemoglobin and myoglobin and accounts for nearly 40% of the iron present in animal tissue, including fish and poultry [72]. Nonheme iron is present in foods of vegetable origin and also accounts for the remaining 60% iron in animal tissue. Other sources of nonheme iron are compounds added to fortify the diet with additional iron above its endogenous level. The most common sources are soluble iron salts or small-particle elemental iron. When taken without food, ferrous salts are better absorbed than ferric forms [73-76]. This is probably related to the fact that ferric iron is insoluble in aqueous solution above pH 3, whereas the majority of ferrous iron remains soluble at pH 8.
Conclusion
Iron is a vital element in the body. It is also toxic when consumed in excess. Hence, its effect in the body is like a two-edged sword. Iron deficiency doesn’t always result in anemia, but it may cause other health problems such as lethargy or a weakened immune system. Iron deficiency occurs when the diet does not include enough iron rich foods, if there is blood loss, or if there is an increased need for iron during adolescence and pregnancy. When the body does not get enough iron, it cannot make enough red blood cells to carry adequate oxygen throughout the body. This condition of having too few red blood cells is called anemia. To prevent this diseases adequate intake of iron should be encouraged.
We are grateful to the farmers of Donbi watershed for allowing us to visit their farm as well as willingness during the household survey. And also great thanks to Southern Nations and Nationalities Peoples Regional state of government for financial support of the research work.
- Malhotra VK (1998) Biochemistry for Students. Tenth Edition. Jaypee. Brothers Medical Publishers (P) Ltd, New Delhi, India. Link: https://goo.gl/UQQ3zd
- Eruvbetine D (2003) Canine Nutrition and Health. A paper presented at the seminar organized by Kensington Pharmaceuticals Nig. Ltd. Link: https://goo.gl/eethw5
- Hays VW, Swenson MJ (1985) Minerals and Bones. In: Dukes’ Physiology of Domestic Animals, Tenth Edition. 449-466. Link: https://goo.gl/973QrZ
- Ozcan M (2003) Mineral Contents of some Plants used as condiments in Turkey. Food Chemistry 84: 437-440. Link: https://goo.gl/AcFJQe
- Darby WJ (1976) Trace elements in human health and disease, Prasad AS. and Oberleas D. Eds. Academic Press, New York, San Francisco, London 1: 17. Link: https://goo.gl/zN3KYb
- Underwood EJ (1977) Trace elements in Human and Animal Nutrition. 4th ed. Academic Press, New York.
- Arora S, Kapoor RK (2012) Iron Metabolism in Humans: An Overview. In Iron Metabolism. InTech. Link: https://goo.gl/5vehwd
- World Health Organization (2008) Centers for Disease Control and Prevention Atlanta. Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia; World Health Organization: Geneva, Switzerland. Link: https://goo.gl/tTojrj
- McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B (2009) Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr 12: 444-454. Link: https://goo.gl/aXw4mb
- Murray-Kolb LE (2011) Iron Status and Neuropsychological Consequences in Women of Reproductive Age: What Do We Know and Where Are We Headed? J Nutr 141: 747S-755S. Link: https://goo.gl/LTFquN
- Scholl TO (2005) Iron status during pregnancy: setting the stage for mother and infant. Am J Clin Nutr 81: 1218S-1222S. Link: https://goo.gl/UcbLCP
- Gibson RS (2005) Principles of nutritional assessment. Oxford university press, USA.
- Miller JL (2013) Iron deficiency anemia: a common and curable disease. Cold Spring Harbor perspectives in medicine. a011866. Link: https://goo.gl/tR7qBn
- Ramakrishnan U, Yip R (2002) Experiences and challenges in industrialized countries: control of iron deficiency in industrialized countries. J nutr 132: 820S-824S. Link: https://goo.gl/cwPCgx
- Bothwell T, Charlton RW, Cook JD, Finch CA (1979) Iron Metabolism in Man. Blackwell Scientific Publications, Oxford. Link: https://goo.gl/oJcqgk
- Hallberg L, Rossander L (1982) Effect of different drinks on the absorption of non-heme iron from composite meals. Hum Nutr Appl Nutr 36: 116–123. Link: https://goo.gl/dJXbfN
- Dallman PR (1986) Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr 6: 13-40. Link: https://goo.gl/n2ZGwb
- World Health Organization (2004) Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements. Folate and Folic Acid [Internet]. Vitamin and Mineral Requirements in Human Nutrition. Second ed. Geneve: World Health Organization. 289-302. Link: https://goo.gl/fWiw76
- Monsen ER (1988) Iron nutrition and absorption: dietary factors which impact iron bioavailability. J Am Diet Assoc 88: 786-790. Link: https://goo.gl/GgbqMH
- Lynch SR (1997) Interaction of iron with other nutrients. Nutrition Reviews: 55: 102-110. Link: https://goo.gl/JVckPt
- Hurrell RF (1997) Preventing iron deficiency through food fortification. Nutr Rev 55: 210-222. Link: https://goo.gl/bgdjRe
- Gillooly M, Bothwell TH, Torrance JD, McPhail AP, Derman DP, et al. (1983) The effects of organic acids, phytates and polyphenols on iron absorption from vegetables. Br J Nutr 49: 331–342. Link: https://goo.gl/ChJmkC
- Hallberg L, Rossander L, Skånberg AB (1987) Phytates and the inhibitory effect of bran on iron absorption in man. Am J Clin Nutr 45: 988-996. Link: https://goo.gl/u1jEqC
- Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, et al. (1992) Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr 56: 573-578. Link: https://goo.gl/uZHo6x
- Disler PB, Lynch SR, Charlton RW, Torrance JD, Bothwell TH, et al. (1975) The effect of tea on iron absorption. Gut 16: 193–200. Link: https://goo.gl/e4nGpq
- Morck TA, Lynch SR, Cook JD (1983) Inhibition of food iron absorption by coffee. American Journal of Clinical Nutrition 37: 416–420. Link: https://goo.gl/cMZtkV
- Hurrell RF, Reddy M, Cook JD (1999) Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br J Nutr 81: 289-295. Link: https://goo.gl/Tm1m2y
- Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF (2008) Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr 87: 881-886. Link: https://goo.gl/dEt3w7
- Taylor PG, Martinez-Torres C, Romano EL, Layrisse M (1986) The effect of cysteine-containing peptides released during meat digestion on iron absorption in humans. Am J Clin Nutr 43: 68-71. Link: https://goo.gl/zomxN2
- Hunt JR (2005) Dietary and physiological factors that affect the absorption and bioavailability of iron. International journal for vitamin and nutrition research 75: 375-384. Link: https://goo.gl/DqK6fc
- Benito P, Miller D (1998) Iron absorption and bioavailability: an updated review. Nutrition Research 18: 581-603. Link: https://goo.gl/XT5zZc
- Tidehag P, Sandberg AS, Hallmans G, Wing K, Türk M, et al. (1995) Effect of milk and fermented milk on iron absorption in ileostomy subjects. Am J Clin Nutr 62: 1234-1238. Link: https://goo.gl/3qg7uZ
- Conrad ME (1987) Iron absorption. In: Physiology of the Gastrointestinal Tract. Johnson LR. 2nd Ed, pp 1437-1453. Raven Press, New York.
- Baynes RD, Bothwell TH (1990) Iron deficiency. Annual Review of Nutrition 10, 133-148. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 131: 568S-579S. Link: https://goo.gl/WV1sLV
- Cook JD (1990) Adaptation in iron metabolism. Am J Clin Nutr 51: 301-308. Link: https://goo.gl/HiXpjY
- Cook JD, Lynch SR (1986) The liabilities of iron deficiency. Blood 68: 803-809. Link: https://goo.gl/VzdD7Q
- Weinberg ED (1989) Cellular regulation of iron assimilation. The Quarterly review of biology 64: 261-290. Link: https://goo.gl/EWFWjW
- Sharp PA (2010) Intestinal iron absorption: regulation by dietary & systemic factors. Int J Vitam Nutr Res 80: 231-242. Link: https://goo.gl/MeCzLz
- McKie AT, Barrow D, Latunde-Dada GO, Rolfs A, Sager G, et al. (2001) An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 291: 1755-1759. Link: https://goo.gl/DjGW3R
- Hallberg L, Rossander-Hulthèn L, Brune M, Gleerup A (1993) Inhibition of haem-iron absorption in man by calcium. Br J Nutr 69: 533-540. Link: https://goo.gl/yuUEF9
- Barton JC, Conrad ME, Parmley RT (1983) Calcium inhibition of inorganic iron absorption in rats. Gastroenterology 84: 90-101. Link: https://goo.gl/4LwtNn
- Cook JD, Dassenko SA, Lynch SR (1991) Assessment of the role of nonheme-iron availability in iron balance. Am J Clin Nutr 54: 717-722. Link: https://goo.gl/XrYyyN
- Gleerup A, Rossander-Hultén L, Gramatkovski E, Hallberg L (1995) Iron absorption from the whole diet: comparison of the effect of two different distributions of the daily calcium intake. Am J Clin Nutr 61: 97-104. Link: https://goo.gl/mWd8HH
- Lönnerdal B (2010) Calcium and iron absorption—mechanisms and public health relevance. International Journal for Vitamin and Nutrition Research 80: 293-299. Link: https://goo.gl/fssG3C
- Hallberg L, Brune M, Erlandsson M, Sandberg AS, Rossander-Hulten L (1991) Calcium: effect of different amounts on nonheme-and heme-iron absorption in humans. Am J Clin Nutr 53: 112-119. Link: https://goo.gl/92bjC3
- Gleerup A, Rossander-Hultén L, Hallberg L (1993) Duration of the inhibitory effect of calcium on non-haem iron absorption in man. Eur J Clin Nutr 47: 875-879. Link: https://goo.gl/TkMxxR
- Sokoll LJ, Hughes BD (1992) Calcium supplementation and plasma ferritin concentrations in premenopausal women. Am J Clin Nutr 56: 1045-1048. Link: https://goo.gl/Y9Sbke
- Harland BF, Oberleas D (1987) Phytate in foods. World Review of Nutrition and Dietetics 52: 235-259. Link: https://goo.gl/FkKNzS
- Sharpe LM, Peacock WC, Cooke R, Harris RS (1950) The Effect of Phytate and other Food Factors on Iron Absorption: Two Figures. J Nutr 41: 433-446. Link: https://goo.gl/Vk8gq8
- Brune M, Rossander-Hultén L, Hallberg L, Gleerup A, Sandberg AS (1992) Iron absorption from bread in humans: inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. J Nutr 122: 442-449. Link: https://goo.gl/ePVs5b
- Brune M, Rossander L, Hallberg L (1989) Iron absorption: no intestinal adaptation to a high-phytate diet. Am J Clin Nutr 49: 542-545. Link: https://goo.gl/DCF4yj
- Larsson M, Sandberg AS (1992) Phytate reduction in oats during malting. Journal of Food Science 57: 994-997. Link: https://goo.gl/73EeGB
- Ranhotra GS, Lee C, Gelroth JA (1979) Bioavailability of iron in some commercial variety breads. Nutrition Reports International 19: 851-856. Link: https://goo.gl/6AvmZJ
- Fairweather Tait SJ (1982) The effect of different levels of wheat bran on iron absorption in rats from bread containing similar amounts of phytate. British Journal of Nutrition 47: 243-249. Link:
- Andersson H, Nävert B, Bingham SA, Englyst HN, Cummings JH (1983) The effects of breads containing similar amounts of phytate but different amounts of wheat bran on calcium, zinc and iron balance in man. Br J Nutr 50: 503-510. Link: https://goo.gl/nwxX9J
- Morris ER, Ellis R (1980) Bioavailability to rats of iron and zinc in wheat bran: Response to low-phytate bran and effect of the phytate/zinc molar ratio. J Nutr 110: 2000-2010. Link: https://goo.gl/WhAaaD
- Bezwoda WR, Torrance JD, Bothwell TH, McPhail AP, Graham B, et al. (1985) Iron absorption from red and white wines. Scand J Haematol 34: 121–127. Link: https://goo.gl/yPJ7Vo
- Cook JD, Reddy MB, Hurrell RF (1995) The effect of red and white wine on non-heme iron absorption in humans. Am J Clin Nutr 61: 800–804. Link: https://goo.gl/zJzBPn
- Gillooly M, Bothwell TH, Charlton RW, Torrance JD, Bezwoda WR, et al. (1984) Factors affecting the absorption of iron from cereals. Br J Nutr 51: 37–46. Link: https://goo.gl/Xj8dLR
- Tuntawiroon M, Sritongkul N, Brune M, Rossander-Hulthen L, Pleehachinda R, et al. (1991) Dose-dependent inhibitory effect of phenolic compounds in foods on nonheme iron absorption in men. Am J Clin Nutr 53: 554–557. Link: https://goo.gl/V9YcZf
- Gaur S, Miller DD (2015) Is Indian tea (chai) detrimental to dietary iron absorption? International Food Research Journal 22(3):1002-1008. Link: https://goo.gl/Xeo798
- Hallberg L, Brune M, Rossander L (1989) Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr 49: 140-144. Link: https://goo.gl/jn9JMC
- Cook JD, Watson SS, Simpson KM, Lipschitz DA, Skikne BS (1984) The effect of high ascorbic acid supplementation on body iron stores. Blood 64: 721-726. Link: https://goo.gl/84MTmT
- Hunt JR, Gallagher SK, Johnson LK (1994) Effect of ascorbic acid on apparent iron absorption by women with low iron stores. Am J Clin Nutr 59: 1381-1385. Link: https://goo.gl/opPYMT
- Teucher B, Olivares, Cori (2004) Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res 74: 403-419. Link: https://goo.gl/gBXGoN
- Rossander L, Hallberg L, Björn-Rasmussen E (1979) Absorption of iron from breakfast meals. Am J Clin Nutr 32: 2484-2489. Link: https://goo.gl/WkvRvK
- Hallberg L, Björn-Rasmussen E, Howard L, Rossander L (1979) Dietary heme iron absorption: a discussion of possible mechanisms for the absorption-promoting effect of meat and for the regulation of iron absorption. Scand J Gastroenterol 14: 769-779. Link: https://goo.gl/WByNLN
- Johnson JM, Walker PM (1992) Zinc and iron utilization in young women consuming a beef-based diet. J Am Diet Assoc 92: 1474-1478. Link: https://goo.gl/xLCEME
- Hurrell RF, Reddy MB, Juillerat M, Cook JD (2006) Meat protein fractions enhance nonheme iron absorption in humans. J Nutr 136: 2808-2812. Link: https://goo.gl/Nbi5RE
- Morris ER (1983) An overview of current information on bioavailability of dietary iron to humans. In Federation proceedings 42: 1716-1720. Link: https://goo.gl/aXA7jd
- Layrisse M, Martinez-Torres C (1971) Iron absorption from food. Iron supplementation of foods. Page 137 in: Brown, E. B., and Moore, C. V., eds. Progress in Hematology, Vol. VI. Grune and Stratton, New York.
- Monsen ER, Hallberg L, Layrisse M, Hegsted DM, Cook JD, et al. (1978) Estimation of available dietary iron. The American journal of clinical nutrition 31: 134-141. Link: https://goo.gl/yKrZJ6
- Brise H, Hallberg L (1962) A method for comparative studies on iron absorption in man using two radioiron isotopes. Acta Medica Scandinavica 171: 7-22. Link: https://goo.gl/8qAY54
- Hallberg L, Hulthén L (2000) Prediction of dietary iron absorption: an algorithm for calculating absorption and bioavailability of dietary iron. Am J Clin Nutr 71: 1147-1160. Link: https://goo.gl/JsDZWH
- Hunt JR (2002) Moving toward a plant‐based diet: are iron and zinc at risk? Nutr Rev 60: 127-134. Link: https://goo.gl/j2bFz1
- Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF (2008) Iron absorption in young Indian women: the interaction of iron status with the influence of tea and ascorbic acid. Am J Clin Nutr 87: 881-886. Link: https://goo.gl/eBuohY
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
