International Journal of Aquaculture and Fishery Sciences
The use of underwater active and passive acoustics to locate and study fishes
Anthony D Hawkins*
Cite this as
Hawkins AD (2022) The use of underwater active and passive acoustics to locate and study fishes. Int J Aquac Fish Sci 8(3): 080-081. DOI: 10.17352/2455-8400.000081Copyright License
© 2022 Hawkins AD. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.This paper describes how fish can be located using sound, especially in the sea, but also in rivers and lakes. It describes the use of sound detections, including both passive and active acoustics, and it reviews each of these technologies and shows how they can be used to understand the distribution of sound-producing species and to examine information on the spawning habitats of fishes, and their spawning behavior, and also their movement patterns. Sounds generated by humans can have detrimental effects upon fishes, and some stocks of fishes are exploited close to their safe biological limits, requiring restrictions upon those human activities that may harm them. There is a need to regulate those human activities that have adverse effects on fish.
Introduction
There are two important goals in studying the biology of fishes, based on their detection and identification, and defining where they are located. Locating and counting fishes is quite difficult, and defining and mapping a particular fish’s habitat can be especially hard to carry out. A fish’s habitat is the physical, chemical, geological, and biological environment in which it resides, or migrates; and it can include the pelagic (open water), or benthic (upon or within the sea floor), regions. With climate change currently damaging the rivers, estuaries, and coastal marine habitats, it is especially important to seek out the waters and substrates that are utilized as spawning, nursery, and feeding areas by fishes. It is important to examine the habitats occupied by fishes, and especially to identify the managed, threatened, and endangered fish species.
Investigating the location and distribution of fish is especially difficult because fish can rarely be seen and counted visually underwater. Fish catches using trawls, or fishing net surveys, can provide an overall picture of fish distribution, but they can be rather imprecise and are often destructive to the species being surveyed. In fact, the use of fishing trawls or fishing net surveys can be quite damaging to the fish species that are being surveyed. One of the greatest problems in studying fish populations is the difficulty of collecting data on their location over large spatial scales, and studying their behavior for long periods of time, without interfering with their lives.
Locating fishes using sound
Sound waves involve particles of the water oscillating in the direction of propagation of the sound – which can be monitored as the particle motion, that fish are especially sensitive to, as they are moved by it. However, sound waves are more often monitored by detecting fluctuations in sound pressure. Underwater animals are not the only ones that can listen to fish. We can also listen to them. However, there are two methods of listening for sounds that can be used for studying fish populations and fish behavior. Passive acoustics involves listening to the actual sounds produced by the fishes themselves, using hydrophones to detect them, and investigating their distribution and also their behavior, as different sounds can be made by them in different circumstances. Active acoustics uses sound generated regularly by transducers attached to the fish, and those sounds are then monitored using several spaced hydrophones, or by moving a single hydrophone around the area. In contrast, passive acoustics is limited to those fishes that make sounds, and to the times and places where they produce them. The common method for listening to fish is to use an aquatic hydrophone that is sensitive to sound pressure. However, tracking a fish sound source is better achieved by employing two or more directional particle motion receivers, which can measure the directions traveled by the sounds from their sources. This can be done by rotating each hydrophone until the direction of a maximum signal is found. The target position is then calculated by triangulation. The sounds from the fish can either be a continuous signal or its output may be pulsed. An alternative technique allows the use of much simpler underwater equipment and can work well in the open sea. In this case, the omnidirectional sound pressure hydrophones are used, and the coordinates of the source are calculated from measurements of the times at which the sounds arrive at three widely spaced hydrophones. Underwater particle motion (acceleration, velocity, and displacement) from human sources has been reported by Erbe, et al. [1].
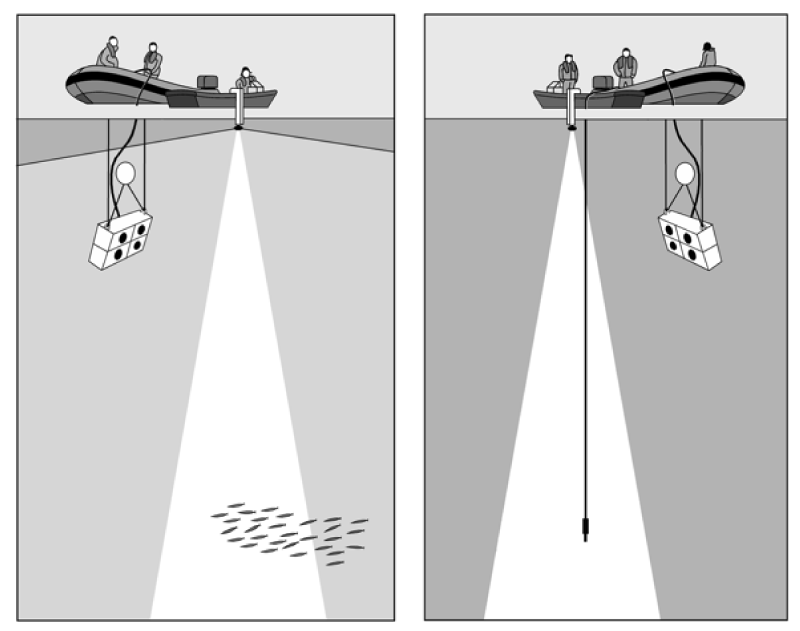
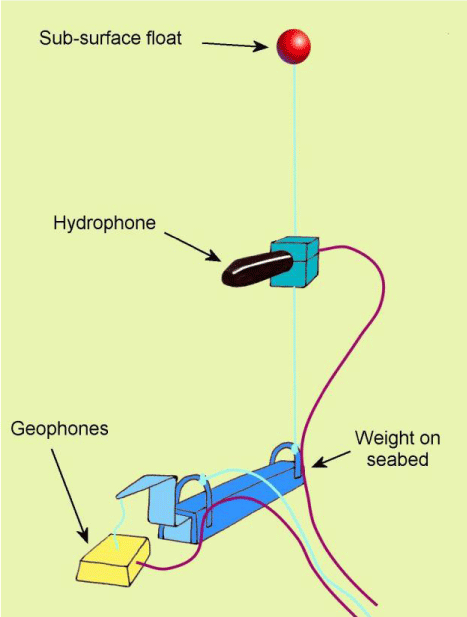
This paper reviews both the active and passive technologies and shows how they can be used to understand both the distribution of sound-producing species and to examine information on the spawning habitat of fishes, and their actual spawning behavior. Figure 1 shows the kind of way that fishes can be monitored and influenced in the sea using boats. The boats can use sonar systems that generate sounds and detect the reflection of the sounds by animals or objects in the water. An alternative method, however, is to mount hydrophones on the sea bed, or on the substrate in rivers and lakes to detect fish sounds (Figure 2).
The nature of underwater sounds
In water, the sound is generated by the movement or vibration of any immersed object and results from the inherent elasticity of the surrounding medium. As the source moves, kinetic energy is imparted to the medium and is passed on as a traveling acoustic wave, within which the component particles of the medium are alternately forced together and then apart. The particles of the medium oscillate back and forth along the line of transmission in waves of compression and rarefaction. The disturbance propagates away from the source at a speed that depends on the density and elasticity of the medium. The Underwater Sound is essentially made up of two elements. Sound is generated by the movement or vibration of some immersed object and results from the inherent elasticity of the surrounding medium. There are waves of compression and rarefaction – termed Sound Pressure. These are monitored by conventional aquatic hydrophones. However, in addition, as a result of the motion of sound sources in water, particles of the water are alternately forced together and then apart. This is termed the Particle Motion, which travels along a line of passage, and is a vector quantity. Particle Motion levels are much higher in the near field, close to the source, especially at low frequencies.
Many investigators who have an interest in the potential effects of man-made (anthropogenic) sounds upon aquatic animals are familiar with the concept of sound pressure and, to a growing degree, the particle motion that is generated in the water column. However, far fewer are aware that some anthropogenic sources such as pile driving, dredging, and seismic exploration, may also generate vibrations within the substrate at the bottom of the water column. Substrate vibration has been dealt with recently, in detail, by Hawkins, et al. [2]. Seismic interface waves may travel along the surface of the substrate generating high levels of particle motion. There is, however, little data on the ambient levels of particle motion close to the seabed and within the substrates of lakes and rivers. Nor is there information on the levels and the characteristics of the particle motion generated by anthropogenic sources in and on the substrate, which may have major effects upon fishes and invertebrates, all of which primarily detect particle motion. There are a number of human activities that can result in the vibration of underwater substrates, together with many natural sources of substrate vibration. Human activities that can generate vibration of the substrate underwater include (among others): pile drivers; explosives; offshore wind-driven electric turbines that are fixed to the seabed rather than floating at the surface; dredging and trawling activities; aircraft-generated sonic booms; air guns used for seismic surveys; and even subsurface transportation tunnels and onshore vehicles on roads close to the water’s edge or on bridges with in-water piling [3]. Natural sources of substrate vibration include volcanos, earthquakes, and breaking waves, also animal movements/interactions, and objects falling and rolling onto the seabed.
A study of Inshore Marine Soundscapes was reported by McWilliams and Hawkins [4]. Acoustic recordings were made in Lough Hyne, Ireland in May 2012, following a nested design in three benthic habitats; Mud, Gravel, and Cliff. Three patches of each habitat were selected using hydro-acoustic and underwater video surveys and within each patch, five different sites were monitored. The high acoustic connectedness of marine habitats underlines the need for evaluating the impact of anthropogenic activities, particularly for ecosystems with unique biophonies in need of protection. There is potential for developing passive acoustic monitoring as a principal method for surveying marine habitats and observing local processes at different spatial and temporal scales. The term ‘soundscape’ describes the physical combination of sounds that prevails at a particular place and time [5,6]. Environmental Noise was also reported by Bruel and Kjaer [7].
Sounds made by fishes
Some fishes produce sounds for many purposes. However, the exact use of sound varies between species. Several fishes only listen to sounds and do this for the detection of prey or predators, and orientation during migration. However, activities in some fishes are facilitated by them actually producing sounds, and detection of the fish sounds is of vital importance to them. These social interactions include mating (e.g. cod and relatives), schooling (e.g. herring), and territoriality (e.g. gurnards and many reef fish). Many fish species are known to produce sounds, and vocalization among teleost fish has been documented for over 40–50 families, mostly in association with social interactions, and especially during reproductive periods [8-11]. Sound-producing mechanisms in teleost fishes have been reported by Kaatz [12].
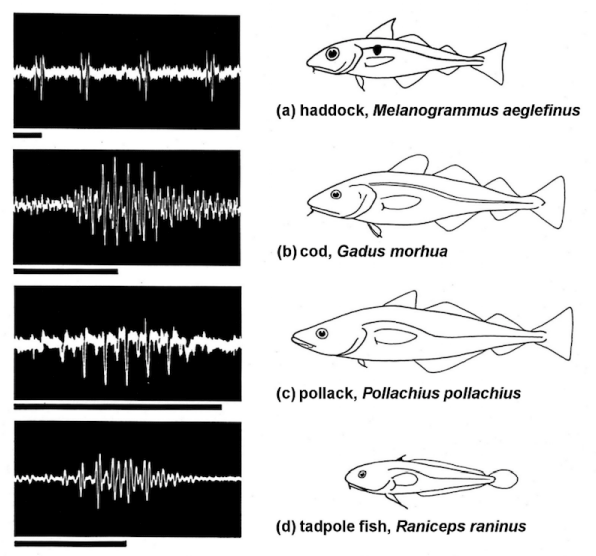
The fish sounds are usually pulsed, with most of their energy below 3 kHz, and they can be produced as grunts or clicks [13]. The sounds may be very intense (. 130 dB re 1 m Pa!), which may be important in predicting the size or the physical strength of an individual. Sound production outside spawning periods commonly occurs during intraspecific and interspecific aggression, or when fish are disturbed or frightened. If sounds are used during agonistic encounters they are usually accompanied by visual agonistic displays [14]. Such sounds vary from low-frequency grunts and drumming sounds to higher frequency creaking sounds, clicks, and stridulating sounds. Within a fish group, the sounds may vary from one species to another. Figure 3 shows the sounds made by several members of the Gadoid family. These fishes make their sounds using drumming muscles attached to their gas-filled swim bladders (that make them buoyant). There is usually a continuous train of short sound pulses, or knocks, with different repetition rates. The knocks are quite low in frequency, which fishes are more sensitive to, but they are repeated in particular patterns by different species. Both males and females can produce sounds. The benthic species use sounds to defend the guarded areas where they live, deterring the entry of intruding animals, with the sounds moving in various directions. However, males especially generate sounds during their spawning behavior, when they use the sounds to attract and influence the females. Such sounds may be quite directional.
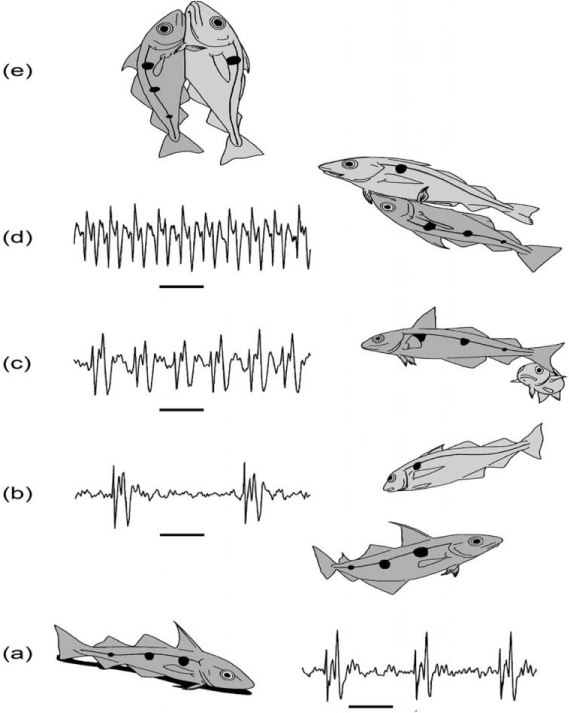
The sound-producing behavior of a particular species, the haddock, Melanogrammus aeglefinis, has been examined in detail. The haddock is widely distributed throughout the deeper shelf waters of the North Atlantic. We studied groups of haddock in a large tank over extended periods and were able to describe the reproductive behavior of the fish, and characterize the sounds associated with particular behavioral acts. We first reported the differences in the sounds of individual male fish. During spawning, the male haddock produce sounds that vary in their characteristics as courtship proceeds [15,16]. Distinctive sounds are associated with particular behavior patterns (Figure 4).
We used the distinctive characteristics of the sounds made by haddock to locate spawning concentrations of this fish in the sea. The more dominant male haddock occupied territories which they defended by means of aggressive displays while producing trains of repeated knocking sounds. These males occupied a particular part of the ground and performed a dance, the Solitary Display (SD). The females tended to occupy mid-water and came down to the areas occupied by the males. Courtship was often prolonged and started when a female entered the territory of a male performing the SD. The female intrusion was followed by a “lead and follow” phase. Female ingressions and retreats were accompanied by the male ceasing his SD and approaching and displaying to the female. As courtship proceeded the male left his territory and moved around the female in tight circles, flicking his fins up and down and emitting humming sounds. The humming sounds were made up of rapidly repeated knocks, separated by intervals as short as 30 ms, and modulated in amplitude and repetition rate. Courtship culminated in the male fish mounting the female. The amount generally occurred higher up outside the male territory. The fish pressed together their ventral surfaces, swam together through the water, and released their eggs and milt into the water together. Some female fish spawned at regular intervals over several weeks. Both the male and female haddock produced short sequences of repeated knocks during agonistic encounters. During the spawning period, however, the male haddock produced much longer sequences of knocks, lasting from several seconds to several minutes, the knocks being produced at intervals varying from 500 ms to 30 ms. At the very fastest rates, with intervals of less than 50 ms, the sounds merged to give a continuous humming. Different behavioral acts leading up to the spawning embrace were associated with particular sounds, as the repetition rate of the knocks varied. The rich diversity of sounds produced by the male haddock appeared to be especially characteristic of this species. It became clear that different individual males produced knocks with different waveforms. The amplitude ratio of the two pulses making up the knocks was different for females and males. Female knocks also showed lower mean frequencies in both the first and second pulses.
Haddock spawning takes place with males spaced out on the seabed in a matrix of defended territories. The solitary display sounds made by the male may attract females to these territories. These sounds, with their individual characteristics which may reflect differences between the males, may then allow the females to choose particular males. The sounds may also enable competing males to judge the fitness of their neighbors. Later, during courtship, variations in the repetition rate of the sounds may assist in synchronizing the release of milt and eggs by the males and females. Mating in the haddock is noisy, lengthy, and easily disrupted. Fishing in the vicinity of spawning haddock at a critical time may have an adverse effect on spawning success. We have shown that listening for sounds offers a very effective and reliable means for detecting the precise areas where sound-emitting fish spawn enabling the protection of the spawning fishes. The location of spawning haddock in the sea was very fully done in a Norwegian fjord. We detected the haddock sounds using a hydrophone held beneath a traveling boat. Listening was carried out at many locations within a fjord, Balsfjord, in Norway [17]. The areas where the haddock sounds were detected were mostly within the inner, most southerly part of the fjord, where a small fishery takes place in the spring for both cod and haddock. Long sequences of repeated knocks were heard at particular locations in the fjord.
Hearing of fishes
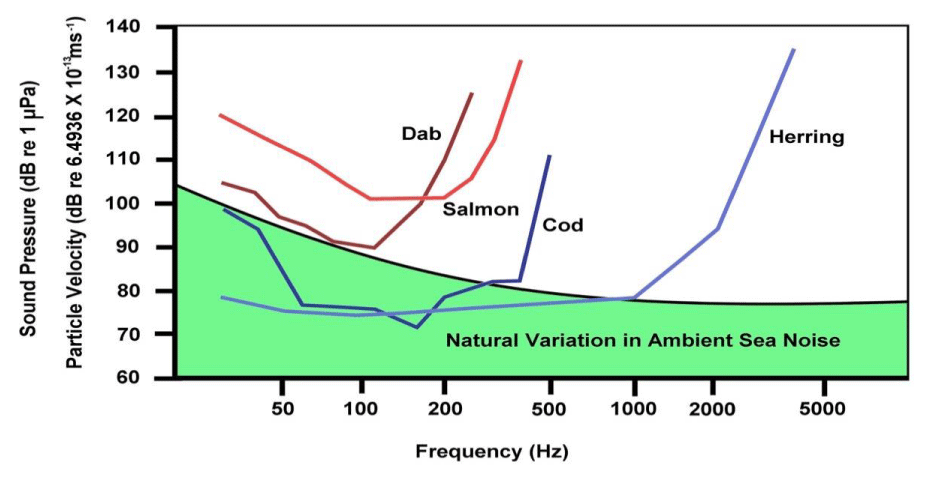
Fishes can hear, and detecting natural sounds in their environment can be critical for their survival and reproduction. Fish hearing thresholds have been determined over a range of pure tone frequencies, providing audiograms from several marine fishes. The experiments were carried out in a Scottish loch [18]. It became apparent that the detection of sounds by fishes like the cod and haddock was often masked by natural variations in the levels of ambient sea noise, and especially by the presence of anthropogenic sounds (Figure 5). The dab and salmon were sensitive to the particle motion, whereas the cod and herring were sensitive to the sound pressure.
Experiments on masking the background noise were carried out using the presentation of pure tone stimuli in the presence of different noise frequency bands [19,20]. Fay, R. R. [21]. Masking of tones by noise for the goldfish was examined by Fay [21].
Hearing is one of the most important senses for some fishes. In water, hearing gains importance compared with seeing, since sounds travel fast in all directions (five times faster than in air) and are not hampered by poor transparency or low light conditions, which can hamper vision.
Fishes have evolved a very substantial variation in the structures of the ear associated with hearing. Speaking of a single ‘fish ear’ is therefore too much of a generalization. However, there is one fundamental similarity: all fishes primarily detect particle motion and use an accelerometer-like system as the basis of hearing [22]. Particle motion is detected in the three pairs of otolith organs within each of the two ears, which consist of a dense mass (the otolith itself) and sensory hair cells that serve as receptors. Essentially the fish itself and the sensory epithelia have approximately the same density as the water and they are moved in a sound field. The otoliths are much denser than the other tissues, and they move with different amplitudes and phases. The hair cell bundles that are in contact with the otolith then undergo a shearing force, which they ‘translate’ into a physiological response. Auditory sensitivity through particle motion is the ancestral way of hearing in fishes [23]. However, in some fish species, sound pressure detection takes place, in addition to particle motion sensitivity. The pressure detection involves a gas-filled swim bladder or other gas bubbles [24-26]. The swim bladder is present to provide fish with buoyancy. Some fishes, including the cod family, gulp air at the surface, and this air is then transported into the swim bladder. Species with a swim bladder may detect the sound pressure component of sound, especially if the swim bladder is close to the inner ear, or connected to the ear. Sound pressure causes volume oscillations in the swim bladder which are transferred to the inner ear, often via a physical connection, e.g. through paired bladder extensions, additional air cavities, or a series of bones (Weberian ossicles), where the particle motion component generated by the sound pressure is registered by the otoliths and sensory epithelia [27].
Hearing differences among different species of fish, related to differences in the peripheral auditory system, have been reported by Coombs and Popper. [28].
The responses of fishes to sounds
It is especially interesting to examine the responses of fish to sounds within the sea. We observed the behavior of wild, pelagic fish in response to sound playback using a sonar/echo sounder [29]. Schools of sprat, Sprattus sprattus and mackerel, Scomber scombrus, were examined at a quiet coastal location in Lough Hyne, Ireland. The fish were exposed to a short sequence of repeated impulsive sounds, simulating the strikes from a pile driver, at different sound pressure levels. The incidence of behavioral responses increased with increasing sound levels. Sprat schools were more likely to disperse and mackerel schools more likely to change depth. The sound pressure levels to which the fish schools responded on 50% of presentations were 163.2 and 163.3 dB re 1 l Pa peak-to-peak, and the single strike sound exposure levels were 135.0 and 142.0 dB re 1 l Pa, for sprat and mackerel, respectively, estimated from dose-response curves. For sounds leading to mackerel responses, particle velocity levels were also estimated. The method of observation by means of a sonar/echo sounder proved successful in examining the behavior of unrestrained fish exposed to different sound levels. This technique may allow further testing of the relationship between responsiveness, sound level, and sound characteristics for different types of man-made sound, for a variety of fish species under varied conditions.
The Effects of a seismic survey on the movement of free-ranging Atlantic cod. Has been carried out by Van der Knaap, et al. [30].
The adverse effects of human activities
Human activities on, in, and near the water introduce potentially adverse sounds into the fish habitat. These human-made (Anthropogenic) sounds may be audible to fishes and they can potentially disturb or deter the fishes, or mask other sounds that are relevant to the animals Exposure to man-made sounds can also have physiological and behavioral effects that may be detrimental to the animals [29,31,32]. Some of these human-made sounds can kill or injure fishes and other aquatic animals, also impairing their hearing and altering their behavior. Death can occur as a result of body damage taking place during sound exposure. Lower damage to body tissues can also take place, including internal hemorrhaging; disruption of gas-filled organs like the swim bladder, and consequent damage to surrounding tissues. The animal may also receive injuries to its auditory system, with the ears themselves being damaged. When the animals are very close to sound sources they may be temporarily deafened by loud noise, and with fishes, this may be the result of damage to the sensory hair cells in the inner ear. At even lower sound levels the hearing abilities may be affected. This may not cause immediate effects but may have longer-term consequences in terms of affecting their communication, reducing their avoidance of predators, or preventing them from capturing prey. Lower levels still may also affect the behavior of the animals: for instance, driving an animal away from, or perhaps attracting it towards, an area. Animals may especially be excluded from key habitats, and this may occur at a ‘bad’ time in terms of their migrations or breeding. Human effects on spawning concentrations of fish may have an especially deleterious effect on stocks. Ambient noise levels are now often much higher in the sea, lakes, and rivers because of human activities. Masking by anthropogenic noise can prevent the detection of the sounds made by fish themselves and other sound signals of importance to them.
Anthropogenic underwater noise impacts have become a major topic for environmental managers and regulators in Europe [33]. Their document focuses on the advances in our knowledge with respect to anthropogenic underwater sound within the Ocean. It is pointed out that the most urgent priority actions/questions are to:
- Develop collaborative international standards applicable to all steps of the risk framework;
- Conduct comprehensive monitoring combined with spatial ecological modeling of marine species’ dynamic habitat use, movements, behavior, and distribution to establish baselines;
- Foster comprehensive monitoring and data collection of current soundscapes/ambient noise, including via joint monitoring programs in existing and new areas;
- Shortlist high-priority (and biologically relevant) sound sources and perform standardized source characterization studies;
- Undertake hearing studies on baleen whales and on selected fish and invertebrate species;
- Conduct field and modeling studies on changes in acoustic habitats to identify masking risks to communication in fishes and marine mammals;
- Conduct further studies on the behavioral response of marine mammals and fishes due to exposure to high-intensity impulsive sounds to assess population consequences;
- Conduct taxa-relevant studies on hearing impairment and physiological stress to address existing knowledge gaps in invertebrates, fishes, and marine mammals;
- Conduct dedicated studies including multi-species investigations, predator-prey interactions, and interaction with other food web levels, addressing the question of how noise impacts combine with other stressors;
- Develop frameworks and conduct studies to allow population-level assessment of effects from the cumulative impact of noise and other pressures;
- Conduct dedicated modeling and field studies to improve understanding of the effectiveness, safety, and cost-effectiveness of noise mitigation devices, mitigation measures, and management options;
- Develop regional action plans and guidelines for Environmental Impact Assessment and policies; and
- Initiate international collaborative trans-disciplinary projects to develop stakeholder and societal capacity in understanding and addressing underwater noise.
There is now a need for more research on aquatic soundscapes, and how they may be deteriorating as a result of human activities. There is a real need for further studies describing the behavioral responses of free-living fish to man-made sounds since neither the short or long-term effects are well understood. Such experiments are best carried out in the sea, but there is also a need to carry them out in lakes and rivers. Work on the effects of ship noise on marine mammals in the sea has recently been carried out by Erbe, et al. [34].
- Erbe C, Parsons M, Duncan AJ, Lucke K, Gavrilov A, Allen K. Underwater particle motion (acceleration, velocity and displacement) from recreational swimmers, divers, surfers and kayakers. Acoustics Australia. 2017; 45: 293-299, doi: 10.1007/s40857-017-0107-6.
- Hawkins AD, Hazelwood RA, Popper AN, Macey PC. Substrate vibrations and their potential effects upon fishes and invertebrates. J Acoust Soc Am. 2021 Apr;149(4):2782. doi: 10.1121/10.0004773. PMID: 33940912.
- Reeder DB, Joseph JE, Rago TA. Underwater sound generated by motor vehicle traffic in an underwater tunnel. J Acoust Soc Am. 2020 Sep;148(3):EL215. doi: 10.1121/10.0001805. PMID: 33003850.
- McWilliam J, Hawkins AD. A comparison of inshore marine soundscapes. Journal of Experimental Marine Biology and Ecology. 2014; 446: 166–176.
- Krause B, Gage SH, Joo W. Measuring and interpreting the temporal variability in the soundscape at four places in Sequoia National Park. Landscape Ecol. 2011; 26(9): 1247–1256.
- Schafer RM. The Tuning of the World. Random House Inc., New York. 1977.
- Brüel and Kjær. Environmental noise. Bruel & Kjaer, Denmark: 2001; 1-67. http://www.bkhome.com/doc/br1626.pdf.
- Brawn VM. Sound production by the cod ~Gadus morhua L.!. Behaviour. 1961;18: 239-255.
- Myrberg AA. Sound communication and interception in fishes. in Hearing and Sound Communication in Fishes, edited by W. N. Tavolga. AN. Popper. RR. Fay ~Springer Verlag, New York! 1981; 395–426.
- Myrberg AA, Ha SJ, Shamblott MJ. The sound of bicolor damselfish ~Pomacentrus partitus!: predictors of body size and spectral basis for individual recognition and assessment. J A Soc Am. 1993; 94: 3067–3070.
- Rice AN, Farina SC, Makowski AJ, Kaatz IM, Lobel PS, Bemis WE, Bass AH. Evolutionary patterns in sound production across fishes. Ichthyology & Herpetology. 2022; 110(1):1-12.
- Kaatz IM. Multiple sound-producing mechanisms in teleost fishes and hypotheses regarding their behavioral significance. Bioacoustics. 2002;. 12(2-3), 230-233.
- Hawkins AD RKJ. The sounds of gadoid fish. J. Mar. Biol. Assoc. U.K. 1978; 58: 891-911.
- Hawkins AD. Underwater sound and fish behavior. In Behavior of Teleost Fishes, edited by TJ. Pitcher (Chapman and Hall, London), 1993; 114–153.
- Hawkins AD, Amorim MCP. Spawning sounds of the male haddock, Melanogrammus aeglefinus. Environmental Biology of Fishes. 2000; 59: 29-41.
- Casaretto L, Picciulin M, Hawkins AD. Seasonal patterns and individual differences in the calls of male haddock Melanogrammus aeglefinus. J Fish Biol. 2015 Sep;87(3):579-603. doi: 10.1111/jfb.12740. PMID: 26333138.
- Casaretto LP, Hawkins AD. Locating spawning haddock (Melanogrammus aeglefinus) at sea by means of sound. Fish Res. 2014; 154:127–134.
- Hawkins AD, Chapman CJ. Studying the behavior of fishes in the sea at Loch Torridon, Scotland. ICES Marine Science. 2020; 118: 1-9.
- Hawkins AD, Chapman CJ. Masked auditory thresholds in the cod Gadus morhua (L). Journal of Comparative Physiology. 1975; 103: 209–226.
- Hawkins AD, Johnstone ADF. The hearing of the Atlantic salmon Salmo salar. Journal of Fish Biology. 1978; 13: 655–673.
- Fay RR. Masking of tones by noise for the goldfish (Carassius auratus). J Comp Physiol Psychol. 1974 Oct;87(4):708-16. doi: 10.1037/h0037002. PMID: 4426992.
- Popper AN, Fay RR. Evolution of the ear and hearing: issues and questions. Brain Behav Evol. 1997;50(4):213-21. doi: 10.1159/000113335. PMID: 9310196.
- Fay RR. Hearing in Vertebrates: A Psychophysics Data book (Hill-Fay Associates, Winnetka, IL). 1988.
- Poggendorf D. The absolute threshold of hearing of the bullhead (Amiurus nebulosus) and contributions to the physics of the Weberian apparatus of the Ostariophysi”), Z. Comp. physiol. 1952; 34: 222–257.
- Fay RR. Behavioral audiogram for the goldfish, Carassius auratus. Journal of Auditory Research. 1969; 9:112- 121.
- Sand O, Hawkins AD. Acoustic properties of the cod swim bladder. Journal of Experimental Biology. 1973; 58: 797-820.
- Popper AN, Fay RR. Rethinking sound detection by fishes. Hear Res. 2011 Mar;273(1-2):25-36. doi: 10.1016/j.heares.2009.12.023. Epub 2009 Dec 23. PMID: 20034550.
- Coombs S, Popper AN. Hearing differences among Hawaiian squirrelfish (family Holocentridae) related to differences in the peripheral auditory system. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1979; 132(2): 203-207.
- Hawkins AD. Responses of free-living coastal pelagic fish to impulsive sounds. Acoustical Society of America. 2014; 3101–3116.
- van der Knaap I, Reubens J, Thomas L, Ainslie MA, Winter HV, Hubert J, Martin B, Slabbekoorn H. Effects of a seismic survey on movement of free-ranging Atlantic cod. Curr Biol. 2021 Apr 12;31(7):1555-1562.e4. doi: 10.1016/j.cub.2021.01.050. Epub 2021 Feb 9. PMID: 33567289.
- Popper AN, Fay RR. Platt C, Sand O. Sound detection mechanisms and capabilities of teleost fishes. In Sensory Processing in Ed. by S. P. Collin, and N. J. Marshall. Springer-Verlag, New York. Aquatic Environments. 2003; 3–38:
- Hawkins AD, Pembroke AE, Popper AN. Information gaps in understanding the effects of noise on fishes and invertebrates. Rev Fish Biol Fisheries. 2014;
- Thomsen F, Mendes S, Bertucci F, Breitzke M, Ciappi E, Cresci A, Debusschere E, Ducatel C, Folegot F, Juretzek C, Lam FP, O’Brien J, dos Santos ME. Addressing underwater noise in Europe: Current state of knowledge and future priorities. 2021. Kellett, P, van den Brand R, Alexander B, Muniz Piniella A, Rodriguez Perez A, van Elslander J, Heymans JJ. [Eds.] Future Science Brief 7 of the European Marine Board, Ostend, Belgium. ISSN: 2593-5232. ISBN: 9789464206104. DOI: 10.5281/zenodo.5534224.
- Erbe C, Marley S, Schoeman R, Smith JN, Trigg L, Embling CB. The effects of ship noise on marine mammals--A review. Frontiers in Marine Science. 2019; 6: 606. doi: 10.3389/fmars.2019.00606.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
