International Journal of Aquaculture and Fishery Sciences
Some epidemiological aspects of Myxosporean infections in Oreochromis niloticus (Linnaeus, 1758) and Hemichromis fasciatus (Peters, 1857), two cultured Cichlid fishes in the West - Cameroon
Fonkwa Georges1,2*, Nack Jacques1, Kouam K Marc3, Tomedi Eyango Minette1 and Tchoumboue Joseph2
2Applied Hydrobiology and Ichthyology Research Unit, Department of Animal Production, Faculty of Agronomy and Agricultural Science, University of Dschang, P.O. Box 222, Dschang, Cameroon
3Physiology and Animal Health Research Unit, Department of Animal Production, Faculty of Agronomy and Agricultural Science, University of Dschang, P.O. Box 118, Dschang, Cameroon
Cite this as
Fonkwa G, Nack J, Kouam KM, Tomedi EM, Tchoumboue J (2022) Some epidemiological aspects of Myxosporean infections in Oreochromis niloticus (Linnaeus, 1758) and Hemichromis fasciatus (Peters, 1857), two cultured Cichlid fishes in the West - Cameroon. Int J Aquac Fish Sci 8(1): 001-009. DOI: 10.17352/2455-8400.000073Copyright License
© 2022 Fonkwa G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.In order to assess epidemiological aspects of myxosporean infection in cultured Oreochromis niloticus and Hemichromis fasciatus fishes in Cameroon to develop efficient prevention and control program, a total of 320 Cichlid fishes (189 Oreochromis niloticus and 131 Hemichromis fasciatus) were collected from June 2019 to April 2020 in the ponds located at the Ngoundoup Village, Koutaba Subdivision, Noun Division, Region of West-Cameroon. They were examined both macroscopically and microscopically for myxosporean infections. The prevalence of infection was determined as a function of fish species, sex, size, target organs, and seasons. Results showed that kidneys and ovaries were the only infected organs and harbored nine and three myxosporean species of the genus Myxobolus respectively. A total of 154 fish were infected (54.38%). Irrespective of the parasite species, Oreochromis niloticus (75.13%) was significantly more infected than Hemichromis fasciatus (24.27%). The prevalence of parasites was very low (<25%) whatever the fish species. The sex and fish size did not significantly influence the prevalence of parasite species. The prevalence of Myxobolus tilapiae was negatively and significantly correlated (r= -0.20; p= 0.02) with Oreochromis niloticus size. The overall prevalence was significantly higher during the dry season (88.76%) than during the rainy (75.31%) and the transitional (20.29%) seasons. Oreochromis niloticus was not infected during the transitional season while Hemichromis fasciatus was more infected (p<0.001) during the dry season (26.84%) followed by the rainy (15.80%) and the transitional (10.32%) seasons. The high prevalence of myxosporeans infection may decrease the fish farming yield. The epidemiological data recorded help develop prevention and control strategies to boost the production of Oreochromis niloticus and Hemichromis fasciatus in Cameroon.
Introduction
Fish farming is an important socio-economic activity in a rural community, contributing to livelihoods, food security, and poverty alleviation [1]. This certainly explains why in Cameroon rural community is paying a lot of attention to fish farming, and investments in this sector are increasing in order to meet the high demand for animal proteins induced by population explosion. Many fish farms have been constructed among which the most important are the Ngoundoup fish ponds, in the Koutaba subdivision, Noun Division, West-Cameroon. In these ponds, two Cichlid fishes are reared and appreciated by households for consumption. These fishes include Oreochromis niloticus and Hemichromis fasciatus commonly called Nile tilapia and banded jewel fishes respectively.
Having the full mastery of farming skills is not the sole prerequisite for success in fish farming. Epidemiology aspects of infections should be taken into account as well. In a natural environment, the balance established during the evolution in the host-parasite system results in the decrease of the pathogenic effect of parasites [2]. On the contrary, the anthropogenic activities in fish farming can modify the water physicochemical characteristics resulting in the disruption of the fish-parasite equilibrium. As a result, fish not only stress but water can become more conducible to epizootics leading to massive fish death and important economic losses [3]. The confinement of fish, the presence of a muddy vase, the weak oxygenation, and the low depth of ponds are also factors favoring the transmission of parasites [4-7].
Among fish parasites, Myxosporeans impedes fish growth [8], their reproduction [4] and are involved in epizootics responsible for massive fish deaths in farms and hatcheries [9- 10]. In Cameroon, studies on fish myxosporeans are essentially descriptive. Therefore, for the last decades, only a few epidemiological data are available comprising those by Lekeufack [11], Lekeufack and Fomena [12], Nchoutpouen, et al. [13], and Nchoutpouen [14]. Qualitative and quantitative data provided by epidemiology are essential in the implementation of prevention and control strategies against myxosporean infections. The goal of this study was to assess the prevalence and some epidemiological aspects of myxosporean infection in Oreochromis niloticus and Hemichromis fasciatus fishes from the Ngoundoup ponds in Koutaba Subdivision, West Cameroon in order to set up a database for their efficient prevention and control.
Materials and methods
Study area and geoclimatic characteristics
Fishes were collected from June 2019 to April 2020 in the ponds located at the village named Ngoundoup (Figure 1), Koutaba Subdivision (North Latitude: 5°37’- 5°52’, East Longitude: 10°44’-10°54’), Noun Division, Region of West-Cameroon. The average altitude is about 1276m above sea level. Climate is of the tropical mountain subset with two seasons: a long rainy season running from March to November and a short dry season from November to March. There are two transitional seasons i.e. from mid - October to mid - November and from mid-March to mid-April. The annual average temperature ranges from 19.80°C to 22.00°C while the rainfall varies between 1313.72 and 1988.60mm. The soil is of ferritic type and rich in organic matter. The sub-highland forest is often degraded by coffee plantations and other food crops [15].
Collection, examination of fishes and Myxosporeans identification
Fishes were captured at day using fish nets and were immediately stored in a vial containing 10% formalin solution and transported to the laboratory for examination. Fishes were identified as described by Stiassny, et al. [16] and examined for the presence of myxosporeans as per Abakar [17]. Thus, standard and total lengths were measured to the nearest millimeter using a slide caliper. Fishes were sex- determined after dissection.
The community structure of fishes (Table 1) reveals that a total of 320 specimens (Figure 2) comprising 189 Oreochromis niloticus and 131 Hemichromis fasciatus were collected. Various organs (skin, eye, kidneys, livers, gonads, spleens, gills, fins, buccal cavity, brain, digestive tract, gall bladder, brain) were examined with naked eyes, then with a stereoscopic microscope using the 10X lens to look for the cysts. Smears of the kidneys, spleen, and gonads were made and examined at a total magnification of 1000X with a light microscope to search for myxospores. Cysts were crushed between a slide and a cover glass in a drop of distilled water and their contents were identified with the light microscope using the 100X lens. Spores were fixed and stained with methanol and May-Grünwald-Giemsa respectively and photographed with a digital camera (Canon Ixus brand).
Myxospores were measured with a calibrated ocular micrometer as recommended by Lom and Arthur [18] and were morphologically identified using the key provided by Lom and Dyková [19,20], Eiras, et al. [21-22], Fomena and Bouix [23].
Epidemiological parameter studied and statistical analysis
The epidemiological parameter studied was the prevalence (Pr) of infection expressed in percentage and defined as the number of fish species infected by a given parasite species divided by the number of fish examined [24]. The prevalence was classified as very low (Pr<25%), low (25% ≤ Pr <50%), high (50% ≤ Pr <75%) and very high (75% ≤ Pr ≤ 100%).
The comparison of prevalence was performed using the Chi-square (X2) test. The Spearman correlation coefficient “r” was calculated to determine a probable relationship between the prevalence of parasite species and the fish size. The significance level of the probability was p < 0.05 and the Graph Pad Prism 5 software was helpful for analysis.
Results
Results are illustrated in Figures 3-7 and Tables 2-3.
Myxosporean fauna of fishes and prevalence of fish species
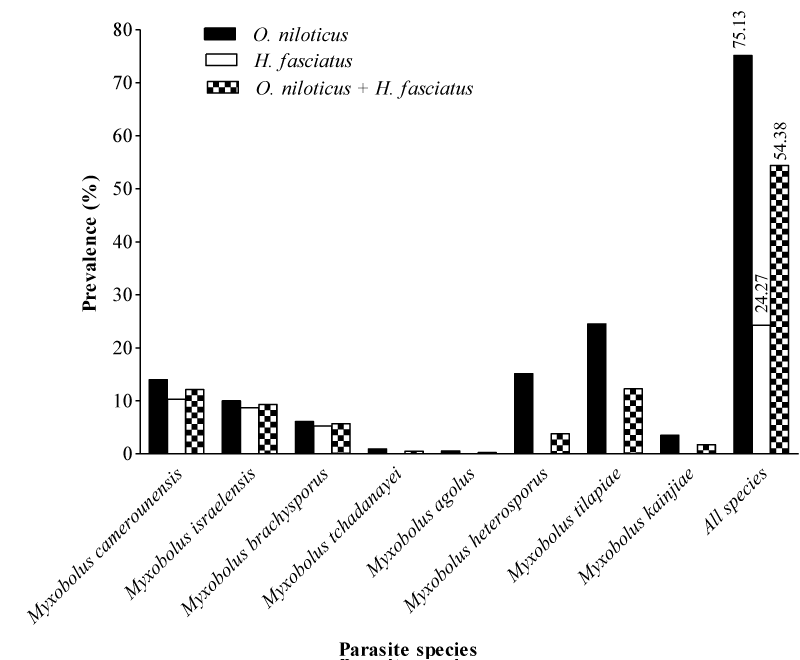
The myxosporean fauna of fishes shown in Table 2 was composed of 8 species of the genus Myxobolus. The prevalence of myxosporeans species in relation to fish species illustrated in Figure 3 reveals a high (54.38%) overall prevalence of infection in the ponds. Irrespective of the parasite species, both fish species were parasitized. In addition, Oreochromis niloticus (75.13%) was significantly (X2= 25.92; p= 0.001) more infected than Hemichromis fasciatus (24.27%). The prevalence of parasites was very low (<25%) whatever the fish species.
Oreochromis niloticus and Hemichromis fasciatus harbored 8 and 3 myxosporean species respectively. Moreover, 5 parasite species (M.tchadanayei, M. agolus, M. heterosporus, M.tilapiae and M. kainjiae) were specific to Oreochromis niloticus while 3 (M. camerounensis, M. israelensis and M. brachysporus) were common to both fish species. In Hemichromis fasciatus, the prevalence varied significantly (X2= 17.41; p<0.001) from 5.30 (M. brachysporus) to 10.30% (M. camerounensis) while in Oreochromis niloticus, Myxobolus tilapiae and M. tchadanayei exhibited the highest (24.50%) and the lowest (0.92%) prevalence respectively (p<0.001).
Prevalence of Myxosporean species as a function of fish sex
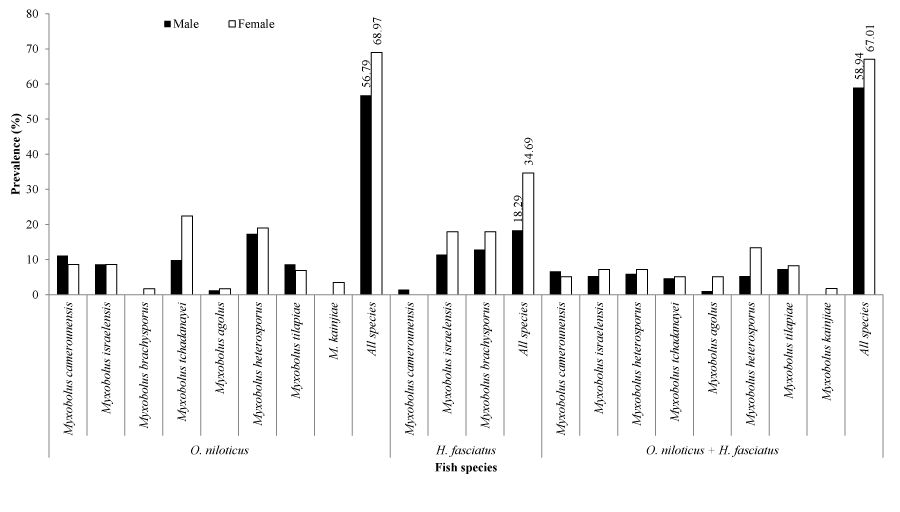
The prevalence of myxosporean species as a function of fish sex exhibited in Figure 4 reveals that, both males and females were infected. Regardless of the fish and parasite species, females (67.01%) were insignificantly (X2= 1.63; p= 0.201) more infected than males (58.94%). Whether in Oreochromis niloticus (X2= 2.12; p= 0.145) or Hemichromis fasciatus (X2= 0.08; p= 0.782), the prevalence of parasite species did not differ between males and females.
Prevalence of Myxosporean species in relation to fish size class
As illustrated in Figure 5, fish of all size classes were infected. Whatever the fish species, the prevalence of myxosporean species did not significantly differ (p>0.05) between size classes. The prevalence of Myxobolus tilapiae (Table 3) was negatively and significantly correlated (r= -0.20; p= 0.02) with Oreochromis niloticus size.
Prevalence of Myxosporean species as a function of the infection sites and fish species
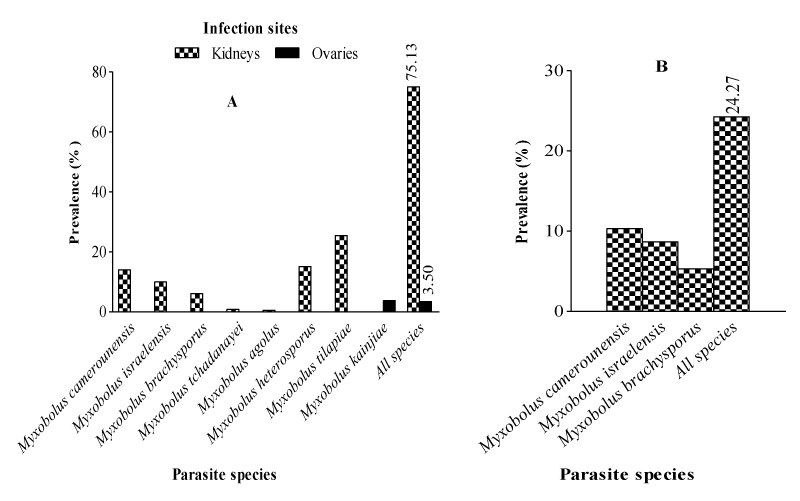
The prevalence of myxosporean species as a function of infection sites and fish species as illustrated in Figure 6 reveals that irrespective of the fish species, only two organs were infected namely kidneys and ovaries. In both fish species, kidneys were infected while ovaries were the only parasitized organs in Oreochromis niloticus. Whether between or within fish species, the prevalence was higher for the kidneys. The comparison of the infection sites in terms of parasites richness shows that all the 8 myxosporean species were encountered in the kidneys while Myxobolus kainjiae was specific to O. niloticus ovaries.
Seasonal prevalence of Myxosporean species
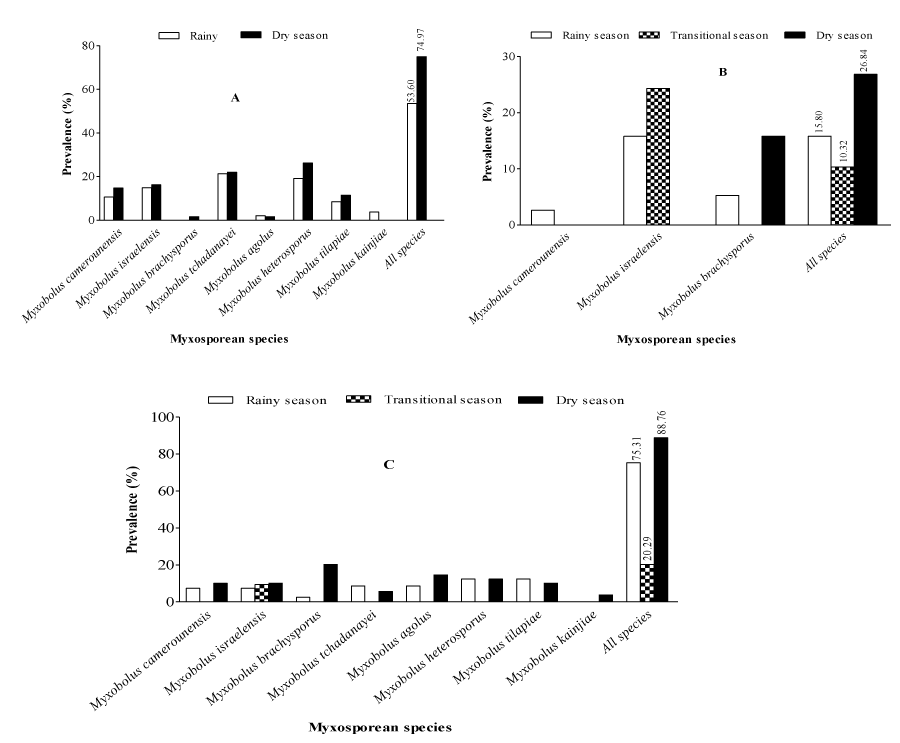
The seasonal prevalence of myxosporean species (Figure 7) shows that irrespective of the fish and parasite species, all the considered seasons were favorable to fish infections. Moreover, the overall prevalence was significantly (X2= 85.84; p<0.001) higher during the dry season (88.76%) than the rainy (75.31%) and transitional (20.29%) seasons. Myxobolus tchadanayei, M. heterosporus and M. tilapiae were the sole parasites exhibiting no significant (p>0.05) seasonal variation of the prevalence. The comparison of the seasonal prevalence of parasites between fish species reveals that Oreochromis niloticus was not infected during the transitional season. On the contrary, Hemichromis fasciatus was significantly more parasitized by Myxobolus israelensis (24.32%) during the transitional season compared to other seasons. In Oreochromis niloticus, the prevalence of parasites did not show a significant seasonal variation (X2= 0.47; p= 0.92) while in Hemichromis fasciatus, parasites were more prevalent (X2= 14.254; p<0.001) during the dry season (28.84%) followed by the rainy (15.80%) and the transitional seasons (10.32%).
Discussion
The predominance of myxosporeans of the genus Myxobolus (8 species over 8) in the study site is well documented. The world myxosporeans fauna is composed of about 2180 species (62 genera) among which the genus is numerically the most abundant with about 35% of species [20]. Kaur [25] reported 55 myxosporean species grouped in 6 genera in various organs of wetlands and cultured carps in Punjab out of which, the genus Myxobolus was the most represented (28 species) compared to other genera namely Thelohanellus (18 species), Henneguya (6 species), Triangula (2 species), Neothelohanellus and Unicauda (1 species each). The same observation was made by Anu [26] who recorded up to 64% of Myxobolus species infecting cultured native and exotic carps in Punjab. Abakar [17] reported 60% of Myxobolus species in Myxosporeans fauna of Chad freshwater fishes. Meanwhile, in Cameroon, Lekeufack and Fomena [12] collected 54.55% of the myxosporeans of the genus Myxobolus in the River Sangé infecting several hosts. Nchoutpouen, et al. [13] reported 100% Myxobolus species (10 species over 10) in Foumban fish ponds. The broad spectrum of hosts experienced by the genus Myxobolus, its preponderance, wide geographic distribution, and ecological plasticity might be due to the versatility of its metabolic pathway and the genetic background enabling it to get adapted to various biotopes.
Myxobolus camerounensis, M. israelensis and M. brachysporus could be of stenoxenous specificity since they commonly infected Oreochromis niloticus and Hemichromis fasciatus which belong to the same family (Cichlidae). Myxosporean species that were specific to O. niloticus probably might be oїoxenous (narrow specificity). The fish/parasite specificity can be explained by the fact that different hosts constitute different habitat options (ecological niches) for parasites. Each parasite is thus adapted to the host which provides a maximum resource for the parasite survival.
The high overall prevalence of infection (54.38%) in the ponds is in agreement with Nchoutpouen, et al. [13] who reported a high prevalence (64.80%) in Oreochromis niloticus (Nile tilapia) in Foumban fish ponds (West-Cameroon). Moreover, the latter authors also observed a prevalence of 61.10% in O. niloticus from the natural environment (Noun River). In Punjab, 34.71% of fishes were infected in three wetlands (Harike, Kanjali, and Ropar) and 26.28% in aquaculture [25]. Anu [26] reported 44.20% of infected native Indian carps in aquaculture. In Egypt, Mohammed, et al. [27] recorded in the Nile River the prevalence of 25.00% and 24.20% in O. niloticus and Tilapia zilli respectively. In the natural environment, the prevalence of infection is generally low (compared to the farming situation) because the balance established during the evolution of host/parasite system reduces the pathogenic effects of parasites [2]. The prevalence of the parasite species vary geographically [28] as per the host species [29]. In aquaculture, the anthropogenic activities, the confinement of fish, the presence of muddy vase, the low oxygenation and lo water depth are factors increasing the prevalence of parasites [4-7].
In ponds, the low water flow may result in heavy myxosporean infections. In fact, Ray, et al. [30], Ray and Bartholomew [31] claimed that the water flow was the most important abiotic factor after the water temperature influencing the transmission of myxosporeans. High flows may scour and remove preferred oligochaete habitat, dilute infectious stages and decrease transmission of actinospores to fish [32]. On the other hand, lower flows encourage higher retention of spores and transmission of myxospores to oligochaete hosts [33] resulting in a higher prevalence of infection in both oligochaete and fish hosts, as well as higher infection severity in fish [32]. Actinospores transmission as well as the prevalence of myxosporean infections are greatly reduced above a velocity threshold of about 0.2–0.3m/s [34- 35]. If the soil of our study area was clayey or silty, one might think that it would favor the myxosporean infections. Indeed, silt and clay harbor more infected oligochaetes than other substrates [36]. At the same water flow, oligochaetes inhabiting silt or mud produce more actinospores than those in the sand [37-38]. However, Neudecker, et al. [39] found no significant association between fine sediment abundance and infection severity.
The infection rate of Oreochromis niloticus (75.13%) was significantly higher than that of Hemichromis fasciatus (24.27%). This may be due to the difference in fish genetic background. If we assume that the genetic background has nothing to do with the fish’s susceptibility to myxosporean infections, then the infection rate will not vary between fishes sharing the same confined biotope.
The fish sex did not significantly influence the prevalence of parasite species. The same observation was made by Abakar [17], Milanin, et al. [40], Lekeufack and Fomena [12]. Fomena [5] did not observe any significant difference between the prevalence of myxosporean in male and female Oreochromis niloticus at Mélen fish ponds in Cameroon. Viozzi and Flores [41] also claimed that the prevalence of Myxobolus biliare in Galaxias maculates was not sex-related. They further opined that it is a general situation with myxosporean infection. Anu [26] instead reported that female carps were more infected (38.25%) compared to males (26.21%). Gbankoto, et al. [42] thought that the prevalence of myxosporidiosis is often higher in males than in females probably due to the fact that males lose a huge amount of energy for testosterone synthesis, resulting in a weaker immune system [43]. The effect of fish sex on the prevalence of myxosporean infections is still to be thoroughly investigated [44].
Fishes of all size classes were infected without any significant difference in the prevalence among size classes. Obiekezie and Okaeme [4] made the same remark. According to Viozzi and Flores [41], Tombi and Bilong Bilong [7], and Abakar [17], young fishes are more vulnerable to myxosporean infections than older ones. On the contrary, Nchoutpouen, et al. [13] outlined that in ponds, older Oreochromis niloticus were more infected than the younger ones. As Oreochromis niloticus grows, the prevalence of Myxobolus tilapiae decreased (r= -0.20; p= 0.02). A similar phenomenon was observed in Finland where the prevalence of infection of Rutilus rutilus by Myxobolus rhodei and M. pseudodispar decreased with the fish size; this might be due to the increase of the immune system response with the fish size [29].
The infections of the kidneys with all the 8 myxosporean species suggest that those organs offer suitable micro biotopes with optimal life conditions for parasites. Since kidneys filter blood and secrete many solutes [45], parasites converge there for the metabolites they need, this may be the reason why they harbored more parasite species than ovaries. The specificity of Myxobolus kainjiae to O. niloticus ovaries probably is because ovaries provide a suitable environment for the M. kainjiae survive.
The prevalence was higher in the dry season than in the rainy and transitional seasons. In Foumban ponds (Cameroon), Nchoutpouen, et al. [13] noticed that the infection rates were higher in the rainy season and low in the dry season. Moreover, Sitjà and Alvarez [46] observed that in Spain, the prevalence of Sphaeropora dicentrarchi, in Dicentrarchus labrax varied with seasons in the fish ponds higher, being during the summer than the autumn. Also, the prevalence of Myxidium biliaire was reported to be higher in summer than in winter [41]. Thus there appear to be contradictory results regarding the season of higher infection rate suggesting that other factors may be intrinsic (parasite and host) or extrinsic (water Physico-chemical characteristics, general management of ponds) regulate parasite prevalence. For instance, during the dry season, the increase in water temperature and the presence of a muddy vase raise the prevalence of the myxosporean and the oligochaetes (definitive host). Özer, et al. [47] revealed that mud subtracts favors rapid growth and multiplication of oligochaetes. Consequently, during the dry season, the definitive hosts are very abundant and their infecting stages (actinospores) multiply rapidly. This situation is favorable to fish infection [48].
Conclusion
The overall prevalence of myxosporean infection in the ponds was high. The main risk factors of myxosporean infection were the seasons and fish species. Kidneys and ovaries were the only infected organs. Fish kidneys being mixed organs with hematopoietic, reticuloendothelial, endocrine, and excretory functions, its infection can lead to fish death. Also, the infection of the ovaries may result in the barrenness of fishes and the decrease of their productivity. The epidemiological data recorded help develop control strategies in order to increase the production of Oreochromis niloticus and Hemichromis fasciatus in the ponds.
We do not advise the exportation of O. niloticus and H. fasciatus fingerlings of our ponds for any aquaculture purpose without implementing a control program. Since the prevalence of myxosporean species significantly decreased from the dry, rainy to transitional seasons, it is better to rear those Cichlids during the transitional season.
Ethical approval and consent to participate
Fishes used followed a protocol approved and authorized by the Institutional Animal Care and Use Committee at the Department of Animal Production, Faculty of Agronomy and Agricultural Science, University of Dschang, Cameroon. Fish farmers agreed to the study.
The authors are grateful to fish farmers of Koutaba Subdivision, West Region of Cameroon for their collaboration.
Availability of data and material
The raw data used to support the findings are available from the corresponding author upon reasonable request.
- Nounagnon D, Gbankoto A, Siko EJE, Canal C, Vouvé F, et al. (2016) Pattern and pathophysiological effects of myxosporean infection in the gills of Tilapia species (Telesotei: Cichlidae) from Bénin. IJMCR 4: 1229–1238. Link: https://bit.ly/3gQMZ9L
- Euzet L, Pariselle A (1996) Le parasitisme des poisons siluroidei: un danger pour l’aquaculture? Aquatic Living resour 9: 145-151. Link: https://bit.ly/3BmJpxE
- Boungou M, Sinaré Y, Mano K, Kabré GB (2013) Parasitic Copepods (Arthropoda, Crustacea, Copepoda) from fishes in Burkina Faso, Africa. Int J Fish Aquat Sci 2: 58-64. Link: https://bit.ly/3gQMYTf
- Obiekezie AI, Okaeme AN (1990) Myxosporea (Protozoa) infections of cultured tilapias in Nigeria. J Afr Zool 104: 77- 91. Link: https://bit.ly/3oRydE8
- Fomena A (1995) Les Myxosporidies et Microsporidies des poissons d’eau douce du Sud–Cameroun : Etude faunistique, Ultrastructure et Biologie. Thèse de Doctorat d’Etat. Université de Yaoundé I. 397.
- Barassa B, Adriano EA, Arana S, Cordeiro NS (2003) Henneguya curvata sp. n. (Myxosporea; Myxobolidae) parasitizing the gills of Serrasalmus spilopleura (Characidae: Serrasalmidae), South American fresh water fish. Folia Parasitologica 50: 151-153. Link: https://bit.ly/3uO4ika
- Tombi J, Bilong CF (2004) Distribution of gills parasites of the freshwater fish Barbus martorelli Roman, 1971 (Teleostei: Cyprinidae) and tendency to inverse intensity evolution Between Myxosporidia and Monogenea as a function of the host age. Revue Elev réd Vét Pays trop 57: 71-76. Link: https://bit.ly/3HVFzhB
- Longshaw M, Freak PA, Nunn AD, Cowx IG, Feist SW (2010) The influence of Parasitism on fish population success. Fish Manage Ecol 17: 426–434. Link: https://bit.ly/3t3idjZ
- Gbankoto A, Pampoulie C, Marques A, Sakiti GN (2001) Occurrence of Myxosporean parasites in gills of tilapia species from Lake Nokoue (Benin, West Africa). Effect of host size and sex, and seasonal pattern of infection. Dis Aqua Organ 44: 217-222. Link: https://bit.ly/3Bn4vfg
- Feist SW, Lonshaw M (2005) Myxozoan diseases of fish and effects on host population. Acta zool Sin 51: 758–760. Link: https://bit.ly/3I6bHik
- Lekeufack Folefack GB (2010) Faunistique et Biologie des Myxosporidies (Myxozoa: Myxosporea) parasites de quelques poissons téléostéens dans la rivière Sangé ( afluent du Wouri). Thèse de Doctorat/Ph.D, Université de Yaoundé I 181.
- Lekeufack Folefack GB, Fomena A (2013) Structure et dynamique des infracommunautés de myxosporidies parasites de Ctenopoma petherici Günther, 1864 (Anabantidae), Clarias pachynema Boulenger, 1903 (Clariidae) et Hepsetus odoe (Bloch, 1794) (Hepsetidae) dans la rivière Sangé au Cameroun. Int J Biol Chem Sci 7: 2301–2316. Link: https://bit.ly/3HTv91L
- Nchoutpouen E, Lekeufack Folefack GB, Fomena A (2011) Structure and population dynamics of Myxobolus infections in wild and cultured Oreochromis niloticus Linnaeus, 1758 in the Noun division (West- Cameroon). J Cell Anim Biol 5: 254-264. Link: https://bit.ly/3gQY2Qg
- Nchoutpouen E (2015) Myxosporidies (Myxozoa:Myxosporea) parasites de quelques téléostéens du Bassin du Noun (Région de l’Ouest, Cameroun): taxonomie et biologie des espèces inféodées à Oreochromis niloticus Linneaus, 1758 et Labeo parvus Boulenger, 1902. Thèse de Doctorat/Ph.D, Université de Yaoundé I 171.
- Olivry JC (1986) Fleuves et rivières du Cameroun. O.R.S.T.O.M. (éd): 733. Link: https://bit.ly/3oTlm4j
- Stiassny MLG, Teugels GG, Hopkins CD (2007) Poissons d’eaux douces et saumâtres de la Basse Guinée, Ouest de l’Afrique Centrale. Collection faune et flore tropicales. IRD (éd.) Paris I: 797. Link: https://bit.ly/34Le6R9
- Abakar O (2006) Les myxosporidies (Myxozoa : Myxosporea) parasites des poissons D’eau douce du Tchad : Faunistique et biologie des espèces inféodées à Oreochromis niloticus (Linné, 1758) et Sarotherodon gallilaeus (Linné, 1758) Cichlidae. Thèse de Doctorat d’État. Université de Yaoundé I 163.
- Lom J, Arthur JR (1989) A guideline for the preparation of species description in Myxosporea. J Fish Dis 12: 151–156. Link: https://bit.ly/3GUi2MH
- Lom J, Dyková I (1992) Myxosporidia (phylum Myxozoa). In Elsevier, Amsterdam – London–NewYork-Tokyo (Ed). Protozoan parasite of fishes developments in aquaculture and fisheries Science. 26: 159-235.
- Lom J, Diková I (2006) Myxozoan genera: definition and notes on taxonomy, life – cycle terminology and pathogenic species. Folia Parasitol 53: 1-36. Link: https://bit.ly/3HW1F3w
- Eiras JC, Molnár KJ, Lu YS (2005) Synopsis of the species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea, Myxobolidae). Syst Parasitol 61: 1-46. Link: https://bit.ly/3GMJKLa
- Eiras JC, Zhang J, Molnár K (2014) Synopsis of the species of Myxobolus Bütschli, 1982 (Myxozoa: Myxosporea, Myxobolidae) described between 2005 and 2013. Syst Parasitol 88: 11-36. Link: https://bit.ly/3sMAGRo
- Fomena A, Bouix G (1997) Myxosporea (Protozoa: Myxozoa) of freshwater fishes in Africa: Keys to genera and species. Syst Parasitol 37: 161-178. Link: https://bit.ly/3HSJFa4
- Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms. Margolis et al. revisited. J Parasitol 83: 575-583. Link: https://bit.ly/3uUr5ee
- Kaur H (2014) Myxozoan infestation in freshwater fishes in wetlands and aquaculture in Punjab, India. Adv Anim Vet Sci 2: 488-502. Link: https://bit.ly/3oRy6sc
- Anu K (2017) Myxozoan parasitic infestation of aquaculture fishes in Punjab, India. 3rd World Conference on Parasitology & Pathogenesis. Chicago, USA. J Bacteriol Parasitol 8: 3.
- Mohammed NI, Rabie SA, Hussein NA, Hussein NM (2012) Infestation of Oreochromis niloticus and Tilapia zilli fresh-water fishes with myxosporean parasites, Qena Province, Egypt. Egypt Acad J Biolog Sci 4: 235-246. Link: https://bit.ly/3gU0B40
- El-Tantawi Sam (1989) Myxosporidian parasites fishes in lakes Dgal Weielki and warniak (Mazurian Lakeland, Poland). I. Survey of parasites. Acta Parasitol Polonica 34: 203–219. Link: https://bit.ly/352QIhK
- Brummer –Korvenkontio H, Valtonen ET, Pugachev ON (1991) Myxosporean parasites in roach, Rutilus rutilus (Linnaeus) from four lakes in central Finland. J Fish Biol 38: 573–586. Link: https://bit.ly/3JqF8Mq
- Ray RA, Holt RA, Bartholomew JL (2012) Relationship between temperature and Ceratomyxa shasta - induced mortality in Klamath River salmonids. J Parasitol 98: 520–526. Link: https://bit.ly/3JnjQzo
- Ray RA, Bartholomew JL (2013) Estimation of transmission dynamics of the Ceratomyxa shasta actinospore to the salmonid host. Parasitol 140: 907-916. Link: https://bit.ly/34Zh58C
- Hallett SL, Bartholomew JL (2008) Effects of water flow on the infection dynamics of Myxobolus cerebralis. Parasitol 13: 371-384. Link: https://bit.ly/3oU7dUB
- Kerans BL, Zale AV (2002) Review: the ecology of Myxobolus cerebralis. In: Bartholomew J.L., Wilson J.C. (eds) Whirling disease: reviews and current topics. Symposium 29. American Fisheries Society. Bethesda, Maryland 145-166.
- Ray RA, Alexander JD, De Leenheer P, Bartholomew JL (2013) Modeling abiotic influences on disease dynamics for the complex life cycle of the Myxozoan parasite Ceratomyxa shasta. PhD thesis. Oregon State. Link: https://bit.ly/3rS1sZu
- Bjork SJ, Bartholomew JL (2009) Effects of Ceratomyxa shasta dose on a susceptible strain of rainbow trout and comparatively resistant Chinook and coho salmon. Dis Aqu Org 86: 29-37. Link: https://bit.ly/3oS8NGA
- Krueger RC, Kerans BL, Vincent ER, Rasmussen C (2006) Risk of Myxobolus Cerebralis infection to rainbow trout in the Madison River, Montana, USA. Ecol Appl 16: 770-783. Link: https://bit.ly/3gMsqeP
- Arndt RE, Wagner EJ, Cannon Q, Smith M (2002) Triactinomyxon production as related to rearing substrate and diel light cycle. In: American Fisheries Society Symposium American Fisheries Society 87–92.
- Blazer VS, Waldrop TB, Schill WB, Ensmore CL, Smith D (2003) Effects of water temperature and substrate type on spore production and release in Eastern Tubifex tubifex worms infected with Myxobolus cerebralis. J Parasitol 89: 21-26. Link: https://bit.ly/3rPTz6T
- Neudecker RA, McMahon TE, Vincent ER (2012) Spatial and temporal variation of whirling disease risk in Montana spring creeks and rivers. J Aqua Anim Health 24: 201–212. Link: https://bit.ly/33n9Uq6
- Milanin T, Eiras JC, Arana S, Maia AAM, Alves AL, et al. (2010) Phylogeny, ultrastructure, histopathology and parasite of Brycon hilarii (Characidae) in the Pantanal Wetland, Brazil. Mem Inst Oswaldo Cruz, Rio de Janeiro 105: 762-769. Link: https://bit.ly/3LAeEdp
- Viozzi G, Flores V (2003) Myxidium biliare sp.n. (Myxozoa) from gall bladder of Galaxias maculatus (Osmeriformes : galaxidae) in patagonia (Argentina). Folia Parasitol 50: 190–194. Link: https://bit.ly/36jFczp
- Gbankoto A, Pampoulie C, Marques A, Sakiti GN, Draman KL (2003) Infections patterns of Myxobolus heterospora in two Tilapia species (teleostei: Cichlidae) and its Potential effects. Diseases Aquatic Organisms 55: 125-131. Link: https://bit.ly/3rTemXl
- Poulin R (2006) Variation in infection parameters among populations within parasite species: Intrinsic properties versus local. Int J Parasitol 20: 887-885. Link: https://bit.ly/3537Vbb
- Simkova A, Jarkovsky J, Koubkouva B, Barus V, Prokes M (2005) Association between fish reproductive cycle and the dynamics of metazoan parasite infection. Parasitol Res 95: 65–72. Link: https://bit.ly/3BqqHp5
- Ellis AE, Robert RJ, Tytler P (1978) The anatomy and physiology of teleost. In: Roberts R.J. Ed., Fish pathology. London, UK, Baillière Tindall 13-54.
- Sitja-Bobadilla A, Alvarez-Pellitero P (1990) Population dynamic of Sphaeropora dicentratrarchi Sitjà – Bobadilla A. et Alvarez – Pelletero P. (1992) and S.testicularis Sitjà – Bobadilla A. et Alvarez – Pelletero P (1990) (Myxosporea : Bivalvulida) infectionsin wild and cultured Mediterranean sea bass (Dicentrarchus labrax L.). Parasitol 106: 39–45. Link: https://bit.ly/3gPmPnT
- Özer A, Wootten R, Shinn AP (2002) Survey on actinosporean types (Myxozoa) belonging to seven collective groups found in a freshwater salmon in Northern Scotland. Folia Parasitol 49: 189-210. Link: https://bit.ly/3gQMTPr
- Abakar O, Bilong CF, Njine T, Fomena A (2007) Structure and dynamics of myxosporean parasites component communities in two fresh water Cichlids in the Chari River (Republic of Chad). Pak J Biol Sci 10: 692-700. Link: https://bit.ly/3LAMrD7

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.



 Save to Mendeley
Save to Mendeley
