International Journal of Aquaculture and Fishery Sciences
Salinity effects on oocytes, fertilized egg density and the reproduction rate of the tilapia Sarotherodon melanotheron heudelotii (Duméril, 1859) in natural and controlled environments
Moussa Guèye1*, Mbaye Tine2 and Justin Kantoussan2
2UFR des Sciences Agronomiques, de l’Aquaculture et des Technologies Alimentaires (UFR S2ATA), Université Gaston Berger (UGB), Route de Ngalléle BP 234, Saint-Louis, Sénégal
Cite this as
Guèye M, Tine M, Kantoussan J (2020) Salinity effects on oocytes, fertilized egg density and the reproduction rate of the tilapia Sarotherodon melanotheron heudelotii (Duméril, 1859) in natural and controlled environments. Int J Aquac Fish Sci 6(2): 035-042. DOI: 10.17352/2455-8400.000054The effects of salinity on the variation of egg and oocyte densities and reproduction rate in the euryhaline tilapia Sarotherodon melanotheron heudelotii was investigated under natural and controlled conditions. The study was conducted on fish from a single population maintained under laboratory conditions for several generations. Fish were exposed to three different salinities conditions: freshwater (0 psu), seawater (35 psu) and hypersaline water (70 psu) while other environmental factors and feeding conditions were held constant. The wild population analyses were conducted on fish collected in environments with different salinities: Guiers lake (0 psu), Gambia estuary (21 psu), Hann Bay (35 psu) and Saloum estuary (54-130 psu). In both natural and controlled environments, the eggs and oocyte densities in freshwater and seawater are much higher than the density of water, which ensures a successful fertilization of eggs and therefore lead to a high reproduction. However in hypersaline condition, opposite results are obtained depending on the environment. In natural hypersaline conditions, the reproduction rate remains optimal despite the reduced oocyte density compared to the density of water. By contrast, in controlled hypersaline environment, the reduced egg density is accompanied with a low reproduction rate. These results suggest that under hypersaline conditions, the reduced oocyte density compared to that of water can prevent fertilization and reduce the reproduction rate. The high reproduction rate obtained in the hypersaline zone of the Saloum estuary in the wild suggests an adoption of a reproductive strategy consisting of a short migration of the spawners towards the oligohaline zones to ensure a successful reproduction.
Introduction
Tilapias are characterized by their robustness and their flexibility that allowed them to colonize coastal marine, estuarine and freshwater environments [1]. They are opportunistic species in their feeding habits and have the ability to reproduce in wide range of environmental conditions [2-4]. This plasticity of the reproduction is often attributed to the adoption of a specific reproductive strategy which consists in producing a large number of small eggs or a small number of large eggs depending on the environmental conditions [5-7]. Environmental factors affecting tilapia reproduction in the wild or under controlled conditions include temperature, oxygen and salinity. Although their remarkable tolerance to salinity variations, few tilapia species are able to reproduce in seawater or hypersaline waters [8-11].
The tilapia Sarotherodon melanotheron heudelotii is a euryhaline species that tolerate high salinities. The species occurs abundantly in West African ecosystems in a wide range of environmental salinities [12-15]. It is found in estuaries such as the Saloum estuary in Senegal where it is exposed to salinities ranging from brackish water to hypersaline waters (up to 130 psu in the upstream part of the estuary) and to marked seasonal variations in salinity [12,16,17]. The reproduction success in these extremely saline conditions is facilitated by the specific strategies adopted by the species but also to the success of egg fertilization, which is a very important step in improving the larvae recruitment of fish species [17]. Fertilization of eggs in both natural and controlled environments occurs on an aqueous substrate (water) and is possible only when the density of eggs is higher than that of water. In general, the density of eggs in freshwater and seawater is higher than that of water [18]. However, when the salinity exceeds a certain threshold, the density of the water could exceed that of the eggs.
The main question now is how do fish adapt to ensure the fertilization of eggs laid by females when the salinity increases? Do they adjust the density of their oocytes in controlled or natural hyperhaline environment or do they migrate from hyperhaline to oligohaline zones to ensure reproduction in natural environments? These questions need to be seriously answered, by performing rigorous analyses. This will allow knowing the real adaptation mechanisms adopted by S. melanotheron heudelotii under hypersaline conditions to ensure its reproduction and therefore its sustainability. In has been demonstrated that egg density of S. melanotheron heudelotii individuals reared in freshwater and seawater environments differ significantly [18]. Then egg density of the species in the upstream part of the Sine Saloum estuary may be lower compared to that of water, which could be a limiting factor for fertilization and for the success of the reproduction.
The study aimed at investigating the impact of salinity on the oocyte and egg density and reproduction rate of both wild and experimental population of S. melanotheron heudoletii. The experiments were conducted on fish that are from a single population maintained under laboratory conditions for several generations. Fish were exposed to three different salinities conditions: freshwater (0 psu), seawater (35 psu) and hypersaline water (70 psu) while other environmental factors and feeding conditions were held constant. The wild population analyses were conducted on fish collected in environments with different salinities: Guiers lake (0 psu), Gambia estuary (21 psu), Hann bay (35 psu) and Saloum estuary (54-130 psu). More specifically, we assessed whether exposition to these salinities in both experimental and natural conditions induce differences in egg density and reproduction rate.
Materials and methods
Sampling of wild populations
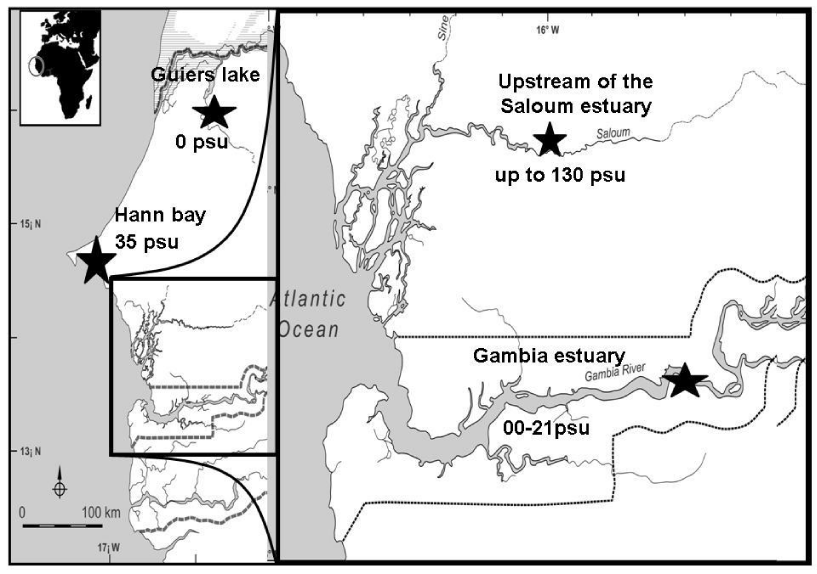
Fish samples were monthly collected at the locations (Figure 1) of Hann bay and Guiers lake in Senegal from January 2003 to august 2004 whereas, in the Saloum and Gambia estuaries, samples were collected during the reproduction period since the reproduction cycle of the species in these ecosystems was clearly determined by previous studies [19, 20]. Thus, eight sampling campaigns were conducted in the Saloum estuary during the reproductive period of the species (one in October 2002; three in March, June and Jury 2003 and four in May, June, July and August 2004). The salinity of each location was measured in situ with a refractometer (ATAGO). Fish were sampled by local fishermen using beach seine net or castnet with small mesh size. After capture, all individuals were killed by anesthetization with a lethal dose of 2-phenoxyethanol, and then preserved in 95% ethanol until processing by dissection. In the laboratory, fish were measured (fork length, FL, in mm), weighed (total mass, W, in g), sexed and the stage of gonad maturity was recorded according to sexual maturity scale of fish described by Legendre and Ecoutin [5]. According to these authors, six maturity stages can be distinguished in the tilapia S. melanotheron. The stage 1 corresponds to immature individuals, the stage 2 to beginning of maturation and stage 3 to mature individuals. Stage 4 corresponds to females ready to reproduce, stage 5 to ripe females and stage 6 to post-spawning individuals. The gonads were extracted, weighted and then preserved in 95% ethanol.
Oocyte density measurements
The total number of samples analyzed was 122, 56, 21, and 122 for the Saloum estuary, Hann Bay, Guiers Lake and the Gambia estuary, respectively (Table 1). The oocyte weight, which is most determinant parameter for the measurements of oocyte density, was determined from these samples for each location. The gonads of wild fish used for the study of oocyte density must complete their maturation to avoid any biased comparison. Consequently, only female gonads of stage 4 and 5 were chosen for the estimation of the density of oocytes. Oocytes measurements in wild females were carried out with samples preserved in alcohol for several weeks. Indeed, a previous comparison of the size of oocytes (diameter and weight) stored in alcohol for several weeks and freshly collected oocytes from the same gonad did not revealed significant difference (p > 0.05) (Table 2). Thus, the storage in alcohol does not affect the quality of the samples and the comparisons will not be biased.
The average weight of an oocyte was determined from the weight of 100 oocytes weighed with a balance accurate to a one-hundredth of a gram. These 100 oocytes are then placed in a 10 ml tube filled with water at a given level. The volume occupied by these 100 oocytes is read, which allows deducing the average oocyte volume and then the average oocyte density.
with Do = oocyte density; Wo = oocyte weight and Vo = oocyte volume
Experimental design
Experiments were performed on adult individuals obtained by natural breeding. The initial broodstock of these individuals was collected from the Niayes zone (Dakar, Senegal) and maintained at GAMET (Groupe Aquaculture Méditerranéenne Et Tropicale) experimental facility in Montpellier, France. Fish were reared during 16 weeks in duplicate treatment tanks of 430 liters each at three different salinities (freshwater: 0 psu, seawater: 35 psu and hypersaline water: 70 psu). Each tank was equipped with a thermostat to maintain a constant temperature of 27°C. The tanks functioned within a recirculated thermoregulated water system and each of them was equipped with mechanical and biological filters that maintained water quality high. Water was aerated vigorously to ensure optimal oxygen saturation. All tanks were initially filled with freshwater from a storage tank and then salt was progressively added until the desired salinity (35 or 70 psu) was reached. The commercial salt mixture that contains all the components of seawater was initially dissolved in a bucket with water from the system before being diluted into the tank. For the hypersaline tanks, the salinity was increased by 2g of salt per liter of water every day until 70 psu. Before stocking the tanks, fish were anesthetized by placing them in a bath containing 2-phenoxyethanol at a concentration of 0.3 mg/l. They were then measured, weighed and marked individually with an electronic mark (PIT tag). Each tank was filled with ten fish comprised of five females and five males of equivalent size. Portions of PVC pipes were placed at the bottom of the tanks to provide shelter and reduce aggressiveness between individuals of this territorial fish species. Fish were fed with commercial processed fish feed containing 40% crude proteins and distributed six days a week for a fixed ration equivalent to 0.5-1% of the biomass. Water was partially changed (about 1/3 of volume) at regular intervals (when the quality was poor) to maintain the quality high. The effective duration of the experimental follow-up phase was ten weeks and the phase of rise in salinity from 35 to 70 psu (considered as an acclimation phase) took six weeks. All individuals were monitored weekly to collect eggs in the male mouth brooders.
Physicochemical parameters (salinity, temperature, dissolved oxygen, ammonia and nitrite) were monitored regularly. Salinity was measured and adjusted once or twice per week using an optical refractometer. The water temperature of the tanks was set at 27°C and maintained constant during the whole experimentation period. The concentration of dissolved oxygen was maintained around the saturation. These two parameters were monitored once or twice every week with an oximeter probe used for simultaneous measurements of water temperature and dissolved oxygen. The rate of nitrite and ammonium were controlled once or twice a week depending on the state of pollution in the tanks. Control of nitrate and nitrite was performed using a commercial kit used for simultaneous measurements of nitrite and ammonium concentrations.
Control of incubation and egg collection
The fish were monitored at the beginning of each week. During each control, the male incubators were identified by morphological and ethological criteria such as swelling of the lower part of the oral cavity, lack of aggressiveness, reduced feeding activity and mobility. Male incubators were captured with a net, and then forced to release the offspring in a water bucket by gently opening the mouth. The eggs were immediately recovered and stored in tubes containing water from the same tank.
Treatment of collected eggs
After collection, eggs were maintained alive in gently aerated water at their appropriate salinity. Developmental stages of eggs were immediately determined by observation under an optical microscope, based on the Shaw and Aronson [21] scale in Tilapia macrocephala (synonym of the S. melanotheron). The embryonic development of S. melanotheron includes four major stages from fertilization to hatching [22]. These four stages are: the young stage which corresponds to eggs that have just (or after few hours) been fertilized, the melanophore stage characterized by the appearance of the first melanophores on the surface of the eggs, the eyed stage which is characterized by the pigmentation of the optic buds and the hatching stage which corresponds to the hatching of the egg.
The diameter of the eggs was determined by image analysis with the ImageJ software (Wayne Rasband, National Institutes of Health, USA). The eggs were then counted manually and weighed to determine their total mass. A sample of 100 eggs was removed, weighed and the volume determined for the determination of egg density. These 100 eggs are then placed in a 10 ml test tube filled with water at a given level. The volume occupied by these 100 eggs was read, which allowed determining the average egg volume and then the average egg density.
The average egg density in freshwater, seawater and hypersaline water was calculated for each of these four stages. The average egg densities of each stage in each salinity condition (freshwater, seawater and hypersaline water) were compared.
The average egg density all stages combined and that of each stage were calculated in each salinity condition using the following formula:
with DE = egg density; WE = egg weight and VE = egg volume
Measurements of reproduction rate
The number of incubations per individual over time was calculated for each salinity condition. The reproduction rate at each level of salinity corresponds to the number of incubations relative to the number of females or males. This reproduction rate is indicated by the percentage of male incubators observed during successive samplings carried out during the experiment. This percentage also gives an idea of the number of sexually active females. The reproduction rate of individuals from different populations was determined in natural and controlled environments.
In natural environments, the reproduction rate was defined as the number of mature females compared to the total number of females. The following formula was used to calculate the reproduction rate in natural populations:
with Rn = reproduction rate in natural environments; Nm = number of mature males and Sm = total number of males sampled.
Under controlled conditions, the reproduction rate was defined as the number of males in incubation compared to the total number of males.
with Rc = reproduction rate in controlled environments; Ni = number of males in incubation and Sm = total number of males.
The average number of eggs incubated by males in freshwater, seawater and hypersaline water was estimated. The quality of the incubation was estimated by calculating the proportion of white eggs present within the whole incubated eggs. White eggs are either unfertilized eggs or fertilized eggs that have aborted during embryonic development. The number of white eggs compared to the total number of eggs incubated could provide indication on the quality of the eggs produced.
Statistical analysis
Comparisons of oocyte densities between sites in natural environment and salinity level in controlled conditions were performed using the multiple comparison tests by Duncan [23] and kruskal wallis nonparametric tests [24]. The same tests were used for the comparisons of the reproduction rate.
Results
Density of oocytes
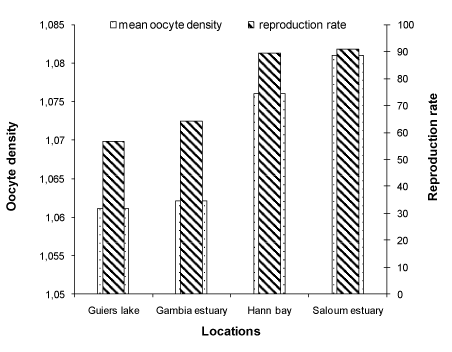
The average density of oocytes calculated in the sampling locations are 1.061 ± 0.0.03, 1.062 ± 0.50, 1.076 ± 0.28 and 1.081 ± 0.02 for the Guiers lake, Gambia estuary, Hann bay and the upstream Saloum, respectively (Table 1). The comparison of the oocyte density between populations did not show significant difference (Kruskal Wallis test, p > 0.05). In all sampling locations (Saloum and Gambia estuaries, Hann bay and Guiers lake), the density of oocytes was always higher than the density of water. On the other hand, at the upstream Saloum, we note that if the salinity exceeds 81, the density (1.081) may be higher than that of the laid oocytes. The oocyte density can be a limiting factor for the quality and success of fertilization, and therefore for reproduction and recruitment.
Density of fertilized eggs
The average egg densities calculated in the three experimental salinity conditions are 1.049 ± 0.02, 1.044 ± 0.03 and 1.065 ± 0.05 in freshwater, seawater and hypersaline water, respectively (Table 3). Comparisons of the egg density between these salinity conditions did not revealed significant difference (Kruskal Wallis test, p > 0.05). Comparison of egg density between developmental stages also revealed no significant difference (Kruskal Wallis, p > 0.05).
Egg density and developmental stage
The results of the analysis of egg density for each developmental stage are indicated in table 4. The comparisons of the egg density in each salinity condition did not show significant difference (Table 4) between the four embryonic stages (young, melanophore, eyed- and hatching stages). The egg density of young stage did not show significant difference between freshwater, seawater and hypersaline water (Table 4). Likewise, the comparison of egg density of melanophore stage did not reveal significant difference between salinity conditions. Similar results were obtained for the eyed-stage and hatching stage.
Reproduction rate
The reproduction rates determined in the wild populations are 64.10%, 89.28% and 90.10% at Guiers lake (freshwater), Gambia estuary (brackish water), Hann bay (seawater) and in the upstream Saloum estuary (hypersaline water), respectively (Table 5). The reproduction rate in wild populations was significantly higher at the Hann bay and Saloum estuary locations and lower at the Guiers lake (Kruskal Wallis test, p < 0.05). The reproduction rate in the wild populations increases significantly with the ambient salinity.
Under controlled conditions, the percentage of incubation estimated using the number of females in the different salinities is 0.30 ± 0.06, 0.33 ± 0.13 and 0.16 ± 0.10 in freshwater, seawater and in hypersaline water, respectively. This percentage calculated using the number of males in the different salinity levels is 0.30 ± 0.06 and 0.26 ± 0.098 in freshwater and seawater, respectively whereas it is 0.08 ± 0.06 in hypersaline water. The average reproduction rates are 28.70%, 32.80% and 1.66% in freshwater, seawater and in hypersaline environment, respectively (Table 5). The reproduction rate was significantly higher in freshwater and seawater and lower in hypersaline water. There was no significant differences in the reproductive rate between freshwater and seawater (Table 5).
Number of eggs incubated and number of eggs per incubation
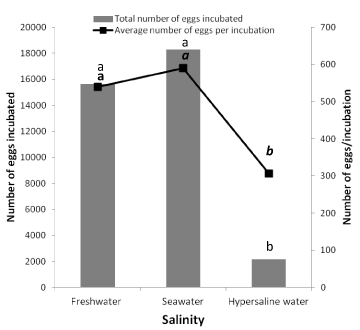
The total number of eggs incubated was significantly higher (Kruskal Wallis test, p < 0.05) in freshwater and seawater and lower in hypersaline water (Figure 2). There is no significant difference in the number of incubated eggs between freshwater and seawater. Likewise, the total number of eggs per incubation was higher in freshwater and seawater compared to hypersaline water where the lowest number of eggs per incubated was recorded (Figure 2). The number of eggs per incubation was not significantly different between freshwater and seawater.
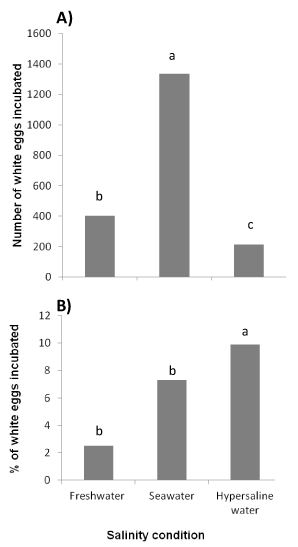
The number of white eggs incubated was significantly higher (Kruskal Wallis test, p > 0.05) in seawater compared to freshwater and hypersaline water (Figure 3A). It was lower in hypsersaline water compared to freshwater. On the other hand, the percentage of white eggs incubated was higher in hypersaline water and seawater and lower in freshwater (Figure 3B). The percentage of white eggs incubated was not significantly different between freshwater and seawater.
Patterns of density and reproduction rate
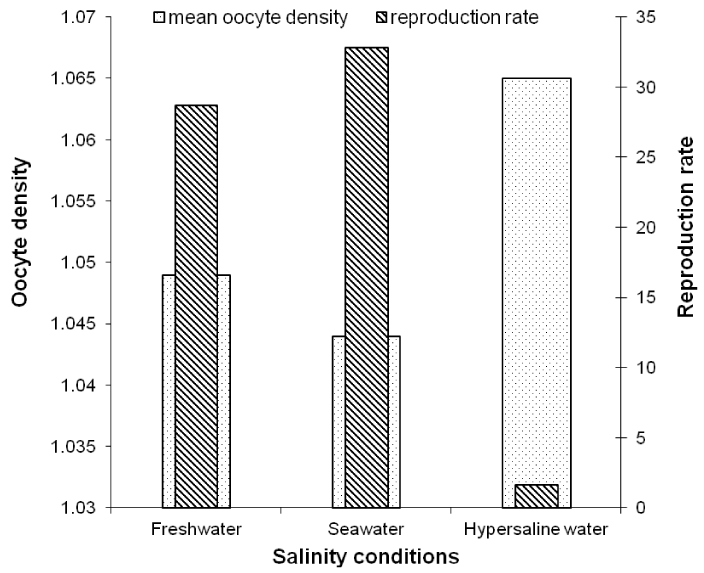
There is an inverse relationship of the density of oocytes and the rate of reproduction with the ambient salinity in the controlled conditions (Figure 4). The reproduction rate decreases with the salinity whereas the oocytes density increases as a function of the ambient salinity. While no significant difference (Kruskal Wallis test, p > 0.05) in oocyte density was found between salinity conditions (Table 3, Figure 4), the reproduction rate was significantly lower in hypersaline water (Kruskal Wallis test, p > 0.05) compared to freshwater and seawater (Table 5, Figure 4). There was no significant difference in reproduction rate between freshwater and seawater.
The density of eggs in wild populations was not significantly different between locations (Kruskal Wallis test, p > 0.05), but there is an increasing tendency with the ambient salinity (Figure 5). The reproduction rate in wild population increases as a function of ambient salinity.
Discussion
The density of oocytes and fertilized eggs are very important reproductive parameters that can provide indications on the success of fertilization and the survival of fertilized eggs [25-27]. The densities of oocytes and fertilized eggs calculated in freshwater and seawater in both natural and controlled environments in this study are always higher than the density of water. Even at salinities much higher than seawater (up to 50-60 psu), the density of fertilized eggs or oocytes remains higher than that of water in both controlled and natural environments. On the other hand, in natural and controlled hypersaline environments, the density of water can exceed that of oocytes or fertilized eggs when the salinity exceeds 70 psu. These results are in accordance with a previous study that has demonstrated that the oocytes density of S. melanotheron heudelotii was identical between freshwater and seawater and significantly exceeded the density of water [18]. Thus, in Guiers lake (freshwater), Hann bay (seawater) and Gambia (brackish-water) ecosystems where the density of oocytes is much higher than that of water, there is no risk of oocytes to float to the water surface, which can prevent their fertilization. By contrast, in the upstream of the Saloum estuary (hypersaline environment) where the density of water most often exceeds that of oocytes, these later may float to the surface of water. Despite the lower oocyte density compared to that of water, the populations of S. melanotheron heudelotii successfully reproduce in this ecosystem even at extremely high salinities (up to 130 psu). The lower reproduction rates recorded at these salinities can be explained by the fact that most of the eggs were not fertilized.
The density of fertilized eggs in rearing environments is much higher than the density of water in freshwater and seawater and lower than that of water in hyper saline conditions. By contrast, this lower density of fertilized eggs compared to that of water is associated with a low reproduction rate contrary to what was observed in upstream of the Saloum estuary where the low density of fertilized eggs is associated with high reproduction rate. This reduced reproductive success of S. melanotheron heudelotii in hypersaline condition in rearing environment can be explained either by a low fertilization rate of oocytes or by a poor development success of fertilized eggs [28, 29]. Indeed, the low density of eggs relative to that of water may cause the low fertilization rate in this hypersaline condition. Egg density in tilapia should not vary greatly before and after fertilization. Indeed, the perivitelline space is very small and its formation does not induce large variations in density between the oocytes and the fertilized eggs (Legendre, pers. Comm.). Consequently, most of the eggs laid are not fertilized, which could explain the low incubation rate (low number of eggs incubated) of individuals reared in hypersaline conditions. This interpretation is in accordance with the presence of a larger amount of white eggs encountered in hypersaline water during egg incubation. These white eggs would be unfertilized oocytes which have to float because of their low density. They can also be embryonated eggs that aborted during embryogenesis. On the other hand, since the fertilized eggs do not undergo constraints of the external environment during their incubation in the oral cavity of the male, a decreased fertilization rate due to a low egg density seems to be the more valuable cause of the decline in reproduction in hypersaline controlled condition. However, the success of the reproduction in natural hypersaline condition associated with the oocyte densities lower than that of water despite the high salinity conditions, could reflect migration strategies of the spawners towards the less salty water to reproduce.
In conclusion, this study shows that the reproductive function is one of the most plastic life history traits in the tilapia S. m. heudelotii. Reproductive characteristics, such reproduction rate, number of incubated eggs and egg density varied in wild populations of this species depending on the ecosystem studied. A comparative experimental approach of reproductive parameters allowed highlighting the effects of changes in ambient salinity on reproductive parameters. Indeed, fish reared in hypersaline environment displayed a lower incubation frequency and number of incubated eggs than those reared in freshwater and seawater. It can, therefore, be concluded that the reproductive function is one of the most sensitive vital functions to salinity in S. m. heudelotii.
- Kirk RG (1972) A review of recent developments in Tilapia culture, with spezial reference to fish farming in the heated effluents of power stations.Aquaculture 1: 45-60. Link: https://bit.ly/3ehw5hx
- Tesfahun A, Temesgen M (2018) Food and feeding habits of Nile tilapia Oreochromis niloticus (L.) in Ethiopian water bodies: A review. International Journal of Fisheries and Aquatic Studies 6: 43-47. Link: https://bit.ly/3cS4PWl
- El-Sayed AFM (2006) Tilapia Culture in Salt Water: Environmental Requirements, Nutritional Implications and Economic Potentials. En: Editores: L. Elizabeth Cruz Suárez, Denis Ricque Marie, Mireya Tapia Salazar, Martha G. Nieto López, David A. Villarreal Cavazos, Ana C. Puello Cruz y Armando García Ortega. Avances en Nutrición Acuícola VIII .VIII Simposium Internacional de Nutrición Acuícola. 15 - 17 Noviembre. Universidad Autónoma de Nuevo León, Monterrey, Nuevo León, México ISBN 970-694-333-5.
- Baroiller JF, Toguyeni A (2009) The Tilapiini Tribe: Environmental and Social Aspects of Reproduction and Growth. Fisheries and Aquaculture- Vol III 2009, Edited by Patrick Safran 230-258. Link: https://bit.ly/3bSRRGu
- Legendre M, Ecoutin JM (1996) Aspects of the reproductive strategy of Sarotherodon melanotheron: comparison between a natural population (Ébrié lagoon, Côte d’Ivoire) and different cultured populations. In: Pullin, RSV, Lazard, J, Legendre, M, Amon Kothias, J;-B, Pauly, D (Eds), Proceeding of the 3rd international symposium on tilapia in aquaculture ICLARM, Manila:320-325.
- Duponchelle F (1997) Reproduction du tilapia (Pisces, Cichlidae) Oreochromis niloticus (Linnaeus, 1758) dans les retenues artificielles de Côte d’Ivoire: analyse comparative des modalités de reproduction et approche expérimentale de leur déterminisme. PhD Thesis Université de Bretagne Occidentale, Rennes.
- De Silva SS (1986) Reproductive biology of Oreochromis mossambicus populations of man-made lakes in Sri Lanka: a comparative study. Aquaculture and Fisheries Managment 17: 31-38. Link: https://bit.ly/2zgPVKO
- Popper D, Lichatowich T (1975) Preliminary success in predator control of Tilapia Mossambica. Aquaculture 5: 213-214. Link: https://bit.ly/36iretk
- Hora SL, Pillay TVR (1962) Handbook of fish culture in the Indo-Pacific region. FAO Fish Biol Tech Pap 204. Link: https://bit.ly/36maaTA
- Chervinski J (1982) Environmental physiology of tilapias. In: Pullin, R.S.V., Lowe-McConnell, R.H. (Eds.), The Biology and Culture of Tilapias, ICLARM Conference Proceedings, vol. 7. International Center for Living Aquatic Resources Management, Manila, Philippines 432. Link: https://bit.ly/3cQWDFY
- Albaret JJ (1987) Les peuplements de poissons de la Casamance (Sénégal) en période de sécheresse. Revue d’HydrobiolOGY Tropicale 20: 291-310. Link: https://bit.ly/3d23gFe
- Panfili J, Thior D, Ecoutin JM, Ndiaye P, Albaret JJ (2006) Influence of salinity on the size at maturity for fish species reproducing in contrasting West African estuaries. Journal of Fish Biology 69: 95-113. Link: https://bit.ly/2zT7wZa
- Panfili J, Mbow A, Durand JD, Diop K, Diouf K, et al. (2004) Influence of salinity on the life-history traits of the West African black-chinned tilapia (Sarotherodon melanotheron): comparison between the Gambia and Saloum estuaries. Aquatic Living Resourses 17: 65-70. Link: https://bit.ly/2WQ3RVe
- Falk TM, Teugels GG, Abban EK, Villwock W, Renwrantz L (2003) Phylogeographic patterns in populations of the blackchinned tilapia complex (Teleostei, Cichlidae) from coastal areas in West Africa: support for the refuge zone theory. Molecular Phylogenetics and Evolution 27: 81-92. Link:
https://bit.ly/2z60Ff3 - Diouf K, Panfili J, Labonne M, Aliaume C, Tomas J, et al. (2006) Effects of salinity on strontium: calcium ratios in the otoliths of the West African blackchinned tilapia Sarotherodon melanotheron in a hypersaline estuary. Environmental Biology of Fish 77: 9e20. Link: https://bit.ly/3bRvhOG
- Tine M, McKenzie DJ, Bonhomme F, Durand JD (2011) Salinity-related variation in gene expression in wild populations of the black-chinned tilapia from various West African coastal marine, estuarine and freshwater habitats. Estuarine, Coastal and Shelf Science 91: 102e-109. Link: https://bit.ly/36iAm1a
- Guèye M, Tine M, Kantoussan J, Ndiaye P, Thiaw OT, et al. (2012) Comparative Analysis of Reproductive Traits in Black-Chinned Tilapia Females from Various Coastal Marine, Estuarine and Freshwater Ecosystems. PLoS ONE 7: e29464. Link: https://bit.ly/3bTF7iW
- Gilles S, Zamora L, Amiel C, Rodriguez JL (2004) Comparative study of reproductive characteristics of the euryhaline tilapia, Sarotherodon melanotheron heudelotii in fresh and sea waters. Journal of aquaculture in the tropics 19: 277-284. Link: https://bit.ly/3bPrelV
- Panfili J, Durand JD, Mbow A, Durand JD, Diop K, Diouf K, et al. (2004) Influence of salinity on the life-history traits of the West African black-chinned tilapia (Sarotherodon melanotheron): Comparison between the Gambia and Saloum estuaries. Aquatic living Resources 17: 65-74. Link: https://bit.ly/3bPrdhR
- Diouf K (2003) Etude comparative de la reproduction des populations de Sarotherodon melanotheron heudelotii (Dumeril, 1859) des estuaire du Saloum et de la Gambie. DEA de Biologie Animale, Institut des Sciences de l’Environnement de l’UCAD 56p.
- Shaw ES, Aronson LR (1954) Oral incubation in Tilapia macrocephala.. Bulletin of the Americain Museum of Natural History 103. Link: https://bit.ly/3bJsGpW
- Legendre M, Trébaol L (1996) Mouthbrooding efficiency and spawn-ing frequency of Sarotherodon melanotheron (Rüppel, 1852) inculture environments (Ebrié Lagoon, Côte d’Ivoire). In: PullinR.S.V., Lazard J., Legendre M., Amon Kothias J.-B., Pauly D.(Eds.). The Third Symposium on Tilapia in Aquaculture Manila,ICLARM Conf Proc 41: 339-334.
- Scherrer B (1984) Biostatistique. Gaëtan Morin, Boucherville. 850. Link: https://bit.ly/2yjX9NA
- Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. Journal of the American Statistical Association 47: 583-621.Link: https://bit.ly/3gbzLTo
- Lehtonen TK (2015) Density effects on fish egg survival and infections depend on salinity. Marine Ecology Progress Series 540: 183-191.Link: https://bit.ly/3g7ytJe
- Bobe J, Labbe C (2010) Egg and sperm quality in fish. General and Comparative Endocrinology 165: 535-548. Link: https://bit.ly/3gaKEF6
- Bobe J (2015) Egg quality in fish: Present and future challenges Animal Frontiers 5: 66-72. Link: https://bit.ly/3bNtjPm
- Guèye M (2006) Stratégie de la reproduction du tilapia estuarien Sarotherodon melanotheron heudelotii (Duméril, 1859) en milieux naturels: approche expérimentale du rôle de la salinité sur la fonction de reproduction. Doctorat de troisième cycle Université Cheikh Anta Diop, Dakar.
- Duguè R, Baras E, Gueye M, Avarre JC, Combes Y, et al. (2014) Egg production in the euryhaline tilapia, Sarotherodon melanotheron heudelotii, experimentally maintained in fresh, sea and hypersaline waters. Aquatic Living Resources 27:63-72. Link: https://bit.ly/3cPrnHr

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
