Global Journal of Zoology
Spider limb regeneration: Cost and benefits
Akamu Jude Ewunkem*, Agee Kyle and Rivera Kayse
Department of Biological Sciences, Winston Salem State University, Winston Salem, North Carolina, USA
*Corresponding author: Akamu Jude Ewunkem, Assistant Professor, Molecular and Cellular Biology, Department of Biological Sciences, Winston Salem State University, Winston Salem, North Carolina, USA, Tel: (+1) 336-750-2201; Fax: Fax: 336-750-3094; E-mail: ewunkemaj@wssu.edu
DOI: 10.17352/gjz.000023
Received: 25 July, 2022 | Accepted: 03 August, 2022 | Published: 04 August, 2022
Keywords: Regeneration; Spider; Heteropoda venatoria; Autotomy
Cite this as
Ewunkem AJ, Kyle A, Kayse R (2022) Spider limb regeneration: Cost and benefits. Glob J Zool 7(1): 015-018. DOI: 10.17352/gjz.000023
Copyright
© 2022 Ewunkem AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
One of the most phenomenal innate powers of organisms is their ability to repair injured or lost body parts better known as regeneration. Regeneration is the natural process of replacing or restoring missing body parts and is a primary attribute of all living organisms. Studying regeneration may be a potential for use in biomedical sciences. Closely associated with regeneration in the arthropods is autotomy an anti-predator behavior in animals. Autotomy is one of the most remarkable features of many arthropods, however, autotomy is not well known in spiders. Also, the cost and benefits of regeneration of lost limbs have not received much attention in recent decades. Understanding the cost-benefits dynamics of regeneration of lost limbs in spiders will undoubtedly increase our understanding of the evolutionary trajectory. Spiders are remarkable for their ability to regenerate limbs with apparent ease during young stages. We used the huntsman spider Heteropoda venatoria as a model to address this. This mini-review also addresses the ecological implications of regeneration for spiders themselves. The study is of great importance because understanding the molecular mechanisms associated with regeneration could be exploited to reconstitute regeneration from constituent parts.
Introduction
Living organisms must be resilient to survive. Animals usually suffer injuries at some point in their lifetimes which may affect certain behaviors. The speed and success of their recovery may be consequential i.e., may determine whether the animals live or die. Most organisms have an amazing ability to recover from substantial bodily damage, a secret of tapping into the powers of recovery: regeneration [1]. Regeneration is the process by which lost or damaged cells, tissues, organs, or limbs are re-formed from the remaining tissue.
The astonishing ability of some animals to replace body parts following injury has captured the interest of humans for millennia and has fascinated scientists for years because it can be exploited. The restorative properties of organisms have drawn the attention of great minds of ancient like the regenerating eye of the ancient Egyptians’ god Horus; the regenerative features of the Aztec deity Xolotl and the regenerating liver of the ancient Greeks’ Prometheus just to name a few [2]. Regeneration is reckoned as a powerful tool for biologists to investigate the fundamental aspects of development [3]. The goal to understand the regenerative potential of organisms is to provide regenerative medicines which can be used to replace or repair damaged limbs by inducing endogenous regenerative responses in humans and other organisms. Regeneration typically involves cell recruitment, growth proliferation, and differentiation which require signaling from limb nerves (as described in the next section) to replace missing tissues [4].
To regrow lost or injured body parts is a reality for a significant proportion of living organisms and arthropod limbs are no exception. Arthropods, (phylum Arthropoda) are the largest phylum in the animal kingdom and are commonly divided into four subphyla which include, Crustacea (crustaceans), Hexapoda (insects and springtails), Myriapoda (millipedes and centipedes) and Chelicerata (arachnids). Chelicerata contains more than 100 000 described species recent species and the number continues to increase substantially year on year [5]. Chelicerata contains animals such as scorpions, harvestmen, mites, ticks, and spiders. Spiders are exceptionally diverse and abundant with more than 50,000 known species with 130 families found in habitats in terrestrial ecosystems where they serve as beneficial predators keeping populations of many insect pests in check [6-9]. This review will consider not only the regenerative ability of the spiders using the huntsman spider Heteropoda venatoria as a model but also the ecological implications of regeneration of lost limbs.
The hunstman spider, Heteropoda venatoria (Linnaeus, 1767)
The huntsman spiders, which are a part of the family Sparassidae (formerly Heteropodidae), are the eleventh-most diverse family of spiders with 1,319 species described from 91 genera [10]. Members of the family sparassidae are famously known by this name because of their speed and mode of hunting. Furthermore, the sparassidae are identified by their legs, which, rather than being jointed vertically relative to the body, are twisted in such a way that in some attitudes the legs extend forward in a crab-like fashion. Their bodies are on average about 2.5 centimeters long with a leg span of 12.7 cm.
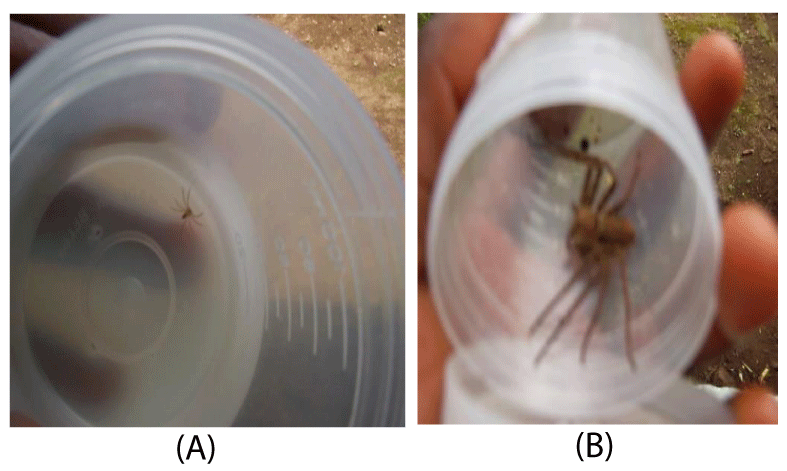
The giant crab huntsman spider, Heteropoda venatoria (L.) is a sit-and-wait predator that consumes a variety of insects. H. venatoria is a large brown spider with a flattened body structure and a leg span of 7 to 12 cm (Figure 1). Sexual dimorphism is highly variable among H. venatoria (Figure 1). Females (Figure 1A) are usually larger than the males (Figure 1B) as physical demands and accompanying energy requirements to produce webs and broods are far greater. Males are smaller because once mature; their only function is to mate. Where males are similar in size to the females, they are generally slenderer, with longer legs. In addition, males have a high contrast pattern on their dorsal shield of prosoma, whereas females are almost uniformly brown [11].
H. venatoria lives in warm climates throughout the world, especially in the tropics and subtropics, and their long flexible legs and flattened bodies enable them to fit surprisingly under loose backs on trees, crevices on under rocks, and slabs of bark on the ground [12-15]. This ability and adaptability of H. venatoria help explain their frequent appearance in marketed bananas in grocery stores each year. Due to several complaints from the international markets relating to banana infestations by H. venatoria, we conducted a study in a commercial banana plantation agroecosystem to generate basic biological and ecological on H. venatoria as a prelude to designing appropriate spider population control strategies [14,15]. Our data showed that H. venatorai are known to be ubiquitous in the banana plantations and thrive in microhabitats such as spaces in the flower bracts, banana bunces, leaf petioles and in loose banana pseudostem barks [14,15].
Courtship, and mating of H. venatoria generally occurred at night. When a male H. venatoria mets a female, he launches into an elaborate courtship dance, including rhythmic flailing of limbs and complex vibrations through stridulation by rubbing the bristles on its first two legs and pedipalps together [15]. Thereafter the male successfully approaches the female and meticulously mounts her (Figure 2) and repeatedly inserts his embolus into her epigynum for about 2 hours 3 minutes
About 15 days after mating, the abdomens of the gravid female H. venatoria becomes distended (Figure 3A). After about 28 days she produces a flat, oval egg sac of white papery silk, and lays about 200 eggs. She carries the egg sac under her body with an aid of the pedipalps (Figure 3B) without eating for about 30 days. However, during the egg incubation period female H. venatoria can be quite aggressive and will rear up in a defensive display if provoked. The female may often moisten tear the egg sac open helping the spiderlings to emerge. To grow, the spiderlings (Figure 4) must shed their hard-exterior exoskeleton through a process known as molting. Each molt was associated with an incremental increase in body size. The spider number of instar molts varies with sex. Females molted 4 additional times compare to the males. The spiderling molted averagely once monthly until they reach sexual maturity, The total developmental duration for the females (~392 days) was significantly longer compared to the males (~304 days) [15]. Molting and regeneration of lost appendages are tightly coupled as demonstrated in the next section.
Limb loss and limb regeneration of huntsman spider, Heteropoda venatoria
In a laboratory study, we demonstrated the phenomenon of regeneration of lost limbs by studying the model organism, H. venatoria. Briefly, the spiderlings or juveniles of the wild-caught H. venatoria were raised in the laboratory. Spiderlings that had accidentally lost their limbs and spiderlings whose limbs were purposely amputated were observed critically after each subsequent molt [15].
Regeneration of lost limb was associated with molting. After subsequent molts, the spiderlings were able to regenerate the lost appendages. After one molt, the new legs appeared smaller than the full-grown size and slowly became indistinguishable from the others. However, fully mature H. venatoria were not able to regenerate their lost legs and amputation was permanent. A discourse on regeneration of loss limb is incomplete without addressing the subject of autotomy. Autotomy, self-induced limb loss, is one of the most effective antipredator mechanisms that allow juveniles to escape if captured [16]. Autotomy has been observed in diverse animal taxa including both invertebrates and vertebrates that detach a variety of body parts [17-20]. Forms of autotomy are autospasy, limb loss by the action of an outside agent against resistance provided by the organism, and autotilly when limb loss is obtained with the assistance of other limbs [21]. The experiments with H. venatoria were more associated with autospasy because it may refer to a situation where the limb could be lost to a predator, or the limb could be lost during molting, or the limb loss could be due do accidental.
Regardless of the form of autotomy, it is of great importance to discuss the ecological implication of autotomy. There are multiple benefits associated with the ability to shed an appendage with the most prevalent being predation avoidance which allows an organism to survive an encounter with a predator. This might be significant in spiders because they use their legs extensively for prey capture, sensory detection of physical restrains of prey (due to the presence of sensitive setae) and constructing webs. Moreover, autotomy can be beneficial since molting can lead to appendages becoming stuck in the exuviating [22]. Also, the legs can be used for mating as seen when a male tufted golden orb weaver spider Nephila inaurata offers a female one of his front legs to snack on during sex to appease the female [23]. However, autotomy can also have some disadvantages. The most obvious cost of autotomy is an impediment to efficient locomotion which can be translated into a decreased survivorship or the ability to forage/capture prey or escape from predators. Furthermore, spiders with autotomized limbs are easier to be preyed upon by other predators, some predators are known to be actively seeking automized arthropods during foraging [24]. Missing appendages may also affect behaviors associated with a reduction in the foraging rate [3].
Insect, crustacean and amphibian models have been used to shed light on the molecular mechanisms of regeneration [25-28]. In Gryllus bimaculatus (Insecta; De Geer, 1773), and Leptuca pugilator (Crustacea; Bosc, 1802), The re-growth of limbs is initiated through the formation of blastema accompanied by proliferation, dedifferentiation, and redifferentiation of blastemal cells [25]. These processes are also associated with transcription factors and ecdysteroid hormone signaling [29].
The regeneration of lost body parts has been widely studied in axolotl salamander Ambystoma mexicanum (Shaw and Nodder, 1798) and entails different growth phases during limb regeneration. The early ‘early tiny limb’ phase involves a new limb with right shape and structure and the ‘late tiny limb’ phase creates a growing limb which will eventually reach the right size. Regenerating tissue occur more rapidly during the early limb phase compared to the late tiny limb’ and growth observed during these phases is due to a decrease in cell death and an increase in cell number and size [30]. Growing evidence shows that intact nerve supply is essential for limbs to regrow and may regulate allometric growth and size of a regenerating limb following amputation [30].
In summary, regeneration is the natural process of replacing or restoring damaged or missing body parts. Although Regeneration is widely spread throughout the animal kingdom, much is not known about arthropods. the largest phylum in the animal kingdom. In the Arthropod, powers of regeneration is for the most part confined to the appendages as observed in crabs, insects and spiders. Spiders are remarkable for their ability to regenerate lost limbs with apparent ease which is often associated with molting. The capacity to regenerate a lost limb has immediate advantages principally connected with permitting an individual to escape. However, once a limb has been lost the spider may become a less effective predator itself which may also lead to a decrease in foraging rates. Invertebrates and vertebrate models provided insight into the molecular mechanisms of regeneration which may have potential uses in medicine, such as treating a variety of injuries and diseases.
- Bideau L, Kerner P, Hui J, Vervoort M, Gazave E. Animal regeneration in the era of transcriptomics. Cell Mol Life Sci. 2021 Apr;78(8):3941-3956. doi: 10.1007/s00018-021-03760-7. Epub 2021 Jan 30. PMID: 33515282.
- Elliott SA, Alvarado AS. Planarians and the History of Animal Regeneration: Paradigm Shifts and Key Concepts in Biology. Methods Mol Biol. 2018;1774:207-239. doi: 10.1007/978-1-4939-7802-1_4. PMID: 29916157.
- Maginnis TL. The costs of autotomy and regeneration in animals: a review and framework for future research. Behavioral Ecology. 2006; 17(5):857-872
- Santos Jr AR, Nascimento VA, Genari SC, Lombello CB. Mechanisms of Cell Regeneration-From Differentiation to Maintenance of Cell Phenotype. Cells and Biomaterials in Regenerative Medicine. 2014; 37-69.
- Dunlop JA. Geological history and phylogeny of Chelicerata. Arthropod Struct Dev. 2010 Mar-May;39(2-3):124-42. doi: 10.1016/j.asd.2010.01.003. Epub 2010 Mar 20. PMID: 20093195.
- Rajeevan S, Kunnath SM, Varghese T, Kandambeth PP. Spider diversity (Arachnida: Araneae) in different ecosystems of the Western Ghats, Wayanad region, India. South Asian Journal of Life Science. 2019; 7(2), 29-39.
- Gajski D, Pekár S. Assessment of the biocontrol potential of natural enemies against psyllid populations in a pear tree orchard during spring. Pest Manag Sci. 2021 May;77(5):2358-2366. doi: 10.1002/ps.6262. Epub 2021 Jan 21. PMID: 33415804.
- Sharma S. Different techniques used for chromosomal analysis of spiders. Recent Advancements and Research in Biological Sciences. 2021;178.
- World Spider Catalog. Natural History Museum Bern. retrieved 2022.
- Gorneau JA, Rheims CA, Moreau CS, Rayor LS. Huntsman spider phylogeny informs evolution of life history, egg sacs, and morphology. Mol Phylogenet Evol. 2022 Sep;174:107530. doi: 10.1016/j.ympev.2022.107530. Epub 2022 May 27. PMID: 35636670.
- Jäger P. Spiders from Laos with descriptions of new species (Arachnida: Araneae). Acta arachnologica. 2007; 56(1):29-58.
- Edwards Jr GB. Huntsman Spider, Heteropoda venatoria (Linnaeus)(Arachnida: Araneae: Sparassidae): EENY-160/IN317. EDIS 2003; (16).
- GB E. Huntsman Spider, Heteropoda venatoria (Linnaeus)(Arachnida: Araneae: Sparassidae). Florida Cooperative Extension Service. Gainesville: University of Florida IFAS. 2009.
- Ntonifor NN, Parr MC, Ewunkem JA. Seasonal abundance and distribution of the huntsman spider, Heteropoda venatoria (Sparassidae: Araneae) in banana agro-ecosystems in Cameroon. Journal of Entomology. 2012; 9(2):79-88.
- Ewunke JA, Ntonifor NN, Parr MC. Bioecology of Heteropoda venatoria (Linnaeus)(Araneae: Sparassidae) and its implications in a tropical banana agroecosystem. Journal of Global Agriculture and Ecology. 2016; 5(3):164-175
- Smith LD. Effects of limb autotomy and tethering on juvenile blue crab survival from cannibalism. Marine ecology progress series. Oldendorf. 1995; 116(1):65-74.
- Fleming PA, Muller D, Bateman PW. Leave it all behind: a taxonomic perspective of autotomy in invertebrates. Biol Rev Camb Philos Soc. 2007 Aug;82(3):481-510. doi: 10.1111/j.1469-185X.2007.00020.x. PMID: 17624964.
- Higham TE, Russell AP, Zani PA. Integrative biology of tail autotomy in lizards. Physiol Biochem Zool. 2013 Nov-Dec;86(6):603-10. doi: 10.1086/673875. Epub 2013 Oct 31. PMID: 24241059.
- Mitoh S, Yusa Y. Extreme autotomy and whole-body regeneration in photosynthetic sea slugs. Curr Biol. 2021 Mar 8;31(5):R233-R234. doi: 10.1016/j.cub.2021.01.014. PMID: 33689716.
- Baban NS, Orozaliev A, Kirchhof S, Stubbs CJ, Song YA. Biomimetic fracture model of lizard tail autotomy. Science. 2022 Feb 18;375(6582):770-774. doi: 10.1126/science.abh1614. Epub 2022 Feb 17. PMID: 35175822.
- Maruzzo D, Bortolin F. Arthropod regeneration. In Arthropod biology and evolution 2013 ; 149-169). Springer, Berlin, Heidelberg.
- Dunoyer LA, Seifert AW, Van Cleve J. Evolutionary bedfellows: Reconstructing the ancestral state of autotomy and regeneration. J Exp Zool B Mol Dev Evol. 2021 Mar;336(2):94-115. doi: 10.1002/jez.b.22974. Epub 2020 Jun 18. PMID: 32558244; PMCID: PMC7746608.
- Neumann R, Schneider JM. Males sacrifice their legs to pacify aggressive females in a sexually cannibalistic spider. Animal behaviour. 2020; 159:59-67.
- Davenport J, Spikes M, Thornton S, Kelly B. Crab eating in the diamondback terrapin Malaclemys terrapin: dealing with dangerous prey. J Mar Bio1992;l72:835–48.
- Das S. Morphological, Molecular, and Hormonal Basis of Limb Regeneration across Pancrustacea. Integr Comp Biol. 2015 Nov;55(5):869-77. doi: 10.1093/icb/icv101. Epub 2015 Aug 20. PMID: 26296354.
- Bando T, Mito T, Hamada Y, Ishimaru Y, Noji S, Ohuchi H. Molecular mechanisms of limb regeneration: insights from regenerating legs of the cricket Gryllus bimaculatus. Int J Dev Biol. 2018;62(6-7-8):559-569. doi: 10.1387/ijdb.180048ho. PMID: 29938767.
- Ožana S, Pyszko P, Dolný A.). Determination of suitable insect part for non‐lethal DNA sampling: case study of DNA quality and regeneration capability of dragonflies. Insect Conservation and Diversity. 2020;13(4):319-327.
- Ren Z, Fu Y, Liu L, Liu X, Wang C. Expression and function of WNT4 involved in larvae development and limb regeneration in Portunus trituberculatus. Journal of Oceanology and Limnology. 2021; 39(1):306-316.
- Elchaninov A, Sukhikh G, Fatkhudinov T. Evolution of regeneration in animals: A tangled story. Frontiers in Ecology and Evolution. 2021; 9:621686.
- Wells KM, Kelley K, Baumel M, Vieira WA, McCusker CD. Neural control of growth and size in the axolotl limb regenerate. Elife. 2021 Nov 15;10:e68584. doi: 10.7554/eLife.68584. PMID: 34779399; PMCID: PMC8716110.
Cite this as
Ewunkem AJ, Kyle A, Kayse R (2022) Spider limb regeneration: Cost and benefits. Glob J Zool 7(1): 015-018. DOI: 10.17352/gjz.000023Copyright
© 2022 Ewunkem AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.One of the most phenomenal innate powers of organisms is their ability to repair injured or lost body parts better known as regeneration. Regeneration is the natural process of replacing or restoring missing body parts and is a primary attribute of all living organisms. Studying regeneration may be a potential for use in biomedical sciences. Closely associated with regeneration in the arthropods is autotomy an anti-predator behavior in animals. Autotomy is one of the most remarkable features of many arthropods, however, autotomy is not well known in spiders. Also, the cost and benefits of regeneration of lost limbs have not received much attention in recent decades. Understanding the cost-benefits dynamics of regeneration of lost limbs in spiders will undoubtedly increase our understanding of the evolutionary trajectory. Spiders are remarkable for their ability to regenerate limbs with apparent ease during young stages. We used the huntsman spider Heteropoda venatoria as a model to address this. This mini-review also addresses the ecological implications of regeneration for spiders themselves. The study is of great importance because understanding the molecular mechanisms associated with regeneration could be exploited to reconstitute regeneration from constituent parts.
Introduction
Living organisms must be resilient to survive. Animals usually suffer injuries at some point in their lifetimes which may affect certain behaviors. The speed and success of their recovery may be consequential i.e., may determine whether the animals live or die. Most organisms have an amazing ability to recover from substantial bodily damage, a secret of tapping into the powers of recovery: regeneration [1]. Regeneration is the process by which lost or damaged cells, tissues, organs, or limbs are re-formed from the remaining tissue.
The astonishing ability of some animals to replace body parts following injury has captured the interest of humans for millennia and has fascinated scientists for years because it can be exploited. The restorative properties of organisms have drawn the attention of great minds of ancient like the regenerating eye of the ancient Egyptians’ god Horus; the regenerative features of the Aztec deity Xolotl and the regenerating liver of the ancient Greeks’ Prometheus just to name a few [2]. Regeneration is reckoned as a powerful tool for biologists to investigate the fundamental aspects of development [3]. The goal to understand the regenerative potential of organisms is to provide regenerative medicines which can be used to replace or repair damaged limbs by inducing endogenous regenerative responses in humans and other organisms. Regeneration typically involves cell recruitment, growth proliferation, and differentiation which require signaling from limb nerves (as described in the next section) to replace missing tissues [4].
To regrow lost or injured body parts is a reality for a significant proportion of living organisms and arthropod limbs are no exception. Arthropods, (phylum Arthropoda) are the largest phylum in the animal kingdom and are commonly divided into four subphyla which include, Crustacea (crustaceans), Hexapoda (insects and springtails), Myriapoda (millipedes and centipedes) and Chelicerata (arachnids). Chelicerata contains more than 100 000 described species recent species and the number continues to increase substantially year on year [5]. Chelicerata contains animals such as scorpions, harvestmen, mites, ticks, and spiders. Spiders are exceptionally diverse and abundant with more than 50,000 known species with 130 families found in habitats in terrestrial ecosystems where they serve as beneficial predators keeping populations of many insect pests in check [6-9]. This review will consider not only the regenerative ability of the spiders using the huntsman spider Heteropoda venatoria as a model but also the ecological implications of regeneration of lost limbs.
The hunstman spider, Heteropoda venatoria (Linnaeus, 1767)
The huntsman spiders, which are a part of the family Sparassidae (formerly Heteropodidae), are the eleventh-most diverse family of spiders with 1,319 species described from 91 genera [10]. Members of the family sparassidae are famously known by this name because of their speed and mode of hunting. Furthermore, the sparassidae are identified by their legs, which, rather than being jointed vertically relative to the body, are twisted in such a way that in some attitudes the legs extend forward in a crab-like fashion. Their bodies are on average about 2.5 centimeters long with a leg span of 12.7 cm.
The giant crab huntsman spider, Heteropoda venatoria (L.) is a sit-and-wait predator that consumes a variety of insects. H. venatoria is a large brown spider with a flattened body structure and a leg span of 7 to 12 cm (Figure 1). Sexual dimorphism is highly variable among H. venatoria (Figure 1). Females (Figure 1A) are usually larger than the males (Figure 1B) as physical demands and accompanying energy requirements to produce webs and broods are far greater. Males are smaller because once mature; their only function is to mate. Where males are similar in size to the females, they are generally slenderer, with longer legs. In addition, males have a high contrast pattern on their dorsal shield of prosoma, whereas females are almost uniformly brown [11].
H. venatoria lives in warm climates throughout the world, especially in the tropics and subtropics, and their long flexible legs and flattened bodies enable them to fit surprisingly under loose backs on trees, crevices on under rocks, and slabs of bark on the ground [12-15]. This ability and adaptability of H. venatoria help explain their frequent appearance in marketed bananas in grocery stores each year. Due to several complaints from the international markets relating to banana infestations by H. venatoria, we conducted a study in a commercial banana plantation agroecosystem to generate basic biological and ecological on H. venatoria as a prelude to designing appropriate spider population control strategies [14,15]. Our data showed that H. venatorai are known to be ubiquitous in the banana plantations and thrive in microhabitats such as spaces in the flower bracts, banana bunces, leaf petioles and in loose banana pseudostem barks [14,15].
Courtship, and mating of H. venatoria generally occurred at night. When a male H. venatoria mets a female, he launches into an elaborate courtship dance, including rhythmic flailing of limbs and complex vibrations through stridulation by rubbing the bristles on its first two legs and pedipalps together [15]. Thereafter the male successfully approaches the female and meticulously mounts her (Figure 2) and repeatedly inserts his embolus into her epigynum for about 2 hours 3 minutes
About 15 days after mating, the abdomens of the gravid female H. venatoria becomes distended (Figure 3A). After about 28 days she produces a flat, oval egg sac of white papery silk, and lays about 200 eggs. She carries the egg sac under her body with an aid of the pedipalps (Figure 3B) without eating for about 30 days. However, during the egg incubation period female H. venatoria can be quite aggressive and will rear up in a defensive display if provoked. The female may often moisten tear the egg sac open helping the spiderlings to emerge. To grow, the spiderlings (Figure 4) must shed their hard-exterior exoskeleton through a process known as molting. Each molt was associated with an incremental increase in body size. The spider number of instar molts varies with sex. Females molted 4 additional times compare to the males. The spiderling molted averagely once monthly until they reach sexual maturity, The total developmental duration for the females (~392 days) was significantly longer compared to the males (~304 days) [15]. Molting and regeneration of lost appendages are tightly coupled as demonstrated in the next section.
Limb loss and limb regeneration of huntsman spider, Heteropoda venatoria
In a laboratory study, we demonstrated the phenomenon of regeneration of lost limbs by studying the model organism, H. venatoria. Briefly, the spiderlings or juveniles of the wild-caught H. venatoria were raised in the laboratory. Spiderlings that had accidentally lost their limbs and spiderlings whose limbs were purposely amputated were observed critically after each subsequent molt [15].
Regeneration of lost limb was associated with molting. After subsequent molts, the spiderlings were able to regenerate the lost appendages. After one molt, the new legs appeared smaller than the full-grown size and slowly became indistinguishable from the others. However, fully mature H. venatoria were not able to regenerate their lost legs and amputation was permanent. A discourse on regeneration of loss limb is incomplete without addressing the subject of autotomy. Autotomy, self-induced limb loss, is one of the most effective antipredator mechanisms that allow juveniles to escape if captured [16]. Autotomy has been observed in diverse animal taxa including both invertebrates and vertebrates that detach a variety of body parts [17-20]. Forms of autotomy are autospasy, limb loss by the action of an outside agent against resistance provided by the organism, and autotilly when limb loss is obtained with the assistance of other limbs [21]. The experiments with H. venatoria were more associated with autospasy because it may refer to a situation where the limb could be lost to a predator, or the limb could be lost during molting, or the limb loss could be due do accidental.
Regardless of the form of autotomy, it is of great importance to discuss the ecological implication of autotomy. There are multiple benefits associated with the ability to shed an appendage with the most prevalent being predation avoidance which allows an organism to survive an encounter with a predator. This might be significant in spiders because they use their legs extensively for prey capture, sensory detection of physical restrains of prey (due to the presence of sensitive setae) and constructing webs. Moreover, autotomy can be beneficial since molting can lead to appendages becoming stuck in the exuviating [22]. Also, the legs can be used for mating as seen when a male tufted golden orb weaver spider Nephila inaurata offers a female one of his front legs to snack on during sex to appease the female [23]. However, autotomy can also have some disadvantages. The most obvious cost of autotomy is an impediment to efficient locomotion which can be translated into a decreased survivorship or the ability to forage/capture prey or escape from predators. Furthermore, spiders with autotomized limbs are easier to be preyed upon by other predators, some predators are known to be actively seeking automized arthropods during foraging [24]. Missing appendages may also affect behaviors associated with a reduction in the foraging rate [3].
Insect, crustacean and amphibian models have been used to shed light on the molecular mechanisms of regeneration [25-28]. In Gryllus bimaculatus (Insecta; De Geer, 1773), and Leptuca pugilator (Crustacea; Bosc, 1802), The re-growth of limbs is initiated through the formation of blastema accompanied by proliferation, dedifferentiation, and redifferentiation of blastemal cells [25]. These processes are also associated with transcription factors and ecdysteroid hormone signaling [29].
The regeneration of lost body parts has been widely studied in axolotl salamander Ambystoma mexicanum (Shaw and Nodder, 1798) and entails different growth phases during limb regeneration. The early ‘early tiny limb’ phase involves a new limb with right shape and structure and the ‘late tiny limb’ phase creates a growing limb which will eventually reach the right size. Regenerating tissue occur more rapidly during the early limb phase compared to the late tiny limb’ and growth observed during these phases is due to a decrease in cell death and an increase in cell number and size [30]. Growing evidence shows that intact nerve supply is essential for limbs to regrow and may regulate allometric growth and size of a regenerating limb following amputation [30].
In summary, regeneration is the natural process of replacing or restoring damaged or missing body parts. Although Regeneration is widely spread throughout the animal kingdom, much is not known about arthropods. the largest phylum in the animal kingdom. In the Arthropod, powers of regeneration is for the most part confined to the appendages as observed in crabs, insects and spiders. Spiders are remarkable for their ability to regenerate lost limbs with apparent ease which is often associated with molting. The capacity to regenerate a lost limb has immediate advantages principally connected with permitting an individual to escape. However, once a limb has been lost the spider may become a less effective predator itself which may also lead to a decrease in foraging rates. Invertebrates and vertebrate models provided insight into the molecular mechanisms of regeneration which may have potential uses in medicine, such as treating a variety of injuries and diseases.
- Bideau L, Kerner P, Hui J, Vervoort M, Gazave E. Animal regeneration in the era of transcriptomics. Cell Mol Life Sci. 2021 Apr;78(8):3941-3956. doi: 10.1007/s00018-021-03760-7. Epub 2021 Jan 30. PMID: 33515282.
- Elliott SA, Alvarado AS. Planarians and the History of Animal Regeneration: Paradigm Shifts and Key Concepts in Biology. Methods Mol Biol. 2018;1774:207-239. doi: 10.1007/978-1-4939-7802-1_4. PMID: 29916157.
- Maginnis TL. The costs of autotomy and regeneration in animals: a review and framework for future research. Behavioral Ecology. 2006; 17(5):857-872
- Santos Jr AR, Nascimento VA, Genari SC, Lombello CB. Mechanisms of Cell Regeneration-From Differentiation to Maintenance of Cell Phenotype. Cells and Biomaterials in Regenerative Medicine. 2014; 37-69.
- Dunlop JA. Geological history and phylogeny of Chelicerata. Arthropod Struct Dev. 2010 Mar-May;39(2-3):124-42. doi: 10.1016/j.asd.2010.01.003. Epub 2010 Mar 20. PMID: 20093195.
- Rajeevan S, Kunnath SM, Varghese T, Kandambeth PP. Spider diversity (Arachnida: Araneae) in different ecosystems of the Western Ghats, Wayanad region, India. South Asian Journal of Life Science. 2019; 7(2), 29-39.
- Gajski D, Pekár S. Assessment of the biocontrol potential of natural enemies against psyllid populations in a pear tree orchard during spring. Pest Manag Sci. 2021 May;77(5):2358-2366. doi: 10.1002/ps.6262. Epub 2021 Jan 21. PMID: 33415804.
- Sharma S. Different techniques used for chromosomal analysis of spiders. Recent Advancements and Research in Biological Sciences. 2021;178.
- World Spider Catalog. Natural History Museum Bern. retrieved 2022.
- Gorneau JA, Rheims CA, Moreau CS, Rayor LS. Huntsman spider phylogeny informs evolution of life history, egg sacs, and morphology. Mol Phylogenet Evol. 2022 Sep;174:107530. doi: 10.1016/j.ympev.2022.107530. Epub 2022 May 27. PMID: 35636670.
- Jäger P. Spiders from Laos with descriptions of new species (Arachnida: Araneae). Acta arachnologica. 2007; 56(1):29-58.
- Edwards Jr GB. Huntsman Spider, Heteropoda venatoria (Linnaeus)(Arachnida: Araneae: Sparassidae): EENY-160/IN317. EDIS 2003; (16).
- GB E. Huntsman Spider, Heteropoda venatoria (Linnaeus)(Arachnida: Araneae: Sparassidae). Florida Cooperative Extension Service. Gainesville: University of Florida IFAS. 2009.
- Ntonifor NN, Parr MC, Ewunkem JA. Seasonal abundance and distribution of the huntsman spider, Heteropoda venatoria (Sparassidae: Araneae) in banana agro-ecosystems in Cameroon. Journal of Entomology. 2012; 9(2):79-88.
- Ewunke JA, Ntonifor NN, Parr MC. Bioecology of Heteropoda venatoria (Linnaeus)(Araneae: Sparassidae) and its implications in a tropical banana agroecosystem. Journal of Global Agriculture and Ecology. 2016; 5(3):164-175
- Smith LD. Effects of limb autotomy and tethering on juvenile blue crab survival from cannibalism. Marine ecology progress series. Oldendorf. 1995; 116(1):65-74.
- Fleming PA, Muller D, Bateman PW. Leave it all behind: a taxonomic perspective of autotomy in invertebrates. Biol Rev Camb Philos Soc. 2007 Aug;82(3):481-510. doi: 10.1111/j.1469-185X.2007.00020.x. PMID: 17624964.
- Higham TE, Russell AP, Zani PA. Integrative biology of tail autotomy in lizards. Physiol Biochem Zool. 2013 Nov-Dec;86(6):603-10. doi: 10.1086/673875. Epub 2013 Oct 31. PMID: 24241059.
- Mitoh S, Yusa Y. Extreme autotomy and whole-body regeneration in photosynthetic sea slugs. Curr Biol. 2021 Mar 8;31(5):R233-R234. doi: 10.1016/j.cub.2021.01.014. PMID: 33689716.
- Baban NS, Orozaliev A, Kirchhof S, Stubbs CJ, Song YA. Biomimetic fracture model of lizard tail autotomy. Science. 2022 Feb 18;375(6582):770-774. doi: 10.1126/science.abh1614. Epub 2022 Feb 17. PMID: 35175822.
- Maruzzo D, Bortolin F. Arthropod regeneration. In Arthropod biology and evolution 2013 ; 149-169). Springer, Berlin, Heidelberg.
- Dunoyer LA, Seifert AW, Van Cleve J. Evolutionary bedfellows: Reconstructing the ancestral state of autotomy and regeneration. J Exp Zool B Mol Dev Evol. 2021 Mar;336(2):94-115. doi: 10.1002/jez.b.22974. Epub 2020 Jun 18. PMID: 32558244; PMCID: PMC7746608.
- Neumann R, Schneider JM. Males sacrifice their legs to pacify aggressive females in a sexually cannibalistic spider. Animal behaviour. 2020; 159:59-67.
- Davenport J, Spikes M, Thornton S, Kelly B. Crab eating in the diamondback terrapin Malaclemys terrapin: dealing with dangerous prey. J Mar Bio1992;l72:835–48.
- Das S. Morphological, Molecular, and Hormonal Basis of Limb Regeneration across Pancrustacea. Integr Comp Biol. 2015 Nov;55(5):869-77. doi: 10.1093/icb/icv101. Epub 2015 Aug 20. PMID: 26296354.
- Bando T, Mito T, Hamada Y, Ishimaru Y, Noji S, Ohuchi H. Molecular mechanisms of limb regeneration: insights from regenerating legs of the cricket Gryllus bimaculatus. Int J Dev Biol. 2018;62(6-7-8):559-569. doi: 10.1387/ijdb.180048ho. PMID: 29938767.
- Ožana S, Pyszko P, Dolný A.). Determination of suitable insect part for non‐lethal DNA sampling: case study of DNA quality and regeneration capability of dragonflies. Insect Conservation and Diversity. 2020;13(4):319-327.
- Ren Z, Fu Y, Liu L, Liu X, Wang C. Expression and function of WNT4 involved in larvae development and limb regeneration in Portunus trituberculatus. Journal of Oceanology and Limnology. 2021; 39(1):306-316.
- Elchaninov A, Sukhikh G, Fatkhudinov T. Evolution of regeneration in animals: A tangled story. Frontiers in Ecology and Evolution. 2021; 9:621686.
- Wells KM, Kelley K, Baumel M, Vieira WA, McCusker CD. Neural control of growth and size in the axolotl limb regenerate. Elife. 2021 Nov 15;10:e68584. doi: 10.7554/eLife.68584. PMID: 34779399; PMCID: PMC8716110.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.




 Save to Mendeley
Save to Mendeley
