Global Journal of Zoology
Assessment of the ameliorative roles of Vitamin E on the histopathology of Clarias Gariepinus (Burchell, 1822) fingerlings exposed to lead nitrate
Samuel PO1*, Arimoro FO1, Ayanwale AV1 and Mohammad HL2
1Fisheries and Hydrobiology Unit, Department of Animal Biology, Federal University of Technology, Minna, Nigeria
2Department of Biochemistry, Federal University of Technology, Minna, Nigeria
*Corresponding author: Samuel PO, Fisheries and Hydrobiology Unit, Department of Animal Biology, Federal University of Technology, Minna, Nigeria, Tel: 07033753561;
E-mail: ajakopatrick@yahoo.com
DOI: 10.17352/gjz.000021
Received: 16 March, 2021 | Accepted: 06 April, 2022 | Published: 07 April, 2022
Keywords: Fish organs; Histopathological alterations; Ameliorative roles; Clarias gariepinus and Lead nitrate
Cite this as
Samuel PO, Arimoro FO, Ayanwale AV, Mohammad HL (2022) Assessment of the ameliorative roles of Vitamin E on the histopathology of Clarias Gariepinus (Burchell, 1822) fingerlings exposed to lead nitrate. Glob J Zool 7(1): 001-009. DOI: 10.17352/gjz.000021
Copyright
© 2022 Samuel PO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Pollution of aquatic ecosystems is constantly increasing with the increase in anthropogenic activities all over the world with negative effects on the constituent biota. The current study addressed the possibility of remedying the effects posed to the tissues of Clarias gariepinus fingerlings (3-11g initial weight) exposed to lead nitrate over 12 weeks. The treatment groups were Pb only and PbVE (Pb+vitamin E) with T1-T4 and replicate in each case. The sub-lethal treatments of lead nitrate concentrations are as follow: 00 (control), 26mg/L (T1), 44mg/L (T2), 61mg/L (T3) and 79mg/L (T4). 26mg/L concentration of the vitamin was applied to every treatment and replicate of the PbVE group. At the end of the exposure period, gills, liver, and kidneys were excised from the samples and preserved in 10% formalin for histopathological analyses. From the results; the livers of the samples of C. gariepinus exposed to sub-lethal concentration of Pb only group displayed aggregation and lumping together of the hepatocytes, massive necrosis and shattering of the hepatocytes, vacoulation with greater severity as the concentration increased. The samples of the PbVE treatment group showed preserved hepatocytes, reduced aggregation and vacoulation of the cells, gradual recovery of the cell nucleus and cytoplasm, normal tissue architecture, and hepatocytes similar to control samples in T1-T3. In the kidneys of the Pb-only group, there were massive necrosis and vacoulation, shattering of cells, tissue edema, and massive lumping of cells together, especially in higher concentrations. The PbVE treatments displayed reduced necrosis and cells aggregating together coupled with reduced vacoulation, preserved cells, and cells with cytoplasm returning to normal. But these were not sustained in the highest concentration. In the gills of the Pb-only group, there was rarefied gill filament with ruptured lamellae, shattered gill arch, and filaments, and ruptured primary and secondary lamellae with greater severity in higher concentrations. The PbVE group displayed how the gill arch and filaments with the primary and secondary lamellae were gradually restored to a certain extent similar to the control. In all the organs the alteration and amelioration of the architecture were concentration-dependent. Therefore, a proportionate concentration of the vitamin can facilitate faster tissue damage recovery in heavy metal exposure.
Introduction
Fish is a known rich source of animal protein throughout the world. Due to its nutritional value [1], the demand for fish food has been on the increase with the increasing human population [2,3]. African catfish, Clarias gariepinus is an important commercial fish due to its high growth rate, high consumer acceptability, ability to withstand poor water quality, and oxygen depletion [4,5]. Also fish is one of the chief protein sources for a man that plays a major role in lowering the blood cholesterol level and offers omega-3 fatty acids that minimize the danger of stroke and heart-related disorders [6]. This is why fish is good for all categories of people _ both old and young in providing the necessary protein and nutritional balance at one point or the other. Clarias species is a widely distributed fish in Asia and Africa. In these areas, the fish is extremely popular on account of its tasty flesh, its unparalleled hardness, its rapid growth, and its somewhat acceptable market price [7]. In Nigeria, the Clarias species is an indigenous fish occurring in freshwater throughout the country and also cultured by many fish farmers. It is suspected that apart from tilapia, Clarias is the most abundant cultivated fish species in Nigeria [7]. Also, Samuel and Uwada [8] posited that C. gariepinus is a hardy species known to be capable of surmounting various environmental challenges; and are omnivorous in nature. It is also known to tolerate difficult conditions in aquaculture [9].
The presence of pollutants in the environment of an aquatic organism such as fish can lead to the production of reactive oxygen species and consequently, oxidative stress. Fish are particularly vulnerable and heavily exposed to pollutants due to feeding and living in aquatic ecosystems because they cannot avoid pollutants’ harmful effects [10]. Heavy metals enter fish by direct absorption from water through their gills and skin, or by ingestion of contaminated food [11]. Heavy metals are known to elicit oxidative stress in organisms when the threshold is exceeded. Heavy metals are also known to promote oxidative damage by increasing the cellular concentration of reactive oxygen species (ROS) in fish, consequently, in a response to antioxidative defenses [12]. In order to cope with a plethora of environmental challenges and ensure survival fishes are endowed with antioxidants provided the threshold is not exceeded. Fishes survive oxidative stress by mobilizing enzymatic as well as non-enzymatic antioxidant defenses [13,14]. Also, Vitamins C and E supplementations have been reported to play a positive role in the detoxification of mercury toxicity, especially at lower concentrations [15,16] demonstrated that vitamin E could improve daily food intake, body weight gain, and feed efficiency ratio.
Vitamin E protects the body from free radical damage. Vitamin E boosts the immune system and facilitates the usage of vitamin K in blood clotting. This fat-soluble vitamin also contributes to the formation of red blood cells. Tocopherol (vitamin E) is a useful indicator of exposure to metals and organic contaminants that generate oxidative stress [17]. Vitamin E has also been reported as a strong inhibitor of apoptosis and a stabilizer of biological membranes [18]. The main biological function of vitamin E is its direct influence on cellular responses to oxidative stress through modulation of the signal transduction pathway [19]. Administration of vitamins can ameliorate or at best attenuate and reduce to the barest minimum the effects of toxicants and the ROS generated from them in the environment of organisms. For instance, Sajitha, et al. [20]. Reported that administration of vitamin E decreased the histopathological and biochemical alterations induced by Pb intoxication in female Sprague-Dawley albino rats. Likewise, Vitamins C and E, or in combination (as antioxidants) ameliorated the hepato-renal and testicular toxicity of abamectin, but were not completely protective, especially in liver tissue [21-23] also reported that tomato paste and vitamin E expressed high protective potentials against cadmium-induced biochemical changes especially liver transaminases and liver histopathological alterations. Chavan and Muley [24] reported that the major histopathological changes in the liver included loss of cellular architecture, necrosis in hepatocytes, and accumulation of fat in parenchymal cells. They also observed congestion of blood vessels. In addition to this, Makinde, et al. [25]. Reported advancing hepatic necrosis in the liver of Clarias gariepinus exposed to 2, 4-D amine. Likewise, Paul and Sengupta [26] demonstrated how sub-lethal concentration of lead acetate has the capacity to bio-accumulate, thereby altering the normal functional activities of freshwater fish, C. punctata. As an important detoxifying organ in fish, the liver is generally considered the richest accumulation position of heavy metals [27].
It is known that in the aquatic environment there are myriads of pollutants at play at one point or the other. There is a paucity of information on the long-run interactions between these elements and other pollutants in the aquatic matrix and the physiological effects of such complex interactions. There is also a dearth of information on how the vitamins are capable of attenuating the deleterious effects of specific toxicants. The presence of toxicants in the environment of organisms is also known to initiate a cascade of reactions bearing on the tissue architecture [28,29], but there is a paucity of information on the effects of specific toxicants such as Pb and when supplemented with vitamins. This is why full knowledge of the mechanisms of action of specific toxicants in the body of vertebrates in terms of alterations of the tissue architecture and how such effects can be ameliorated to a certain degree by the presence of certain concentrations of vitamin E would go a long way in addressing the problems of bioaccumulation and magnification of pollutants from the environment.
Materials and methods
Samples/materials collection and acclimatization
A total number of four hundred (400) fingerlings of Clarias gariepinus were purchased from a commercial fish farmer in Ilorin, Kwara State, and transported in 50L containers filled with water to the Old Farm Research Unit of the Department of Water, Aquaculture and Fisheries Technology, Bosso Campus, Federal University of Technology, Minna, Nigeria. The fishes were placed in fish ponds with water for acclimatization. The fishes were fed twice daily (morning and evening) with vital feed (3 mm) for 14 days. The holding water was changed every three days during the period. About 1.5 kg units of vitamin E granules or pellets were purchased from commercial chemical stores. The toxicant Pb (500g) annular grade was purchased from commercial chemical stores and stored in a cool dry condition throughout the period of the experiment. This toxicant was administered according to the sub-lethal concentrations corresponding to the treatments during the chronic phases of the exposure.
Experimental set-up
The experiment ran for a period of 12 weeks. The first group of treatments was tagged Pb (Pb only) and the second was PbVE (Pb+vitamin E) with T1-T4 and replicates in either case. The five sub-lethal treatments of lead nitrate concentrations used are as follow: 00 (control), 26mg/L (T1), 44mg/L (T2), 61mg/L (T3) and 79mg/L (T4). The minimum concentration of the toxicant (26mg/L) serves the same concentration of the vitamins. The water was changed and fresh toxicants with the same set of concentrations were added every 72 hours according to Organization for Economic Co-operation and Development [30] standards. Each aquarium trough contained 20 samples of the fish. Liver, kidneys, and gills were excised from the Pb only treatment group and the PbVE treatment group, as well as the control for the histopathological analyses of the tissues for possible alterations and amelioration. These organs were preserved in 10% formalin before analyses.
Determination of the histopathology of the tissues of Clarias gariepinus exposed to sub-lethal concentration of lead nitrate
The histopathology of the gills, kidneys, and liver of C. gariepinus from the Pb only treatment group and the PbVE treatment group and replicated in each case were carried out in comparison with the control samples. These organs were preserved in 10 % neutral buffered formalin until required for analyses. The histopathological analyses were carried out in the Histopathology Unit of the University of Ilorin Teaching Hospital, Kwara State, Nigeria. The gills, kidneys, and liver of the fish were fixed in Bouin’s fluid for 24 hours; it was then dehydrated in graded ethanol concentrations and embedded in paraffin wax. Sagittal sections of 3-5µm thickness were cut and mounted on glass slides. The sections were de-paraffinized in xylene, hydrated in ethanol, and stained with hematoxylin-eosin (HE). The possible changes that took place as observed in the gills, liver, and kidneys were indicated from the selected photomicrograph prepared and observed under a light microscope at ×400 magnification.
Results and discussion
Results
Histopathological parameters of C. gariepinus exposed to sub-lethal concentrations of Pb(NO3)2 toxicant and the supplemented treatments with Vitamin E: In the livers of the samples of C. gariepinus exposed to sub-lethal concentration of Pb, the T1 samples displayed aggregation and lumping together of the hepatocytes. The T1 samples of the PbVE treatment group, on the other hand, showed preserved hepatocytes and reduced aggregation and vacoulation of the cells. In T2 samples of the Pb-only group, there were massive necrosis and shattering of the hepatocytes. However, in the T2 samples of the PbVE group, there were also preserved hepatocytes and vacoulation (but not as prominent as PbVE T1 above). There was a gradual recovery of the cell nucleus and cytoplasm. The T3 samples of the Pb-only group indicated massive necrosis, lumping of hepatocytes as well as vacoulation. In the T3 samples of the PbVE treatment group, on the other hand, there were normal tissue architecture and hepatocytes similar to control samples with hepatocytes displaying prominent nucleoli. In the T4 samples of the Pb-only group, there was shattering of the hepatocytes. However, in the T4 samples of the PbVE treatment group, there was aggregation and lumping of the hepatocytes, and normal hepatocytes gradually returned (Plate I).
In the kidneys of the samples exposed to sub-lethal concentrations of Pb, the T1 displayed massive necrosis and vacoulation of the cells. The shattering occurred with the loss of the nucleus and cytoplasm. However, in the T1 samples of the PbVE treatment, there was reduced necrosis and cells show signs of aggregating together coupled with reduced vacoulation. These cells displayed preserved cells and cellular swelling. Likewise, in T2 samples of the Pb only there was massive necrosis and shattering of cells with greater severity than in T1. In the samples of T2 exposed to the PbVE treatment group, there were preserved cells and reduced vacoulation with the slight recovery of the cells. The T3 samples exposed to Pb only group also displayed massive necrosis and severe shattering of cells. Upon supplementation, with vitamin E there were cellular swelling and aggregation of cells. The viable areas with cellular swelling took in much of the stains, and cells with cytoplasm returned to normal. Furthermore, the T4 samples showed massive necrosis, tissue edema, and massive lumping of cells together. The T4 samples exposed to PbVE treatment, on the other hand, displayed massive necrosis and shattering of the cells with minimal effects of the vitamin (Plate II).
Furthermore, gills of the samples of T1 in the Pb only group showed rarefied gill filament with ruptured lamellae. Those supplemented with vitamin E displayed how the gill arch and filaments were restored to a certain extent similar to the control. In T2 samples of the Pb-only group, there were shattered gill arch and filaments and ruptured primary and secondary lamellae. On the other hand, in the T2 samples of the PbVE treatment group, there were ruptured gill arch and filament slightly different from the Pb only group. In the T3 samples, the primary and secondary lamellae have been destroyed or appear shattered. However, in the PbVE group, the T3 samples displayed gradual restoration of the primary and secondary lamellae. The T4 samples of the Pb-only treatment group displayed shattered filaments. On the other hand, in the T4 samples of the PbVE treatment group, there was re-alignment of the gill arch and filaments (Plate III).
Discussion
In the livers of the samples of C. gariepinus exposed to sub-lethal concentration of Pb, the T1 samples displayed aggregation and lumping together of the hepatocytes. This is probably because the presence of the toxicant elicited some physiological changes that culminated in tissue distortions, but upon administration of vitamin E with the same concentration of the toxicant such physiological perturbations were probably alleviated. Hence, there were preserved hepatocytes, and reduced aggregation and vacoulation of the cells. In related research, Ahmad, et al. [31]. Reported that the most common changes in the liver of fishes at both doses of cadmium chloride were loosening of hepatic tissue, vacuolated cell cytoplasm, enucleation, and eccentric nuclei. Furthermore, Abdulkareem, et al. [32]. Also reported a reduction in the pathological damage in the liver of the fishes in the group fed on 5% Moringa oleifera leaves (vitamin E is one of the major proximate compositions of this plant) which was an indication that 5% M. oleifera leaves in fish diet could minimize liver damage. Also in line with the ameliorative capacity of vitamin E, it has been shown that lead acetate combined with vitamin C plus vitamin E supplemented rats showed mild congestion of the interstitial blood vessels and the seminiferous tubules with its components appeared normal compared to DMSA treated rats; and that treatment with DMSA combined with vitamin C plus vitamin E showed the more or less normal histological appearance of the testes in lead acetate induced histopathological changes in the affected organ [33]. Similar findings on the morphological differentiation due to the presence of the toxicant and vitamins (especially vitamin E) were also reported by Samuel, et al. [34] in which T1 and T3 had the highest %WG (percentage weight gain) and SGR (specific growth rate) in comparison to the control in both Pb only and PbVE treatment groups. They further reported that in the PbVE (especially in T1 with 83.26g) there was a general improvement in weight values in all treatments and suggested that vitamin E can attenuate the effects of Pb toxicant and out-perform the un-exposed samples. In like manner, the effects of the vitamin were more evident in lower concentrations than in higher concentrations of the toxicant. However, in a related development, the ameliorative effects of the vitamins were minimal in samples of the fish exposed to cadmium chloride [35].
The massive necrosis and shattering of the hepatocytes in T2 samples of the Pb-only group were probably due to the increased concentration of the toxicant. These effects were, however, probably ameliorated to a certain extent in the T2 samples of the PbVE group which displayed preserved hepatocytes and vacoulation but were not as prominent as the amelioration witnessed in PbVE T1 samples. Similar findings by Deore and Wagh [36] indicated vacoulation in the cytoplasm, degeneration of nuclei, vacoulation in the stroma, cloudy swellings, pycnotic nuclei, necrosis, rupture of blood sinusoids, and disarray of hepatic cords and loss of shape of hepatocytes in the liver when C. gachua was exposed to Cu. Also, Maurya, et al. [37]. Reported how histopathological changes in the liver, intestine, gill, muscle, and heart showed increasing degrees of damage in the tissues in correlation with the accumulation pattern of pesticides while the normal architecture of these organs was observed in the control.
Similarly, the T3 samples of the Pb-only group indicated massive necrosis, lumping of hepatocytes as well as vacoulation which were probably attenuated in the T3 samples of the PbVE treatment group that displayed normal tissue architecture and hepatocytes similar to control samples with hepatocytes displaying prominent nucleoli. The succoring effects of the vitamin may have been brought to bear in restoring the normal tissue architecture. Similar results were reported by [38] on how histopathological examination of the liver of exposed fish showed dilation and congestion of blood vessels, fatty degeneration, necrosis, and pyknotic nuclei of hepatocytes; and that fish fed diet supplemented with vitamin E exhibited protective effect by minimizing the atrazine-induced toxicity, through measured values more or less similar to the control group fish. Also, Mahmoud, et al. (2018) demonstrated the protective effects of propolis and vitamin C against histopathological changes in the liver of Clarias gariepinus treated with cypermethrin. Co-administration of vitamin C with Pb acetate has been shown to diminish the severity of pathological changes and reduced the number of affected organs compared to intoxicated rats l-Neweshy [39].
Furthermore, in the T4 samples of the Pb-only group, there was a shattering of the hepatocytes. The increased concentration of the toxicant may have accounted for this. At this concentration, the amelioration of the vitamin was probably minimal as there was aggregation and lumping of the hepatocytes. Similar results were reported by Osisiogu and Aladesanmi [40] on how cadmium caused degeneration of the liver hepatocytes, congestion of the central vein, area of necrosis, cytoplasmic vacuolation, vascular dilation, and dilation of sinusoids in the hepatic cells in exposed fish (C. gariepinus) as compared to that of the control fish. In addition to this, Chavan and Muley [24] reported that there was the loss of cellular architecture in hepatocytes, along with hemolysis due to the destruction of erythrocytes and prominent focal necrosis in Cirrhinus mrigala was due to the presence of heavy metals.
There have been various reports on the adverse effects of heavy metal contamination on fish health, which include histopathological alterations in their internal organs [41]. In the kidneys of the samples exposed to sub-lethal concentrations of Pb, the T1 displayed massive necrosis and vacoulation of the cells. The shattering occurred with the loss of the nucleus and cytoplasm. These alterations may have arisen from the effects elicited by the toxicant altering the physiological status of the fish, which now manifested in the distortion of the tissue architecture. These alterations were probably ameliorated in the presence of vitamin E since the T1 samples of the PbVE treatment group showed reduced necrosis and cells show signs of aggregating together coupled with reduced vacoulation. In line with this, Abd-Elghaffar, et al. [42]. reported that the kidneys treated with lead acetate showed severe tubular necrosis, periglomerular lymphoid cell reaction, and dilation of the renal tubule, hyaline tubular cast associated with hemorrhage. Similarly, in T2 samples there was massive necrosis and shattering of cells with greater severity than in T1 probably due to increased concentration of the toxicant and the inability of the immune system at that point to counteract such effects. In the same vein, these effects were probably alleviated by the presence of the vitamin because the samples of T2 exposed to the PbVE treatment group displayed preserved cells, and reduced vacoulation with the slight recovery of the cells. A similar report by Ahmad et al. [31] also indicated how the kidney of C. batrachus exposed to cadmium chloride was characterized by loosening of hemopoietic tissue, uriniferous tubules have lost their original appearance, vacuolated cytoplasm, degeneration in the epithelial cells of the renal tubule, narrowing of the tubular lumen and damaged glomeruli.
The T3 samples exposed to Pb only group also displayed massive necrosis and severe shattering of cells probably due to the increasing concentration of the toxicant. In line with this, Odo and Ododeyi [43] reported that exposure of juveniles of Clarias gariepinus to selenium toxicity led to hyperplasia and hemorrhage of the gill lamellar, vacoulation of the kidney, and mucosal eruption of the skin which increased with increase in the selenium concentration. Upon supplementation, with vitamin E there were cellular swelling and aggregation of cells. The viable areas with cellular swelling took in much of the stains, and cells with cytoplasm returned to normal. The vitamin probably ensured quick recovery and improvement in the tissue architecture. Meanwhile, the T4 samples showed massive necrosis, tissue edema, and massive lumping of cells together. This is probably due to the high concentration of the toxicant which may have overwhelmed the immune status of the fish; culminating in physiological imbalances that manifested in the destruction of the tissue architecture. Vitamin administration at this high concentration of the toxicant may have had little or no effects on the kidney of the fish since the T4 samples exposed to the PbVE treatment group displayed massive necrosis and shattering of the cells. As the concentration of the toxicant increased there were probably corresponding increases in the deleterious effects experienced by the fish. Likewise, Odo, et al. [44]. Indicated that the toxic effect of Cyperdicot is clear on the behavioral and histopathological aspects of the fish gills, liver, and kidney tissues and that vitamin E had no amelioration effects on them, especially at higher concentrations of the toxicant. Similar histopathological changes were also reported by Nsofor, et al. [45] on how heavy metals like Zn, Fe, Cu, Hg, Cd, Pb, and Arsenic detected in River Niger around Onitsha elicited extensive hyperemia, oedematous sinusoids, hepatocytes in apoptosis with pyknotic nuclei, and widespread necrotic hepatocytes with mononuclear leucocytes infiltrations and pigment deposits in liver tissues, as well as severe hyperemia of the interstices with degenerating and necrotic tubular epithelial cells in kidney tissues of Chrysichthys nigrodigitatus. In another development, Samuel and Uwada [8] indicate how the administration of combined vitamins C and E without any toxicant led to improvements that were more evident in the highest concentration (T3: 400mg/L) far better than the control; and reported that the highest weight (120.88±28.75g), Specific Growth Rate, SGR (5.686g/day) and percentage weight gain (1109%) were also obtained in T3 at the end of the experiment.
Histology provides a rapid method to detect the effects of irritants in various tissues at a different levels, and so, the harmful effect is indicated among histopathological changes in fish organs [46]. In the gills of the samples of T1 in the Pb only group, there were rarefied gill filaments with ruptured lamellae. Those supplemented with vitamin E displayed how the gill arch and filaments were restored to a certain extent similar to the control. These alterations in the gill architecture were probably due to the intake of the toxicant which was remedied in the treatments with vitamin supplements probably due to the low concentration of the toxicant. This conforms with the findings of Olojo, et al.[46] who reported that after 9 days of treatment with 0.006mg/L the gills showed a gradual process of cytoarchitectural distortion of the lamellae with primary and secondary lamellae overlapping, as there was a decrease in the size of gill because of shrinkage in cartilaginous supporting mass in C. gariepinus exposed to lead. Also, the major histological effects reported by Adebayo and Fapohunda [47] were hypertrophy, necrosis of hepatocytes, and secondary lamella of the liver, gills, and kidney when C. gariepinus was exposed to premium motor spirit.
The T2 samples of the Pb-only group displayed shattered gill arch and filaments and ruptured primary and secondary lamellae which were probably alleviated to a certain extent in the T2 samples of the PbVE treatment group which showed slight differences in ruptured gill arch and filament. This may be due to the increased concentration of the toxicant. This reason may also be plausible in T3 with higher concentration since the samples displayed shattered primary and secondary lamellae of the gill. These were probably improved upon in the PbVE group where the samples displayed gradual restoration of the primary and secondary lamellae. A similar report was given by Omirinde, et al. [48] when they showed how grades of chlorpyrifos induced several gill histo-architectural damages such as moderate to severe gill epithelial sloughing, primary and secondary lamellar hyperplasia, and central veinous congestion in the parenchyma with pronounced severity in fish exposed to higher concentrations; and the gill morphometrics (secondary lamellar length, width, interlamellar distance, and surface area) were markedly altered by the graded concentrations of chlorpyrifos. In conformity with this, Osisiogu and Aladesanmi [40] reported that histopathological changes in the muscle were dependent on the concentration of the toxicant and increased with an increase in concentration. Furthermore, Kumar, et al. [49] reported severe histological alterations in the gills of C. batrachus which include mucus cells hyperplasia, bulging of the taste buds, and formation of interlamellar and sub-epithelial spaces in the primary and secondary gill lamellae.
Subsequently, in the T4 samples of the Pb only treatment group, there were shattered filaments which were probably ameliorated in the PbVE treatment group in which there was re-alignment of the gill arch and filaments. This may also be due to the fact that the gills, being the primary portal of entry in exchange of fluid was directly in contact with the medium in which the fish were kept. In line with this, Chavan and Muley [24] reported that the gills of Cirrhinus mrigala exposed to heavy metals showed lamellar degeneration, epithelial lifting, and dilation with congestion in blood vessels of primary filaments and necrosis of lamellar epithelial cells. Also, Mshelbwala, et al. [50]. Reported that the pathologic changes observed in mussels were represented by branchial and intestinal epithelial cells vacuolization, intestinal lipofuscinosis, lamellar necrosis, and mononuclear cell infiltration. In conformity with the foregoing, the ameliorative role of vitamins was evident when Vitamin E and metallothionein treatments protected against Cd-induced damage to the liver in grass carp (Ctenopharyngodon idellus) by decreasing AST and ALT content, repairing organelles, and maintained the antioxidant system by elevating CAT, SOD, and GSH-Px activity and regulating related mRNA transcript expression [51].
Conclusions and recommendations
Conclusion
The livers of the samples of C. gariepinus exposed to sub-lethal concentrations of Pb displayed varying alterations of the tissue architecture which include aggregation and lumping together of the hepatocytes, massive necrosis, and shattering of the hepatocytes as well as vacoulation. These were ameliorated at varying degrees to near normal tissue architecture in the PbVE treatment group samples, especially in T3 where there were preserved hepatocytes, reduced aggregation, and vacoulation of the cells similar to control samples.
Similar trends were established in the kidneys of the samples exposed to sub-lethal concentrations of Pb which displayed massive necrosis and vacoulation of the cells with loss of nucleus and cytoplasm, and tissue edema and massive lumping of cells together in the highest concentration. There were also varying levels of ameliorations in the tissues of the samples of the treatments supplemented with vitamin E.
The various histopathological alterations in the gills of the samples of the Pb only group were rarefied gill filament with ruptured lamellae, shattered gill arch, and filaments, and ruptured primary and secondary lamellae. These were remedied in the samples supplemented with vitamin E in which the gill arch and filaments were restored and re-aligned to a certain extent similar to the control.
The outcome of the histopathological analyses of the tissues indicated how deleterious Pb toxicant is and how they can be ameliorated to a certain extent by administering a certain concentration of vitamin E; this, therefore, can serve as baseline information in exploring other vitamins, especially at concentrations proportional to the concentrations of the toxicant of interest.
- Tingman W, Jian Z, Xiaoshuan Z (2010) Fish product quality evaluation based on temperature monitoring in cold chain. Afr J Biotechnol 9: 6146-6151. Link: https://bit.ly/3KkCUz0
- FAO (2010) Food and Agricultural Organization of the United Nations: World Review of Fisheries and Aquaculture. Link: https://bit.ly/3KilYcF
- FAO (2012) The state of the world fisheries and aquaculture. Fisheries and Aquaculture Department, Rome, 2012.
- Adewolu MA, Adeniji CA, Adejobi AB (2008) Feed utilization, growth and survival of Clarias gariepinus (Burchell, 1822) fingerlings cultured under different photoperiods. Aquaculture 283: 64-67. Link: https://bit.ly/3NTnHaA
- Karami A, Christianus A, Ishak Z, Courtenay SC, Sayed MA, et al. (2010) Effect of triploidization on juvenile African catfish (Clarias gariepinus). Aquac Int 18: 851-858. Link: https://bit.ly/36VmNd1
- Al-Busaidi M, Yesudhanon P, Al-Mughairi S, Al-Rahbi WAK, Al-harthy KS, et al. (2011) Toxic metals in commercial marine fish in Oman with reference of national and international standards. Chemosphere 85: 67–73. Link: https://bit.ly/3r7CTHo
- FAO (2003) Food Security: concepts and measurement. Rome: Food and Agriculture Organization of the United Nations. In FAO (Ed.), Trade Reforms and Food Security 25-34. Link: https://bit.ly/3uXqqqB
- Samuel PO, Uwada MI (2022) Effects of combined vitamins C and E on the growth performance and some antioxidant production levels in Clarias gariepinus (Burchell, 1822) fingerlings. International Journal of Biosciences 20: 329-341. Link: https://bit.ly/3wRDy2M
- Ukwuani-Ko H, Park H, Kang J (2019) Change of growth performance, haematological parameters and plasma component by hexavalent chromium exposure in starry flounder, Platichthys stellatus. Fisheries and Aquatic Sciences 22: 9. Link: https://bit.ly/3z2WCg7
- Ahmed NF, Sadek KM, Soliman MK, Khalil RH, Khafaga AF, et al. (2020) Moringa Oleifera Leaf Extract Repairs the Oxidative Misbalance following Sub-Chronic Exposure to Sodium Fluoride in Nile Tilapia Oreochromis niloticus. Animals10: 626. Link: https://bit.ly/3uZePYi
- Ayyat MS, Ayyat AM, Naiel MA, Al-Sagheer AA (2020) Reversal effects of some safe dietary supplements on lead contaminated diet induced impaired growth and associated parameters in Nile tilapia. Aquaculture 515: 734580. Link: https://bit.ly/3xaECPZ
- Monteiro DA, Rantin FT, Kalinin AL (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxӑ, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19: 105-123. Link: https://bit.ly/3LNL7w8
- Ahmad I, Maria VL, Oliveira M, Serafim A, Bebianno MJ, et al. (2008) DNA damage and lipid peroxidation vs. protection responses in the gill of Dicentrarchus labrax L. from a contaminated coastal lagoon (Ria de Aveiro, Portugal). Sci Total Environ 406: 298-307. Link: https://bit.ly/3uYQxgP
- Van Der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ Toxicol Pharmacol 13: 57-149. Link: https://bit.ly/371iBIC
- Thakur V, Kanshere RR (2014) Comparative Study on the Protective Role of Vitamin C and Vitamin E on Mercury Induced Toxicity in Heteropneusts fossilis. Int Res J Sci Eng 2: 37-43. Link: https://bit.ly/3uVbQQu
- Osfor MMH, Ibrahim HS, Mohamed YA, Ahmed AM, Abd El Azeem AS, et al. (2010) Effect of alpha lipoic acid and vitamin E on heavy metals intoxication in male albino rats. J Anim Sci 6: 56–63. Link: https://bit.ly/3LKhbRs
- Palace VP, Doebel C, Baron CL, Evans RE, Wautier KG, et al. (2005) Caging small-bodied fish as an alternative method for environmental effects monitoring (EEM). Water Qual Res J 40: 328–333. Link: https://bit.ly/3JfawwX
- Gago-Dominguez M, Castelao JE (2006) Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med 40: 721–733. Link: https://bit.ly/3jihJBG
- Pratt TC, Cullen FT, Sellers CS, Thomas WL, Madensen TD, et al. (2010) The empirical status of social learning theory: a meta-analysis. Justice Quarterly 27: 765-802. Link: https://bit.ly/3x7vATN
- Sajitha GR, Regi J, Andrews A, Ajantha KG, Augustine P, et al. (2010) Garlic oil and vitamin E prevent the adverse effects of lead acetate and ethanol separately as well as in combination in the drinking water of rats. Indian J Clin Biochem 25: 280-288. Link: https://bit.ly/3KiXQXj
- Magdy BW, Mohamed FE, Seleem AA, Sarhan-Rana S (2016) Ameliorative effects of antioxidants (vitamins C and E) against abamectin toxicity in liver, kidney and testis of male albino rats. J Basic Appl Zool 77: 69–82. Link: https://bit.ly/3KlkWwj
- Mekkawy IA, Mahmoud UM, Wassif ET, Naguib M (2011) Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus(Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish Physiol Biochem 37: 71-84. Link: https://bit.ly/3LQY5t3
- Mekkawy IAA, Mahmoud UM, Wassif ET, Naguib M (2012) Protective roles of tomato paste and vitamin e on cadmium-induced histological and histochemical changes of liver of oreochromis niloticus (Linnaeus, 1758). J Fish Aquat Sci 7: 240-265. Link: https://bit.ly/3jgO2RL
- Chavan VR, Muley DV (2014) Effects of heavy metals on liver and gill of fish Cirrhus mrigala. Int J Curr Microbiol Appl Sci 3: 277-287. Link: https://bit.ly/3KgSR9x
- Makinde GEO, Olaifa FE, Banjo OT (2015) Acute Toxicity and Histopathological Changes in Gill and Liver of catfish (Clarias gariepinus) Juvenile exposed to 2, 4-D Amine (Herbex D SI). J Biol Agric Healthc 5: 145-150. Link: https://bit.ly/3lM2MJF
- Paul N, Sengupta M (2013) Lead induced overactivation of phagocytes and variation in enzymatic and non-enzymatic antioxidant defenses in intestinal macrophages of Channa punctatus. Mod Res Inflamm 2: 28-35. Link: https://bit.ly/3xayK95
- Al-Balawi HFA, Al-Akel AS, Al-Misned F (2013) Effects of sub-lethal exposure of lead acetate on histopathology of gills, liver, kidney and muscle and its accumulation in these organs of Clarias gariepinus. Braz Arch Biol Technol 56: 45-56. Link: https://bit.ly/36U2hJO
- Saliu JK, Bawa-Allah KA (2012) Toxicological effects of lead and zinc on the antioxidant enzyme activities of post juvenile Clarias gariepinus. Res Environ 2: 21-26. Link: https://bit.ly/3Jbilnm
- Samuel PO, Adakole JA, Suleiman B, Yaro JD (2017) Histopathological alterations in kidney and liver of Clarias gariepinus (burchell, 1822) studied in river galma, Nigeria. Applied Scientific Reports 1-8. Link: https://bit.ly/3jb4hQj
- Organization for Economic Cooperation and Development (2007) Maximum Acceptable Contaminants: guidance safety level. Fresh Water Fish (RPWS 1991) 12-28.
- Ahmad B, Qureshi TA, Manohar S, Kaur P, Khaliq R (2011) Effect of Cadmium Chloride on the Histoarchitecture of Liver and Kidney of a freshwater Catfish, Clarias batrachus. Int J Environ Sci 2: 543-548. Link: https://bit.ly/37lR04D
- Abdulkareem SI, Oyediran OA, Shaibu RD, Raji M (2017) Toxicological effects of Dichlorvos on haematology and histological changes in Clarias gariepinus juveniles and ameliorative potentials of Moringa oleifera leaves. J Aquat Sci 32: 303-315. Link: https://bit.ly/38s629v
- El-Sayed MF, Abdel-Ghafar SK, Adly MA, Salim AA, Abdel-Samei WA (2016) The ameliorative effects of DMSA and some vitamins against toxicity induced by lead in the testes of albino rats. The J Basic Appl Zool 71: 60–65. Link: https://bit.ly/35M0c1I
- Samuel PO, Arimoro FO, Ayanwale AV, Mohammad HL (2021) Remediative roles of vitamins A, C and E on some growth parameters of Clarias gariepinus (Burchell,1822) fingerlings exposed to lead nitrate in semi-static system. International Journal of Fisheries and Aquatic Studies 9(6): 297-308. Link: https://bit.ly/3PO0t6E
- Samuel PO, Arimoro FO, Ayanwale AV, Mohammad HL (2021) Assessment of the Ameliorative Roles of Vitamins A, C and E on Morphometric Parameters of Clarias gariepinus (Burchell, 1822) Fingerlings Exposed to Cadmium Choride. Journal of Agriculture and Aquaculture 3: 1-15. Link: https://bit.ly/3z43muc
- Deore S, Wagh S (2012) Heavy metal induced histopathological alterations in liver of Channa gachua (Ham.). Journal of Experimental Sciences 25: 481-489. Link: https://bit.ly/3NWlpHq
- Maurya PK, Malik DS, Yadav KK, Gupta N, Kumar S (2019) Haematological and histological changes in fish, Heteropneustes fossilis exposed to pesticides from industrial waste water. Human and Ecological Risk Assessment: An International Journal 1-29. Link: https://bit.ly/3x9BiV7
- Kadry S, Marzouk MS, Amer AM, Hana MI, Azmy A, et al. (2012) Vitamin E as an antioxidant in female African catfish (Clarias gariepinus) exposed to chronic toxicity of Atrazine. Egypt J Aquat Biol Fish 16: 83-98. Link: https://bit.ly/3jk0G29
- El-Neweshy M, El-Sayed Y (2011) Influence of VitC supplementation on lead-induced histopathoogical alterations in male rats. Exp Toxicol Pathol 63: 221-227. Link: https://bit.ly/35M05Dk
- Osisiogu CP, Aladesanmi OT (2019) Cadmium-induced toxicity and antioxidant enzyme responses in tissues and organs of African catfish (Clarias gariepinus). African Journal of Biochemistry Research 13: 63-72. Link: https://bit.ly/3jf2Y2B
- Javed M, Usmani N (2019) An Overview of the adverse effects of heavy metal contamination on fish health. The National Academy of Sciences India 89: 389-403. Link: https://bit.ly/3DNjkJi
- Abd-Elghaffar SKH, El-Sayed MF, Adly MA, Abdel-Samei WM (2015) The protective effects of DMSA and some vitamins against toxicity induced by lead in male Albino rats. J Pharm Appl Chem 1: 1-8. Link: https://bit.ly/35M0oxY
- Odo KE, Ododeyi DO (2017) Responses and histopathological studies of Clarias gariepinus exposed to selenium toxicity. Int J Mar Sci 7: 284-291
- Odo GE, Agwu JE, Madu J, Ezea CO, Nnamonu EI, et al. (2016) Histopathological effects of Cyperdicot and vitamin E supplementation on selected organs of Clarias gariepinus (Burchell, 1822) reared in a tropical fish farm in Nigeria. Afr J Biotechnol 15: 303-314. Link: https://bit.ly/3r3BK3t
- Nsofor CI, Ikpeze OO, ngenegbo UC, Ikeogu CF, Okonkwo JC (2014) Histopathological alterations in the liver and kidney of the fish, Chrysichthys nigrodigitatus due to heavy metals in Niger River. Journal of Natural Sciences Research 4: 11-18. Link: https://bit.ly/3Kmnomu
- Olojo EAA, Olurin KB, Mbaka G, Oluwemimo AD (2005) Histopathology of the gill and liver tissues of the African catfish, Clarias gariepinus exposed to lead. Afr J Biotechnol 4: 117-22. Link: https://bit.ly/3upWOU1
- Fapohunda OO Adebayo IA (2016) Haematological and Histological Responses of Clarias gariepinus Fingerlings Exposed to Premium Motor Spirit (PMS). Nigerian Journal of Fisheries and Aquaculture 4: 1–7. Link: https://bit.ly/3KdEGSy
- Omirinde JO, Audu BS, Mohammed OM, Gosomji IJ (2017) Histomorphometric changes in the gill of Clarias gariepinus exposed to acute concentrations of chlorpyrifos. J Morphol Sci 34: 197-202. Link: https://bit.ly/38D2qSp
- Kumar M, Kumar P, Devi S (2015) To study the histopathological changes in the gills of Clarias batrachus, an air-breathing teleost after short term exposure of copper sulphate. J Aquac Res Develop 6: 10. Link: https://bit.ly/3ufN2n0
- Mshelbwala FM, Ibrahim NDG, Saidu SN, Azeez AA, Akinduti PA, et a. (2017) Motile Salmonella serotypes causing high mortality in poultry farms in three South-Western States of Nigeria. Vet Rec Open 4: e000247. Link: https://bit.ly/3NSjAeI
- Feng Y, Huang X, Duan Y, Fan W, Duan J, Wang K, et al. (2018) The Effects of Vitamin E and Metallothionein on the Antioxidant Capacities of Cadmium-Damaged Liver in Grass Carp, Ctenopharyngodon idellus. Biomed Res Int2018: 7935396. Link: https://bit.ly/3LTCkZ7
2Department of Biochemistry, Federal University of Technology, Minna, Nigeria
Cite this as
Samuel PO, Arimoro FO, Ayanwale AV, Mohammad HL (2022) Assessment of the ameliorative roles of Vitamin E on the histopathology of Clarias Gariepinus (Burchell, 1822) fingerlings exposed to lead nitrate. Glob J Zool 7(1): 001-009. DOI: 10.17352/gjz.000021Copyright
© 2022 Samuel PO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Pollution of aquatic ecosystems is constantly increasing with the increase in anthropogenic activities all over the world with negative effects on the constituent biota. The current study addressed the possibility of remedying the effects posed to the tissues of Clarias gariepinus fingerlings (3-11g initial weight) exposed to lead nitrate over 12 weeks. The treatment groups were Pb only and PbVE (Pb+vitamin E) with T1-T4 and replicate in each case. The sub-lethal treatments of lead nitrate concentrations are as follow: 00 (control), 26mg/L (T1), 44mg/L (T2), 61mg/L (T3) and 79mg/L (T4). 26mg/L concentration of the vitamin was applied to every treatment and replicate of the PbVE group. At the end of the exposure period, gills, liver, and kidneys were excised from the samples and preserved in 10% formalin for histopathological analyses. From the results; the livers of the samples of C. gariepinus exposed to sub-lethal concentration of Pb only group displayed aggregation and lumping together of the hepatocytes, massive necrosis and shattering of the hepatocytes, vacoulation with greater severity as the concentration increased. The samples of the PbVE treatment group showed preserved hepatocytes, reduced aggregation and vacoulation of the cells, gradual recovery of the cell nucleus and cytoplasm, normal tissue architecture, and hepatocytes similar to control samples in T1-T3. In the kidneys of the Pb-only group, there were massive necrosis and vacoulation, shattering of cells, tissue edema, and massive lumping of cells together, especially in higher concentrations. The PbVE treatments displayed reduced necrosis and cells aggregating together coupled with reduced vacoulation, preserved cells, and cells with cytoplasm returning to normal. But these were not sustained in the highest concentration. In the gills of the Pb-only group, there was rarefied gill filament with ruptured lamellae, shattered gill arch, and filaments, and ruptured primary and secondary lamellae with greater severity in higher concentrations. The PbVE group displayed how the gill arch and filaments with the primary and secondary lamellae were gradually restored to a certain extent similar to the control. In all the organs the alteration and amelioration of the architecture were concentration-dependent. Therefore, a proportionate concentration of the vitamin can facilitate faster tissue damage recovery in heavy metal exposure.
Introduction
Fish is a known rich source of animal protein throughout the world. Due to its nutritional value [1], the demand for fish food has been on the increase with the increasing human population [2,3]. African catfish, Clarias gariepinus is an important commercial fish due to its high growth rate, high consumer acceptability, ability to withstand poor water quality, and oxygen depletion [4,5]. Also fish is one of the chief protein sources for a man that plays a major role in lowering the blood cholesterol level and offers omega-3 fatty acids that minimize the danger of stroke and heart-related disorders [6]. This is why fish is good for all categories of people _ both old and young in providing the necessary protein and nutritional balance at one point or the other. Clarias species is a widely distributed fish in Asia and Africa. In these areas, the fish is extremely popular on account of its tasty flesh, its unparalleled hardness, its rapid growth, and its somewhat acceptable market price [7]. In Nigeria, the Clarias species is an indigenous fish occurring in freshwater throughout the country and also cultured by many fish farmers. It is suspected that apart from tilapia, Clarias is the most abundant cultivated fish species in Nigeria [7]. Also, Samuel and Uwada [8] posited that C. gariepinus is a hardy species known to be capable of surmounting various environmental challenges; and are omnivorous in nature. It is also known to tolerate difficult conditions in aquaculture [9].
The presence of pollutants in the environment of an aquatic organism such as fish can lead to the production of reactive oxygen species and consequently, oxidative stress. Fish are particularly vulnerable and heavily exposed to pollutants due to feeding and living in aquatic ecosystems because they cannot avoid pollutants’ harmful effects [10]. Heavy metals enter fish by direct absorption from water through their gills and skin, or by ingestion of contaminated food [11]. Heavy metals are known to elicit oxidative stress in organisms when the threshold is exceeded. Heavy metals are also known to promote oxidative damage by increasing the cellular concentration of reactive oxygen species (ROS) in fish, consequently, in a response to antioxidative defenses [12]. In order to cope with a plethora of environmental challenges and ensure survival fishes are endowed with antioxidants provided the threshold is not exceeded. Fishes survive oxidative stress by mobilizing enzymatic as well as non-enzymatic antioxidant defenses [13,14]. Also, Vitamins C and E supplementations have been reported to play a positive role in the detoxification of mercury toxicity, especially at lower concentrations [15,16] demonstrated that vitamin E could improve daily food intake, body weight gain, and feed efficiency ratio.
Vitamin E protects the body from free radical damage. Vitamin E boosts the immune system and facilitates the usage of vitamin K in blood clotting. This fat-soluble vitamin also contributes to the formation of red blood cells. Tocopherol (vitamin E) is a useful indicator of exposure to metals and organic contaminants that generate oxidative stress [17]. Vitamin E has also been reported as a strong inhibitor of apoptosis and a stabilizer of biological membranes [18]. The main biological function of vitamin E is its direct influence on cellular responses to oxidative stress through modulation of the signal transduction pathway [19]. Administration of vitamins can ameliorate or at best attenuate and reduce to the barest minimum the effects of toxicants and the ROS generated from them in the environment of organisms. For instance, Sajitha, et al. [20]. Reported that administration of vitamin E decreased the histopathological and biochemical alterations induced by Pb intoxication in female Sprague-Dawley albino rats. Likewise, Vitamins C and E, or in combination (as antioxidants) ameliorated the hepato-renal and testicular toxicity of abamectin, but were not completely protective, especially in liver tissue [21-23] also reported that tomato paste and vitamin E expressed high protective potentials against cadmium-induced biochemical changes especially liver transaminases and liver histopathological alterations. Chavan and Muley [24] reported that the major histopathological changes in the liver included loss of cellular architecture, necrosis in hepatocytes, and accumulation of fat in parenchymal cells. They also observed congestion of blood vessels. In addition to this, Makinde, et al. [25]. Reported advancing hepatic necrosis in the liver of Clarias gariepinus exposed to 2, 4-D amine. Likewise, Paul and Sengupta [26] demonstrated how sub-lethal concentration of lead acetate has the capacity to bio-accumulate, thereby altering the normal functional activities of freshwater fish, C. punctata. As an important detoxifying organ in fish, the liver is generally considered the richest accumulation position of heavy metals [27].
It is known that in the aquatic environment there are myriads of pollutants at play at one point or the other. There is a paucity of information on the long-run interactions between these elements and other pollutants in the aquatic matrix and the physiological effects of such complex interactions. There is also a dearth of information on how the vitamins are capable of attenuating the deleterious effects of specific toxicants. The presence of toxicants in the environment of organisms is also known to initiate a cascade of reactions bearing on the tissue architecture [28,29], but there is a paucity of information on the effects of specific toxicants such as Pb and when supplemented with vitamins. This is why full knowledge of the mechanisms of action of specific toxicants in the body of vertebrates in terms of alterations of the tissue architecture and how such effects can be ameliorated to a certain degree by the presence of certain concentrations of vitamin E would go a long way in addressing the problems of bioaccumulation and magnification of pollutants from the environment.
Materials and methods
Samples/materials collection and acclimatization
A total number of four hundred (400) fingerlings of Clarias gariepinus were purchased from a commercial fish farmer in Ilorin, Kwara State, and transported in 50L containers filled with water to the Old Farm Research Unit of the Department of Water, Aquaculture and Fisheries Technology, Bosso Campus, Federal University of Technology, Minna, Nigeria. The fishes were placed in fish ponds with water for acclimatization. The fishes were fed twice daily (morning and evening) with vital feed (3 mm) for 14 days. The holding water was changed every three days during the period. About 1.5 kg units of vitamin E granules or pellets were purchased from commercial chemical stores. The toxicant Pb (500g) annular grade was purchased from commercial chemical stores and stored in a cool dry condition throughout the period of the experiment. This toxicant was administered according to the sub-lethal concentrations corresponding to the treatments during the chronic phases of the exposure.Experimental set-up
The experiment ran for a period of 12 weeks. The first group of treatments was tagged Pb (Pb only) and the second was PbVE (Pb+vitamin E) with T1-T4 and replicates in either case. The five sub-lethal treatments of lead nitrate concentrations used are as follow: 00 (control), 26mg/L (T1), 44mg/L (T2), 61mg/L (T3) and 79mg/L (T4). The minimum concentration of the toxicant (26mg/L) serves the same concentration of the vitamins. The water was changed and fresh toxicants with the same set of concentrations were added every 72 hours according to Organization for Economic Co-operation and Development [30] standards. Each aquarium trough contained 20 samples of the fish. Liver, kidneys, and gills were excised from the Pb only treatment group and the PbVE treatment group, as well as the control for the histopathological analyses of the tissues for possible alterations and amelioration. These organs were preserved in 10% formalin before analyses.
Determination of the histopathology of the tissues of Clarias gariepinus exposed to sub-lethal concentration of lead nitrate
The histopathology of the gills, kidneys, and liver of C. gariepinus from the Pb only treatment group and the PbVE treatment group and replicated in each case were carried out in comparison with the control samples. These organs were preserved in 10 % neutral buffered formalin until required for analyses. The histopathological analyses were carried out in the Histopathology Unit of the University of Ilorin Teaching Hospital, Kwara State, Nigeria. The gills, kidneys, and liver of the fish were fixed in Bouin’s fluid for 24 hours; it was then dehydrated in graded ethanol concentrations and embedded in paraffin wax. Sagittal sections of 3-5µm thickness were cut and mounted on glass slides. The sections were de-paraffinized in xylene, hydrated in ethanol, and stained with hematoxylin-eosin (HE). The possible changes that took place as observed in the gills, liver, and kidneys were indicated from the selected photomicrograph prepared and observed under a light microscope at ×400 magnification.
Results and discussion
Results
Histopathological parameters of C. gariepinus exposed to sub-lethal concentrations of Pb(NO3)2 toxicant and the supplemented treatments with Vitamin E: In the livers of the samples of C. gariepinus exposed to sub-lethal concentration of Pb, the T1 samples displayed aggregation and lumping together of the hepatocytes. The T1 samples of the PbVE treatment group, on the other hand, showed preserved hepatocytes and reduced aggregation and vacoulation of the cells. In T2 samples of the Pb-only group, there were massive necrosis and shattering of the hepatocytes. However, in the T2 samples of the PbVE group, there were also preserved hepatocytes and vacoulation (but not as prominent as PbVE T1 above). There was a gradual recovery of the cell nucleus and cytoplasm. The T3 samples of the Pb-only group indicated massive necrosis, lumping of hepatocytes as well as vacoulation. In the T3 samples of the PbVE treatment group, on the other hand, there were normal tissue architecture and hepatocytes similar to control samples with hepatocytes displaying prominent nucleoli. In the T4 samples of the Pb-only group, there was shattering of the hepatocytes. However, in the T4 samples of the PbVE treatment group, there was aggregation and lumping of the hepatocytes, and normal hepatocytes gradually returned (Plate I).
In the kidneys of the samples exposed to sub-lethal concentrations of Pb, the T1 displayed massive necrosis and vacoulation of the cells. The shattering occurred with the loss of the nucleus and cytoplasm. However, in the T1 samples of the PbVE treatment, there was reduced necrosis and cells show signs of aggregating together coupled with reduced vacoulation. These cells displayed preserved cells and cellular swelling. Likewise, in T2 samples of the Pb only there was massive necrosis and shattering of cells with greater severity than in T1. In the samples of T2 exposed to the PbVE treatment group, there were preserved cells and reduced vacoulation with the slight recovery of the cells. The T3 samples exposed to Pb only group also displayed massive necrosis and severe shattering of cells. Upon supplementation, with vitamin E there were cellular swelling and aggregation of cells. The viable areas with cellular swelling took in much of the stains, and cells with cytoplasm returned to normal. Furthermore, the T4 samples showed massive necrosis, tissue edema, and massive lumping of cells together. The T4 samples exposed to PbVE treatment, on the other hand, displayed massive necrosis and shattering of the cells with minimal effects of the vitamin (Plate II).
Furthermore, gills of the samples of T1 in the Pb only group showed rarefied gill filament with ruptured lamellae. Those supplemented with vitamin E displayed how the gill arch and filaments were restored to a certain extent similar to the control. In T2 samples of the Pb-only group, there were shattered gill arch and filaments and ruptured primary and secondary lamellae. On the other hand, in the T2 samples of the PbVE treatment group, there were ruptured gill arch and filament slightly different from the Pb only group. In the T3 samples, the primary and secondary lamellae have been destroyed or appear shattered. However, in the PbVE group, the T3 samples displayed gradual restoration of the primary and secondary lamellae. The T4 samples of the Pb-only treatment group displayed shattered filaments. On the other hand, in the T4 samples of the PbVE treatment group, there was re-alignment of the gill arch and filaments (Plate III).
Discussion
In the livers of the samples of C. gariepinus exposed to sub-lethal concentration of Pb, the T1 samples displayed aggregation and lumping together of the hepatocytes. This is probably because the presence of the toxicant elicited some physiological changes that culminated in tissue distortions, but upon administration of vitamin E with the same concentration of the toxicant such physiological perturbations were probably alleviated. Hence, there were preserved hepatocytes, and reduced aggregation and vacoulation of the cells. In related research, Ahmad, et al. [31]. Reported that the most common changes in the liver of fishes at both doses of cadmium chloride were loosening of hepatic tissue, vacuolated cell cytoplasm, enucleation, and eccentric nuclei. Furthermore, Abdulkareem, et al. [32]. Also reported a reduction in the pathological damage in the liver of the fishes in the group fed on 5% Moringa oleifera leaves (vitamin E is one of the major proximate compositions of this plant) which was an indication that 5% M. oleifera leaves in fish diet could minimize liver damage. Also in line with the ameliorative capacity of vitamin E, it has been shown that lead acetate combined with vitamin C plus vitamin E supplemented rats showed mild congestion of the interstitial blood vessels and the seminiferous tubules with its components appeared normal compared to DMSA treated rats; and that treatment with DMSA combined with vitamin C plus vitamin E showed the more or less normal histological appearance of the testes in lead acetate induced histopathological changes in the affected organ [33]. Similar findings on the morphological differentiation due to the presence of the toxicant and vitamins (especially vitamin E) were also reported by Samuel, et al. [34] in which T1 and T3 had the highest %WG (percentage weight gain) and SGR (specific growth rate) in comparison to the control in both Pb only and PbVE treatment groups. They further reported that in the PbVE (especially in T1 with 83.26g) there was a general improvement in weight values in all treatments and suggested that vitamin E can attenuate the effects of Pb toxicant and out-perform the un-exposed samples. In like manner, the effects of the vitamin were more evident in lower concentrations than in higher concentrations of the toxicant. However, in a related development, the ameliorative effects of the vitamins were minimal in samples of the fish exposed to cadmium chloride [35].
The massive necrosis and shattering of the hepatocytes in T2 samples of the Pb-only group were probably due to the increased concentration of the toxicant. These effects were, however, probably ameliorated to a certain extent in the T2 samples of the PbVE group which displayed preserved hepatocytes and vacoulation but were not as prominent as the amelioration witnessed in PbVE T1 samples. Similar findings by Deore and Wagh [36] indicated vacoulation in the cytoplasm, degeneration of nuclei, vacoulation in the stroma, cloudy swellings, pycnotic nuclei, necrosis, rupture of blood sinusoids, and disarray of hepatic cords and loss of shape of hepatocytes in the liver when C. gachua was exposed to Cu. Also, Maurya, et al. [37]. Reported how histopathological changes in the liver, intestine, gill, muscle, and heart showed increasing degrees of damage in the tissues in correlation with the accumulation pattern of pesticides while the normal architecture of these organs was observed in the control.
Similarly, the T3 samples of the Pb-only group indicated massive necrosis, lumping of hepatocytes as well as vacoulation which were probably attenuated in the T3 samples of the PbVE treatment group that displayed normal tissue architecture and hepatocytes similar to control samples with hepatocytes displaying prominent nucleoli. The succoring effects of the vitamin may have been brought to bear in restoring the normal tissue architecture. Similar results were reported by [38] on how histopathological examination of the liver of exposed fish showed dilation and congestion of blood vessels, fatty degeneration, necrosis, and pyknotic nuclei of hepatocytes; and that fish fed diet supplemented with vitamin E exhibited protective effect by minimizing the atrazine-induced toxicity, through measured values more or less similar to the control group fish. Also, Mahmoud, et al. (2018) demonstrated the protective effects of propolis and vitamin C against histopathological changes in the liver of Clarias gariepinus treated with cypermethrin. Co-administration of vitamin C with Pb acetate has been shown to diminish the severity of pathological changes and reduced the number of affected organs compared to intoxicated rats l-Neweshy [39].
Furthermore, in the T4 samples of the Pb-only group, there was a shattering of the hepatocytes. The increased concentration of the toxicant may have accounted for this. At this concentration, the amelioration of the vitamin was probably minimal as there was aggregation and lumping of the hepatocytes. Similar results were reported by Osisiogu and Aladesanmi [40] on how cadmium caused degeneration of the liver hepatocytes, congestion of the central vein, area of necrosis, cytoplasmic vacuolation, vascular dilation, and dilation of sinusoids in the hepatic cells in exposed fish (C. gariepinus) as compared to that of the control fish. In addition to this, Chavan and Muley [24] reported that there was the loss of cellular architecture in hepatocytes, along with hemolysis due to the destruction of erythrocytes and prominent focal necrosis in Cirrhinus mrigala was due to the presence of heavy metals.
There have been various reports on the adverse effects of heavy metal contamination on fish health, which include histopathological alterations in their internal organs [41]. In the kidneys of the samples exposed to sub-lethal concentrations of Pb, the T1 displayed massive necrosis and vacoulation of the cells. The shattering occurred with the loss of the nucleus and cytoplasm. These alterations may have arisen from the effects elicited by the toxicant altering the physiological status of the fish, which now manifested in the distortion of the tissue architecture. These alterations were probably ameliorated in the presence of vitamin E since the T1 samples of the PbVE treatment group showed reduced necrosis and cells show signs of aggregating together coupled with reduced vacoulation. In line with this, Abd-Elghaffar, et al. [42]. reported that the kidneys treated with lead acetate showed severe tubular necrosis, periglomerular lymphoid cell reaction, and dilation of the renal tubule, hyaline tubular cast associated with hemorrhage. Similarly, in T2 samples there was massive necrosis and shattering of cells with greater severity than in T1 probably due to increased concentration of the toxicant and the inability of the immune system at that point to counteract such effects. In the same vein, these effects were probably alleviated by the presence of the vitamin because the samples of T2 exposed to the PbVE treatment group displayed preserved cells, and reduced vacoulation with the slight recovery of the cells. A similar report by Ahmad et al. [31] also indicated how the kidney of C. batrachus exposed to cadmium chloride was characterized by loosening of hemopoietic tissue, uriniferous tubules have lost their original appearance, vacuolated cytoplasm, degeneration in the epithelial cells of the renal tubule, narrowing of the tubular lumen and damaged glomeruli.
The T3 samples exposed to Pb only group also displayed massive necrosis and severe shattering of cells probably due to the increasing concentration of the toxicant. In line with this, Odo and Ododeyi [43] reported that exposure of juveniles of Clarias gariepinus to selenium toxicity led to hyperplasia and hemorrhage of the gill lamellar, vacoulation of the kidney, and mucosal eruption of the skin which increased with increase in the selenium concentration. Upon supplementation, with vitamin E there were cellular swelling and aggregation of cells. The viable areas with cellular swelling took in much of the stains, and cells with cytoplasm returned to normal. The vitamin probably ensured quick recovery and improvement in the tissue architecture. Meanwhile, the T4 samples showed massive necrosis, tissue edema, and massive lumping of cells together. This is probably due to the high concentration of the toxicant which may have overwhelmed the immune status of the fish; culminating in physiological imbalances that manifested in the destruction of the tissue architecture. Vitamin administration at this high concentration of the toxicant may have had little or no effects on the kidney of the fish since the T4 samples exposed to the PbVE treatment group displayed massive necrosis and shattering of the cells. As the concentration of the toxicant increased there were probably corresponding increases in the deleterious effects experienced by the fish. Likewise, Odo, et al. [44]. Indicated that the toxic effect of Cyperdicot is clear on the behavioral and histopathological aspects of the fish gills, liver, and kidney tissues and that vitamin E had no amelioration effects on them, especially at higher concentrations of the toxicant. Similar histopathological changes were also reported by Nsofor, et al. [45] on how heavy metals like Zn, Fe, Cu, Hg, Cd, Pb, and Arsenic detected in River Niger around Onitsha elicited extensive hyperemia, oedematous sinusoids, hepatocytes in apoptosis with pyknotic nuclei, and widespread necrotic hepatocytes with mononuclear leucocytes infiltrations and pigment deposits in liver tissues, as well as severe hyperemia of the interstices with degenerating and necrotic tubular epithelial cells in kidney tissues of Chrysichthys nigrodigitatus. In another development, Samuel and Uwada [8] indicate how the administration of combined vitamins C and E without any toxicant led to improvements that were more evident in the highest concentration (T3: 400mg/L) far better than the control; and reported that the highest weight (120.88±28.75g), Specific Growth Rate, SGR (5.686g/day) and percentage weight gain (1109%) were also obtained in T3 at the end of the experiment.
Histology provides a rapid method to detect the effects of irritants in various tissues at a different levels, and so, the harmful effect is indicated among histopathological changes in fish organs [46]. In the gills of the samples of T1 in the Pb only group, there were rarefied gill filaments with ruptured lamellae. Those supplemented with vitamin E displayed how the gill arch and filaments were restored to a certain extent similar to the control. These alterations in the gill architecture were probably due to the intake of the toxicant which was remedied in the treatments with vitamin supplements probably due to the low concentration of the toxicant. This conforms with the findings of Olojo, et al.[46] who reported that after 9 days of treatment with 0.006mg/L the gills showed a gradual process of cytoarchitectural distortion of the lamellae with primary and secondary lamellae overlapping, as there was a decrease in the size of gill because of shrinkage in cartilaginous supporting mass in C. gariepinus exposed to lead. Also, the major histological effects reported by Adebayo and Fapohunda [47] were hypertrophy, necrosis of hepatocytes, and secondary lamella of the liver, gills, and kidney when C. gariepinus was exposed to premium motor spirit.
The T2 samples of the Pb-only group displayed shattered gill arch and filaments and ruptured primary and secondary lamellae which were probably alleviated to a certain extent in the T2 samples of the PbVE treatment group which showed slight differences in ruptured gill arch and filament. This may be due to the increased concentration of the toxicant. This reason may also be plausible in T3 with higher concentration since the samples displayed shattered primary and secondary lamellae of the gill. These were probably improved upon in the PbVE group where the samples displayed gradual restoration of the primary and secondary lamellae. A similar report was given by Omirinde, et al. [48] when they showed how grades of chlorpyrifos induced several gill histo-architectural damages such as moderate to severe gill epithelial sloughing, primary and secondary lamellar hyperplasia, and central veinous congestion in the parenchyma with pronounced severity in fish exposed to higher concentrations; and the gill morphometrics (secondary lamellar length, width, interlamellar distance, and surface area) were markedly altered by the graded concentrations of chlorpyrifos. In conformity with this, Osisiogu and Aladesanmi [40] reported that histopathological changes in the muscle were dependent on the concentration of the toxicant and increased with an increase in concentration. Furthermore, Kumar, et al. [49] reported severe histological alterations in the gills of C. batrachus which include mucus cells hyperplasia, bulging of the taste buds, and formation of interlamellar and sub-epithelial spaces in the primary and secondary gill lamellae.
Subsequently, in the T4 samples of the Pb only treatment group, there were shattered filaments which were probably ameliorated in the PbVE treatment group in which there was re-alignment of the gill arch and filaments. This may also be due to the fact that the gills, being the primary portal of entry in exchange of fluid was directly in contact with the medium in which the fish were kept. In line with this, Chavan and Muley [24] reported that the gills of Cirrhinus mrigala exposed to heavy metals showed lamellar degeneration, epithelial lifting, and dilation with congestion in blood vessels of primary filaments and necrosis of lamellar epithelial cells. Also, Mshelbwala, et al. [50]. Reported that the pathologic changes observed in mussels were represented by branchial and intestinal epithelial cells vacuolization, intestinal lipofuscinosis, lamellar necrosis, and mononuclear cell infiltration. In conformity with the foregoing, the ameliorative role of vitamins was evident when Vitamin E and metallothionein treatments protected against Cd-induced damage to the liver in grass carp (Ctenopharyngodon idellus) by decreasing AST and ALT content, repairing organelles, and maintained the antioxidant system by elevating CAT, SOD, and GSH-Px activity and regulating related mRNA transcript expression [51].
Conclusions and recommendations
Conclusion
The livers of the samples of C. gariepinus exposed to sub-lethal concentrations of Pb displayed varying alterations of the tissue architecture which include aggregation and lumping together of the hepatocytes, massive necrosis, and shattering of the hepatocytes as well as vacoulation. These were ameliorated at varying degrees to near normal tissue architecture in the PbVE treatment group samples, especially in T3 where there were preserved hepatocytes, reduced aggregation, and vacoulation of the cells similar to control samples.
Similar trends were established in the kidneys of the samples exposed to sub-lethal concentrations of Pb which displayed massive necrosis and vacoulation of the cells with loss of nucleus and cytoplasm, and tissue edema and massive lumping of cells together in the highest concentration. There were also varying levels of ameliorations in the tissues of the samples of the treatments supplemented with vitamin E.
The various histopathological alterations in the gills of the samples of the Pb only group were rarefied gill filament with ruptured lamellae, shattered gill arch, and filaments, and ruptured primary and secondary lamellae. These were remedied in the samples supplemented with vitamin E in which the gill arch and filaments were restored and re-aligned to a certain extent similar to the control.
The outcome of the histopathological analyses of the tissues indicated how deleterious Pb toxicant is and how they can be ameliorated to a certain extent by administering a certain concentration of vitamin E; this, therefore, can serve as baseline information in exploring other vitamins, especially at concentrations proportional to the concentrations of the toxicant of interest.
- Tingman W, Jian Z, Xiaoshuan Z (2010) Fish product quality evaluation based on temperature monitoring in cold chain. Afr J Biotechnol 9: 6146-6151. Link: https://bit.ly/3KkCUz0
- FAO (2010) Food and Agricultural Organization of the United Nations: World Review of Fisheries and Aquaculture. Link: https://bit.ly/3KilYcF
- FAO (2012) The state of the world fisheries and aquaculture. Fisheries and Aquaculture Department, Rome, 2012.
- Adewolu MA, Adeniji CA, Adejobi AB (2008) Feed utilization, growth and survival of Clarias gariepinus (Burchell, 1822) fingerlings cultured under different photoperiods. Aquaculture 283: 64-67. Link: https://bit.ly/3NTnHaA
- Karami A, Christianus A, Ishak Z, Courtenay SC, Sayed MA, et al. (2010) Effect of triploidization on juvenile African catfish (Clarias gariepinus). Aquac Int 18: 851-858. Link: https://bit.ly/36VmNd1
- Al-Busaidi M, Yesudhanon P, Al-Mughairi S, Al-Rahbi WAK, Al-harthy KS, et al. (2011) Toxic metals in commercial marine fish in Oman with reference of national and international standards. Chemosphere 85: 67–73. Link: https://bit.ly/3r7CTHo
- FAO (2003) Food Security: concepts and measurement. Rome: Food and Agriculture Organization of the United Nations. In FAO (Ed.), Trade Reforms and Food Security 25-34. Link: https://bit.ly/3uXqqqB
- Samuel PO, Uwada MI (2022) Effects of combined vitamins C and E on the growth performance and some antioxidant production levels in Clarias gariepinus (Burchell, 1822) fingerlings. International Journal of Biosciences 20: 329-341. Link: https://bit.ly/3wRDy2M
- Ukwuani-Ko H, Park H, Kang J (2019) Change of growth performance, haematological parameters and plasma component by hexavalent chromium exposure in starry flounder, Platichthys stellatus. Fisheries and Aquatic Sciences 22: 9. Link: https://bit.ly/3z2WCg7
- Ahmed NF, Sadek KM, Soliman MK, Khalil RH, Khafaga AF, et al. (2020) Moringa Oleifera Leaf Extract Repairs the Oxidative Misbalance following Sub-Chronic Exposure to Sodium Fluoride in Nile Tilapia Oreochromis niloticus. Animals10: 626. Link: https://bit.ly/3uZePYi
- Ayyat MS, Ayyat AM, Naiel MA, Al-Sagheer AA (2020) Reversal effects of some safe dietary supplements on lead contaminated diet induced impaired growth and associated parameters in Nile tilapia. Aquaculture 515: 734580. Link: https://bit.ly/3xaECPZ
- Monteiro DA, Rantin FT, Kalinin AL (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxӑ, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19: 105-123. Link: https://bit.ly/3LNL7w8
- Ahmad I, Maria VL, Oliveira M, Serafim A, Bebianno MJ, et al. (2008) DNA damage and lipid peroxidation vs. protection responses in the gill of Dicentrarchus labrax L. from a contaminated coastal lagoon (Ria de Aveiro, Portugal). Sci Total Environ 406: 298-307. Link: https://bit.ly/3uYQxgP
- Van Der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ Toxicol Pharmacol 13: 57-149. Link: https://bit.ly/371iBIC
- Thakur V, Kanshere RR (2014) Comparative Study on the Protective Role of Vitamin C and Vitamin E on Mercury Induced Toxicity in Heteropneusts fossilis. Int Res J Sci Eng 2: 37-43. Link: https://bit.ly/3uVbQQu
- Osfor MMH, Ibrahim HS, Mohamed YA, Ahmed AM, Abd El Azeem AS, et al. (2010) Effect of alpha lipoic acid and vitamin E on heavy metals intoxication in male albino rats. J Anim Sci 6: 56–63. Link: https://bit.ly/3LKhbRs
- Palace VP, Doebel C, Baron CL, Evans RE, Wautier KG, et al. (2005) Caging small-bodied fish as an alternative method for environmental effects monitoring (EEM). Water Qual Res J 40: 328–333. Link: https://bit.ly/3JfawwX
- Gago-Dominguez M, Castelao JE (2006) Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med 40: 721–733. Link: https://bit.ly/3jihJBG
- Pratt TC, Cullen FT, Sellers CS, Thomas WL, Madensen TD, et al. (2010) The empirical status of social learning theory: a meta-analysis. Justice Quarterly 27: 765-802. Link: https://bit.ly/3x7vATN
- Sajitha GR, Regi J, Andrews A, Ajantha KG, Augustine P, et al. (2010) Garlic oil and vitamin E prevent the adverse effects of lead acetate and ethanol separately as well as in combination in the drinking water of rats. Indian J Clin Biochem 25: 280-288. Link: https://bit.ly/3KiXQXj
- Magdy BW, Mohamed FE, Seleem AA, Sarhan-Rana S (2016) Ameliorative effects of antioxidants (vitamins C and E) against abamectin toxicity in liver, kidney and testis of male albino rats. J Basic Appl Zool 77: 69–82. Link: https://bit.ly/3KlkWwj
- Mekkawy IA, Mahmoud UM, Wassif ET, Naguib M (2011) Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus(Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish Physiol Biochem 37: 71-84. Link: https://bit.ly/3LQY5t3
- Mekkawy IAA, Mahmoud UM, Wassif ET, Naguib M (2012) Protective roles of tomato paste and vitamin e on cadmium-induced histological and histochemical changes of liver of oreochromis niloticus (Linnaeus, 1758). J Fish Aquat Sci 7: 240-265. Link: https://bit.ly/3jgO2RL
- Chavan VR, Muley DV (2014) Effects of heavy metals on liver and gill of fish Cirrhus mrigala. Int J Curr Microbiol Appl Sci 3: 277-287. Link: https://bit.ly/3KgSR9x
- Makinde GEO, Olaifa FE, Banjo OT (2015) Acute Toxicity and Histopathological Changes in Gill and Liver of catfish (Clarias gariepinus) Juvenile exposed to 2, 4-D Amine (Herbex D SI). J Biol Agric Healthc 5: 145-150. Link: https://bit.ly/3lM2MJF
- Paul N, Sengupta M (2013) Lead induced overactivation of phagocytes and variation in enzymatic and non-enzymatic antioxidant defenses in intestinal macrophages of Channa punctatus. Mod Res Inflamm 2: 28-35. Link: https://bit.ly/3xayK95
- Al-Balawi HFA, Al-Akel AS, Al-Misned F (2013) Effects of sub-lethal exposure of lead acetate on histopathology of gills, liver, kidney and muscle and its accumulation in these organs of Clarias gariepinus. Braz Arch Biol Technol 56: 45-56. Link: https://bit.ly/36U2hJO
- Saliu JK, Bawa-Allah KA (2012) Toxicological effects of lead and zinc on the antioxidant enzyme activities of post juvenile Clarias gariepinus. Res Environ 2: 21-26. Link: https://bit.ly/3Jbilnm
- Samuel PO, Adakole JA, Suleiman B, Yaro JD (2017) Histopathological alterations in kidney and liver of Clarias gariepinus (burchell, 1822) studied in river galma, Nigeria. Applied Scientific Reports 1-8. Link: https://bit.ly/3jb4hQj
- Organization for Economic Cooperation and Development (2007) Maximum Acceptable Contaminants: guidance safety level. Fresh Water Fish (RPWS 1991) 12-28.
- Ahmad B, Qureshi TA, Manohar S, Kaur P, Khaliq R (2011) Effect of Cadmium Chloride on the Histoarchitecture of Liver and Kidney of a freshwater Catfish, Clarias batrachus. Int J Environ Sci 2: 543-548. Link: https://bit.ly/37lR04D
- Abdulkareem SI, Oyediran OA, Shaibu RD, Raji M (2017) Toxicological effects of Dichlorvos on haematology and histological changes in Clarias gariepinus juveniles and ameliorative potentials of Moringa oleifera leaves. J Aquat Sci 32: 303-315. Link: https://bit.ly/38s629v
- El-Sayed MF, Abdel-Ghafar SK, Adly MA, Salim AA, Abdel-Samei WA (2016) The ameliorative effects of DMSA and some vitamins against toxicity induced by lead in the testes of albino rats. The J Basic Appl Zool 71: 60–65. Link: https://bit.ly/35M0c1I
- Samuel PO, Arimoro FO, Ayanwale AV, Mohammad HL (2021) Remediative roles of vitamins A, C and E on some growth parameters of Clarias gariepinus (Burchell,1822) fingerlings exposed to lead nitrate in semi-static system. International Journal of Fisheries and Aquatic Studies 9(6): 297-308. Link: https://bit.ly/3PO0t6E
- Samuel PO, Arimoro FO, Ayanwale AV, Mohammad HL (2021) Assessment of the Ameliorative Roles of Vitamins A, C and E on Morphometric Parameters of Clarias gariepinus (Burchell, 1822) Fingerlings Exposed to Cadmium Choride. Journal of Agriculture and Aquaculture 3: 1-15. Link: https://bit.ly/3z43muc
- Deore S, Wagh S (2012) Heavy metal induced histopathological alterations in liver of Channa gachua (Ham.). Journal of Experimental Sciences 25: 481-489. Link: https://bit.ly/3NWlpHq
- Maurya PK, Malik DS, Yadav KK, Gupta N, Kumar S (2019) Haematological and histological changes in fish, Heteropneustes fossilis exposed to pesticides from industrial waste water. Human and Ecological Risk Assessment: An International Journal 1-29. Link: https://bit.ly/3x9BiV7
- Kadry S, Marzouk MS, Amer AM, Hana MI, Azmy A, et al. (2012) Vitamin E as an antioxidant in female African catfish (Clarias gariepinus) exposed to chronic toxicity of Atrazine. Egypt J Aquat Biol Fish 16: 83-98. Link: https://bit.ly/3jk0G29
- El-Neweshy M, El-Sayed Y (2011) Influence of VitC supplementation on lead-induced histopathoogical alterations in male rats. Exp Toxicol Pathol 63: 221-227. Link: https://bit.ly/35M05Dk
- Osisiogu CP, Aladesanmi OT (2019) Cadmium-induced toxicity and antioxidant enzyme responses in tissues and organs of African catfish (Clarias gariepinus). African Journal of Biochemistry Research 13: 63-72. Link: https://bit.ly/3jf2Y2B
- Javed M, Usmani N (2019) An Overview of the adverse effects of heavy metal contamination on fish health. The National Academy of Sciences India 89: 389-403. Link: https://bit.ly/3DNjkJi
- Abd-Elghaffar SKH, El-Sayed MF, Adly MA, Abdel-Samei WM (2015) The protective effects of DMSA and some vitamins against toxicity induced by lead in male Albino rats. J Pharm Appl Chem 1: 1-8. Link: https://bit.ly/35M0oxY
- Odo KE, Ododeyi DO (2017) Responses and histopathological studies of Clarias gariepinus exposed to selenium toxicity. Int J Mar Sci 7: 284-291
- Odo GE, Agwu JE, Madu J, Ezea CO, Nnamonu EI, et al. (2016) Histopathological effects of Cyperdicot and vitamin E supplementation on selected organs of Clarias gariepinus (Burchell, 1822) reared in a tropical fish farm in Nigeria. Afr J Biotechnol 15: 303-314. Link: https://bit.ly/3r3BK3t
- Nsofor CI, Ikpeze OO, ngenegbo UC, Ikeogu CF, Okonkwo JC (2014) Histopathological alterations in the liver and kidney of the fish, Chrysichthys nigrodigitatus due to heavy metals in Niger River. Journal of Natural Sciences Research 4: 11-18. Link: https://bit.ly/3Kmnomu
- Olojo EAA, Olurin KB, Mbaka G, Oluwemimo AD (2005) Histopathology of the gill and liver tissues of the African catfish, Clarias gariepinus exposed to lead. Afr J Biotechnol 4: 117-22. Link: https://bit.ly/3upWOU1
- Fapohunda OO Adebayo IA (2016) Haematological and Histological Responses of Clarias gariepinus Fingerlings Exposed to Premium Motor Spirit (PMS). Nigerian Journal of Fisheries and Aquaculture 4: 1–7. Link: https://bit.ly/3KdEGSy
- Omirinde JO, Audu BS, Mohammed OM, Gosomji IJ (2017) Histomorphometric changes in the gill of Clarias gariepinus exposed to acute concentrations of chlorpyrifos. J Morphol Sci 34: 197-202. Link: https://bit.ly/38D2qSp
- Kumar M, Kumar P, Devi S (2015) To study the histopathological changes in the gills of Clarias batrachus, an air-breathing teleost after short term exposure of copper sulphate. J Aquac Res Develop 6: 10. Link: https://bit.ly/3ufN2n0
- Mshelbwala FM, Ibrahim NDG, Saidu SN, Azeez AA, Akinduti PA, et a. (2017) Motile Salmonella serotypes causing high mortality in poultry farms in three South-Western States of Nigeria. Vet Rec Open 4: e000247. Link: https://bit.ly/3NSjAeI
- Feng Y, Huang X, Duan Y, Fan W, Duan J, Wang K, et al. (2018) The Effects of Vitamin E and Metallothionein on the Antioxidant Capacities of Cadmium-Damaged Liver in Grass Carp, Ctenopharyngodon idellus. Biomed Res Int2018: 7935396. Link: https://bit.ly/3LTCkZ7
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
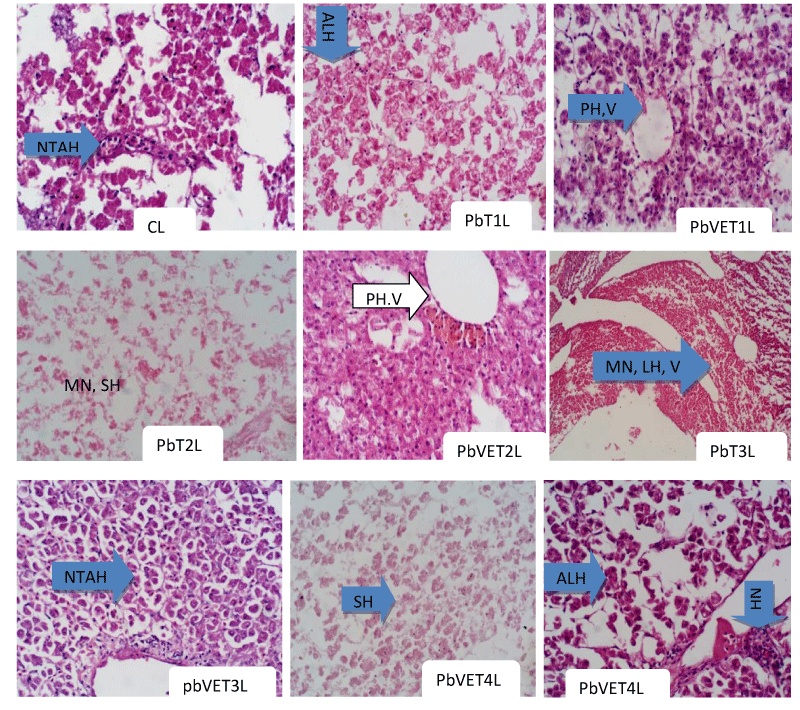
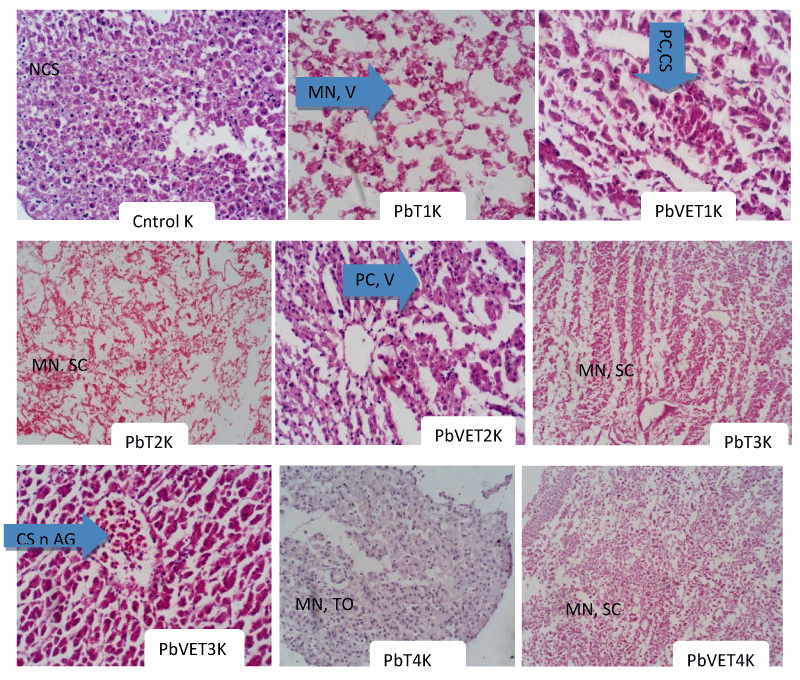


 Save to Mendeley
Save to Mendeley
