Global Journal of Zoology
The effect of freshwater acidification in the tissues of Cyprinus carpio
Ibrahim A1*, Narayanan M2, Khan Salman1 and Sheik Mohamed3
2Aquatic Acidification Research Unit, Department of Zoology, St.Xavier’s College, Palayamkottai, India
3Marine Science Institute, University of Texas, Austin, USA
Cite this as
Ibrahim A, Narayanan M, Salman K, Mohamed S (2020) The effect of freshwater acidification in the tissues of Cyprinus carpio. Glob J Zool 5(1): 009-015. DOI: 10.17352/gjz.000014The extant study was carried out to discern the effect of different acidic pH on the tissues of fishes. The experimental fish Cyprinus carpio were introduced into acidic waters (pH 5.0; 5.8; 6.6 and control 7.2) for 21 days for the histopathological exploration, the pH was upheld vigilantly throughout the investigation, after the experimental period, the results revealed the transfigures in the tissues of gills, intestine and liver. The annihilation of the epithelial layer with hypertrophy and hyperplasia in the gills and the development of necrotic gill disease, the fallouts from the intestine displayed the mucosal necrosis, and massive sloughing off mucosal epithelium, the liver unveiled the massive and intense granulomatous reaction and development of necrotic hepatitis.
Introduction
Histopathological analysis has been documented to be unswerving biomarkers of stress in fish [1]. Histopathological variations have been extensively utilized as biomarkers in the assessment of the healthiness of the fish, which introduced into contaminated habitat, both in the lab and ground studies. It is a great advantage to use the histopathological tissue identities in ecological monitoring and this type of biomarkers permits to analyse the tissues like the gills, kidney and liver; those are accountable for vibrant roles of respiration, excretion and the accretion and bioconversion in the fish [2]. Histology is a powerful technique to find effects of the hazardous substances in various tissues. The testing of fishes with the pollutants in the aquatic environment,which tempts to numerous tissue impairments. The respiratory structure (gills) is a compatible organ for the histopathological observations to conclude the consequences of environmental abnormalities. The gills of the fishes are engaging with the physiological functions of osmotic control, eliminating metabolic wastes and breathing. The gill tissues are observing the abnormal features of the waters. They are highly suitable for the evaluation of aquatic environmental influence [3.4]. Histopathological examinations are useful to observe the effect of the abnormal concentrations of pollutants on fishes in the biometrics [5,6].
The effects of occasional pH decline and inorganic contaminants on aquatic animals are just recounted [7]. Protracted exposure to low pH can cause anomalous progress of laboratory nurtured fish [8-12]. In addition to variations in physiological performances, anatomical variations like abridged development [12-18], skeletal malformations [18,19] reproductive botch [12,15,18,20-24] anatomical mutilation in the gills [17,25-27] and [12] and death [28,29,14,30,31,16]. Amplified an acidity initiated the drop in plasma sodium and chloride and death begin in fishes exposed to an acidic surroundings [32,33] stated that the O2 carrying capacity of fish blood in vitro, fallen abruptly below pH7. The partial pressure of oxygen (pO2) of trout blood declined in acute acid exposure [34-36,12], and found a lessened capability to extract O2 from water of low pH. [37] reported that brook trout got reduction in blood pH when they were exposed to acid waters. In response to long-term acid exposure, the haematocrit, haemoglobin values of blood and haemopoietic activity of fish are all elevated, presumably to uphold O2 carrying ability [38,39,12]. Effect of lethal and sublethal acidic pH medium on Oxygen (O2) consumption and metabolism in Oreochromis mossambicus [12], brook trout (Salvelinus fontinalis) were reported by [40,20] reported that low pH also disturbed the normal action of choriolytic enzymes in embryos of the European perch, [41] reported that the female white suckers from acidified lakes did not release eggs, because of the inactivity of the females to maintain the serum calcium level. Low pH prevents the spawning in some species of fish [42,43] reported that reproduction was impaired in blunt nose minnows. Hatching failure has also been reported [44,45] has done preliminary work on the effect of low pH and behavioural changes in Sarotherodon mossambicus.
Materials and methods
The pH of the experimental freshwater (control) has gradually reduced into pH 6.6, 5.8 and 5.0 by adding 5% Sulfuric acid (H2So4), The prepared pH experimental media has stirred well by electric stirrer and the pH was measured perfectly with a digital pH meter and frequently test with a pen pH meter, and ascertained the constant pH without the fluctuations. Cyprinus carpio were used for histopathological studies. They were exposed to different acidic environmental media (pH 5.0, 5.8, 6.6 and 7.2) for 21 days. Care was taken to observe that all the fishes introduced to each aquarium (20 L capacity), were alive throughout the experimental term. Four fishes were introduced in every aquarium and triplicated manner. Fishes were fed with chopped beef. Fresh test acid media were prepared daily and the experimental fishes were introduced. After exposure for 21 days, the test fish were sacrificed to get the necessary tissues for histological studies. The organs chosen for the present study were gill, liver and intestine. Tissues from these organs of the control and exposed fish were excised from live fish and processed for observations.
Tissue processing
The tissues included in this study were gills, liver and intestine. The tissues were fixed in Zenker’s fluid, for 6 to 8 hours. The fixed tissues were washed in running tap water overnight and then dehydrated in ascending grades of isopropyl alcohol. Finally the tissues were completely dehydrated through two changes of 100% alcohol for 20 to 30 minutes in each. The tissues were, then, dealcoholized by two changes of xylol, 20 to 30 minutes in each change. It was followed by infiltration in xylol saturated with paraffin wax of congealing point 56° to 58°C. The tissues were left in molten wax for at least 30 minutes in the embedding bath. In order to remove all the clearing agent (xylol) three changes in paraffin were made for through infiltration. The tissues were embedded in labelled paper ‘boats’ after proper orientation of the processed tissues. The whole process of embedding was made after Weesner (1968). The embedded tissues were sectioned at 7 mm (microns) thickness in a rotary microtome. The ribbon of sections was cut into strips and floated in warm water which was poured over acid leaned glass slides. The ribbons were then pasted with a thin film of fixative. Egg albumen and Haupt’s fixative were used. Flattening of the sections was achieved by floating the sections on water medium, which was warmed only to the extent required to spread the sections. Neutral formalin (2%) was used as a floating medium for the sections when Haupt’s fixative was used. When the sections became flat and well-spread, the water was drained. After complete air drying for a few hours, the slides were left overnight on slide warmer whose temperature was maintained 10 to 15 °C below the melting point of the paraffin wax. The sections were deparaffinised in xylene and rehydrated in descending grades of iso-propyl alcohol. The tissues fixed in mercuric chloride containing fixative were passed through 70% alcohol with 0.5% iodine to remove the mercury precipitate present in the tissues. Further, rehydration was carried out by bringing the sections to distilled water and the sections were left for 3 minutes in 5% sodium thiosulphate solution to remove the iodine. Later the sections were washed in running tap water for 5 minutes. The slides for Histological and Histopathological observations were stained using one of the following staining methods:
Staining-dichrome staining
Ehrlich’s haematoxylin is a stain which is commonly used in histopathological examination. It is highly suitable for nuclear staining. The cytoplasmic stain is eosin Y (0.2%), Ehrlich’s hematoxylin used to stain the tissue sections. The slides were washed in purified water then they were subjected in to staining procedures, the staining time is approximately 900 seconds (15 minutes), The over stained segments were then distinguished in 1% Hcl in water. After rinsing in Hcl, the sections were, coloured blue in tap water and again observed by microscopic methods. This procedure was recurrent over till the nuclei and basophil cytoplasm was correctly distinguished and the cytoplasmic surroundings were colourless. Then, the sections were splashed in flowing tap water for 60 minutes to eliminate the aluminium ammonium sulphate. After splashing in distilled water, the sections were counter stained in 0.2% Eosin Y for 15 seconds. In order to remove the excess of Eosin Y, the slides were rinsed in distilled water and quickly kept in 95% alcohol. They were finally placed in 100% alcohol I and II, for 5 minutes in each solution and passed through xylol I and II. Sections were, then, astride in DPX [46].
Results
Normal histology
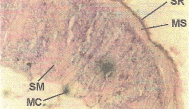
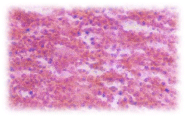
Gills: The rachis of gill from which the individual filaments of gills arise has a core of the cartilaginous skeleton covered by a connective tissue sheath. Many layers of cells are arranged around the core. The cell layers from the parts of the gill, the outermost layer is the gill epithelium (Figure 1), the connective tissue and epithelial cells in several layers envelopes the rachis. The outer most epithelial layer forms the respiratory surface of each finger shaped gill filament. Their epithelial cells are mostly isodiametric and in certain places they are cuboidal. The epithelial cells have large nuclei. The mucus cells are goblet shaped and their vacuole like cavities are filled with mucus.
Histopathological changes
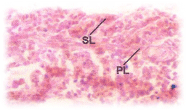
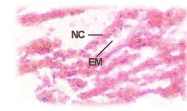
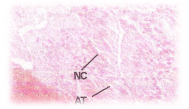
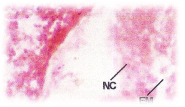
C.carpio when exposed to pH 6.6 media revealed the epithelial necrosis and cellular edema (Figure 2), when exposed to pH 5.8 experimental media, they exhibited extensive gill damage with epithelia capillary separation, the epithelial lining showed severe destruction and there was a hypertrophy of the mucocytes which project on the epithelial surface. The cells showed desquamation. But the severity of acid stress was evident by the formation of mucus hyperplasia, with heavy deposition of mucus on gills (Figure 3), when exposed to pH 5.0 experimental media exhibited destruction of the epithelial layer. Figure 4 illustrates epithelial destruction with degenerative desquamation, hypertrophy (Figure 4) of the mucocytes and hyperplasia of undifferentiated cells. The most prominent feature was degeneration of surface epithelial cells and severe epithelial hyperplasia. The entire architecture of gills were ruptured (Figure 4) resulting in the development of Necrotic gill disease (NGD).
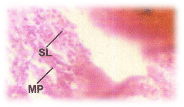
Normal histology of the intestine: The intestine is a long tubular structure. In sectional view it exhibits a very simple histological organization (Figure 5). The innermost lining layer is mucosa consisting of epithelial cells. There is a laminapropria consisting of connective tissue occupying the narrow spaces between the glands and muscularis mucosa. The normal villi show mucosa, submucosa, muscularis and serosa. The mucosa comprises of a layer of epithelial cells establishing the covering of lumen. The submucosa entails of connective tissue. The outermost layer is muscularis coat. The mucosal epithelium entails of columnar cells with basal nuclei, they form a spherical shrill layer of the intestinal wall.
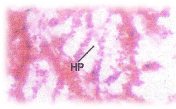
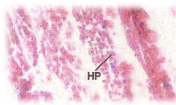
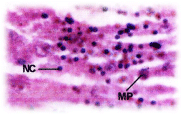
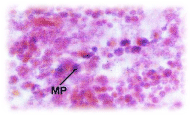
Histopathological changes; The experimental fishes, when exposed to pH 6.6 revealed the chronic inflammation of laminapropria, which led to a massive accumulation of macrophages, necrosis and atrophy of the intestinal villi (Figure 6), when exposed to pH 5.8 experimental media exhibited, the hyper vacuolations of intestinal mucus membrane, mucosal necrosis of absorptive cells and submucosal edema. (Figure 7). C.carpio exposed to pH 5.0 test media showed (Figure 8) massive sloughing off mucosal epithelium and accumulation of macrophages.
Normal Histology of the liver: Liver cells of C. carpio are grouped into lobules. The rows of hepatocytes form laminae. Small bile ducts also find their place in the interlobular connective tissue. The hepatocytes are almost isodiametric and polygonal. The hepatocytes have basiphili cytoplasm and are uninucleated (Figure 9). The nuclei are very prominent in the hepatocytes. Sinusoids are seen and vacuoles are rarely found.
Histopathological changes: Acid stressed (pH 6.6) C.carpio’s liver shown the abnormal histopathological marks of deformed hepatocytes, necrotic debris and macrophages (Figure 10). Exposure of C.carpio in pH 5.8 showed the massive and intense granulomatous reaction, death and debris of cells, aggregates of granular materials and necrotic hepatitis (Figure 11). When C. carpio were exposed to the same acidic environment (pH 5.0) they showed the development of necrotic hepatitis (Figure 12).
Discussion
Though the environmental acidification is a global problem report on the effect of low pH has been focused on temperate fishes, particularly brook trout 10; 39; 6; 65; 48; 64 [47,48,49,25,27,50], rainbow trout 78[51], fathead minnows 39[48] and Atlantic salmon 23 [17]. The morphology and ultrastructure of the gills have been well reviewed by 38 [52].
Gills
The structure of normal gills have been reported in rainbow trout [53,54,55]; striped mullet [56], lamprey [57], brook trout [27], Atlantic salmon [58,17]; Aplocheilus lineatum [59] and Tilapia [12]. Acidic trauma severely impinges the gill, intestine and liver morphology and architecture of the fish in the present investigation. C.carpio when exposed to the acidic environments showed the transfigures of gills such as, epithelial necrosis, epithelial corrosion or destruction, extensive gill damage with epithelio- capillary separation and severe epithelial destruction with degenerative desquamation, mucus hyperplasia, heavy deposition of mucus on gills, epithelial hyperplasia and developmnt of disease called necrotic gill disease (NGD). The results of the present histopathological studies corroborated with the aggravation of gill destruction by lowering the pH of water to less than pH 5.0 as reported by [60]. Supportive evidences were also given by Ibrahim [12] who stated the similar effects in Oreochromis mossambicus exposed at pH 5.0. [11], who reported hypertrophy of mucus cells, excessive mucus secretion and separation of epithelial layer in the gills of brook trout and hypertrophy necrosis of cells in gills of Atlantic salmon; [37] stated elevated mucus secretion at gills; [61,62] have reported hypertrophy of mucus cells and hyper secretion in brook trout; [58] described the presence of brownish mucus on gills and body in Sunpee trout and [27] re-counted the necrotic and degenerating cells in the gills of brook trout. Likewise, [17] also testified morphological mutilation to the gills with impairment being more sombre at low pH. Results obtained in the present investigation force the author of the experiment to suggest that C. carpio is showing higher sensitivity to low pH. [63] also suggested the pH sensitivity of C. carpio. The lesions on the gills and the occurrence of mucus might have disturbed the gill respiration as reported [12]. The gill is the principal target organ and death results from a combination of ionoregulatory, osmoregualatory and respiratory dysfunction [60,64,12].
Intestine
Intestinal pathology also believed to be a very sensitive indicator of acid stress in fishes. C.carpio exposed to different acidic exposures to pH 6.6, 5.8 and 5.0 exhibited the disruption in the intestinal morphology. C.carpio, under various degrees of acidic stress revealed the formation of necrosis and atrophy of intestinal villi, sub mucosal edema and mucosal necrosis and the massive destruction of the mucosal epithelium. As there are no accessible research findings on the histopathological vicissitudes in the fish intestine exposed to acidic waters, the author of the contemporaneous exploration could not associate the present results with the related publications. However, the comparison was made with the pathological changes attained in the intestinal tissues of fishes exposed to other environmental toxicants. Histopathological changes revealed by the acid stressed (pH 6.6, 5.8 and 5.0), C.carpio exhibited the formation of necrosis and atrophy of the intestinal villi, submucosal edema and mucosal necrosis and massive destruction of the mucosal epithelium. The results of the present observations corroborated well with the results reported by [12] in Oreochromis mossambicus, [65] in Scylla serrata when exposed to higher concentrations of heavy metals, [66] in Sarotherodon mossambicus exposed to mercury and [67] in Puntius sarana exposed to malathion. [68] Have also reported similar changes in Oreochromis mossambicus intoxicated with sublethal exposures of phosphomidan and [59] in Aplocheilus lineatum when exposed to effluents from the coconut husk retting pit. Disruption of histological organization in the organ systems reduced the output of enzymes and consequently impairment of digestive and absorptive process and their physiological efficiencies.
Liver
The vertebrate liver has an outstanding role to play in the physiology and wellbeing of the organisms. In addition to taking part in digestion, it serves in storing glycogen and performing most of the anabolic functions. Detoxification is one of the vital roles of the liver. It is testified that smooth endoplasmic reticulum is involved in detoxifying poisonous constituents entering the systems. Maiming to hepatic parenchymal tissue has been the most often stated pathological effect on fishes exposed to various chemical agents [69,70,71,72]. Pollution which has a harmful effect on the organism leaves indelible marks on the structure of the liver and its functioning. The present observations showed that the acidic stress effectively affected the liver tissues of C. carpio. The results of the histopathological examination of acid stressed C.carpio exhibited the formation of vacuolations in hepatocytes and the formation of necrotic hepatitis (NH). The fallouts of the present observations corroborated well with the results reported by [12] in Oreochromis mossambicus were exposed to different acidic pH, [73] in Heteropneustes fossilis when exposed to textile mill effluent; [74] in Notopterus notopterus exposed to phenolic compounds; [68] in Oreochromis mossambicus when exposed to phosphomidan for 120 hrs, [75] in Lebistes reticulates exposed to toxicity of zinc and cadmium for two months [59] in Aplocheilus lineatum exposed to coconut coir retting effluents. Many marks of widespread cellular worsening ensued all over the body. An organ, specifically affected is liver. This happens mainly because of the absence of adequate nutrients to upkeep the normal high rate of metabolism in the liver cells, but also partly because of the dangerous vascular exposure of the liver cells to any toxic or other metabolic influence in jolt or stress. Amid the detrimental cellular effects that are known to occur in most body tissues lessening of active transport of sodium and potassium through the cell membrane is noteworthy. As an outcome of sodium and chloride accretion in cells, potassium is vanished from the cells. In addition, cells instigate to swell and mitochondrial bustle in the liver cells as well as in many other tissues of the body becomes sternly dejected. Lysosomes begin to split open tissues with the intracellular release of hydrolase that cause further intracellular deterioration. Cellular metabolism of nutrients such as glucose ultimately befits significantly depressed in the last stages of shock. The accomplishments of some hormones are depressed as well, including as much as 200 fold depression in the action of insulin. All these effects contribute to further deterioration of many organs of the body, including the liver with depression of its many metabolic and detoxification functions [76]. The Liver is the principal of intercessor metabolism and when it was affected, the entire physiological coordination is abnormal. Finally the pH of the water is very important for the normal growth and development of the fishes, the acidic pH will affect the total physiology of the fishes, especially the growth, nutritive components, metabolism, Hematology and Histology of the fishes [12]; It will lead to the disappearance of the fish species [12]; Aquatic acidification, both freshwater and ocean are the serious global issue; it will affect production of fishes, the aquatic resources and aquaculture practices.
- Baker, J. P. NAPAP Report 13. National Acid precipitation Assessment program. Acid deposition, state of the science and technology. 1990; Volume II.
- Beamish, R. Acidification of lakes in Canada by acid precipitation and the resulting effects on fishes. Water Air and Soil Pollution 1976; 6: 501 – 504.
- Beamish, R. J. and H. H. Harvey. Acidification of Lacoche mountain lakes, Ontario and resulting fish mortalities. J. Fish. Res. Board. Can. 1972; 29: 1131 – 1143.
- Beamish, R. K. Growth and survival of white suckers Catostomus commersoni in an acidified lake. J. Fisheries Research Board of Canada. 1974; 31: 49 – 54.
- Beamish, R. Lethal pH for white sucker Catostomus commensoni (Lacepede). Trans. Am. Fish. Soc. 1972; 101: 355.
- Chevalier, G. L. Gauther and Moreau. Histopathological and electron microscopic studies of gills of brook trout (S. fontinals) from acidified lakes. Can. J. Zool.1985; 63: 2062 - 2070.
- Cleveland, L., E. E. Little, S. J. Hamilton, D. R. Buckler and J. B. Honn. Interactive toxicity of Al and acidity to early life stages of brook trout. Trans. Am. Fish. Soc., 1986; 115: 610 – 620.
- Cleveland. L. Chronic no observed effect concentrations of Al for brook trout exposed to low calcium, dilute acidic water. In : Environmental Chemistry and Toxicology of Aluminium edited by T. E. Lewis publishers. Inc. Chelsea. 1989; 229 - 246.
- Couch J. A. Histopathological effects of pesticides and related chemicals on the livers of fish; In: The pathology of fishes edited by W. E. Ribelin and G. Migaki. University of Wisconsin Press, Madison. 1975; 559 – 584.
- Daye, P. and E. Garside. Histopathologic changes in surficial tissue of brook trout. Salvelinus fontinalis (Mitchill), exposed to acute and chronic levels of pH. Canadian Journal of Zoology. 1976; 54: 2140 - 2155.
- Daye, P. and E. Garside. Structural alterations in embryos and alevins of the Atlantic salmon, Salmo salar L., induced by continuous or short term exposure to acidic levels of pH. Canadian Journal of Zoology.1980; 58: 27 – 43.
- Duis, K. and A.Oberemm. Survival and sublethal response of early life stages of pike exposed to low pH in artificial post-mining lake water. J. Fish. Biology. 2000; 57: 597 – 613.
- Dunson, W. A. and D. R. Martin. Survival of brook trout in bog derived acidity gradient. Ecology.1980; 54: 1370 - 1376.
- Exley, C., J. S. Chappell and J. D.Birchall. A mechanism for acute aluminum toxicity in fish. J. Theor. Biol. 1991; 151: 417 – 428.
- Fanta E, Rios FS, Romão S, Vianna, Freiberger S. Histopathology of the fish Corydoras paleatus contaminated with sublethal levels of organophosphorus in water and food. Ecotoxicology and Environmental Safety. 2003; 54(2):119-130
- Freeman, H.C.and G. B. Salang. The effects of an acid river, caused by acid rain, on weight gain. Steroid genesis and reproduction in the Atlantic salmon (Salmo salar). Aquatic Toxicology and Society for testing and materials, Philadelphia, PA. 1985; 339 – 349.
- Fromm, P.A. Review of some physiological and Toxicological responses of fresh water fish to acid stress. Environmental Biology of Fishes. 1980; 5: 79 – 93.
- Gernhofer M, Pawet M, Schramm M, Müller E, Triebskorn R. Ultrastructural biomarkers as tools to characterize the health status of fish in contaminated streams. Journal of Aquatic Ecosystem, Stress and Recovery. 2001; 8:241-260.
- Gjedrem, T. Growth and survival of fingerlings in acidic water. In: SNSF Project Sandef Jord. Norway.1980; p.309.
- Green, A. A. and R. W. Root.The equilibrium between haemoglobin and oxygen in blood of certain fishes. Biol. Bull., 1933; 64 : 383 – 404
- Gupta, Saraj and R. C. Dalela. Liver damage in Notopterus notopterus following exposure to phenolic compounds. J. Environ. Biol. 1986; 7(2): 75 – 80.
- Guyton, A.C. Textbook of medical physiology. 7th edi. Saunders co. Philadelphia PA. 1986
- Hamilton, J. and Terry A. Haines. Influence of fluoride on aluminium toxicity to Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1995; 52: 2432 - 2444.
- Haya, K. and B. A. Waiwood. Acid, pH and chorionase activity of Atlantic salmon (Salmo salar) eggs. Bull. Environ. Contam. Toxicol. 1981; 27: 7 – 12.
- Horning, W. and T. Neiheisel. Chronic effect of copper on the blunt nose minnow Pimephales notatus (Ratinesque). Archives of Environmental Contamination and Toxicology. 1979; 8: 545 – 552.
- Hosler, R. J. Ruby and T. Mc Ilwain. The gill arch of the mullet Mugil cephalus. I. surface ultrastructure. J. Experimental Zoology. 1979; 208: 379 - 398.
- Huges, G. M. Respiratory responses to hypoxia in fish. Am. Zool. 1976; 13: 474 - 489.
- Ibrahim. A. Investigation on the effect of environmental acidification (low pH) on the eco-physiology of fishes, Ph.D. thesis, 2003, Manonmanium Sundaranar University, India
- Indira.N. P., Ramkumar and V. Meenakshi. Histopathological lesions in Oreochromis mossambicus subjected to acute phosphomidn toxicity, sustainable environment, 1999 ed (N. Sukumaran).1999;196 – 201.
- Jagoe, C. H. and T. A. Haines. Alternations in gill epithelial morphology of yearling sunpee trout exposed to acute acid stress. Trans. Am. Fish. Soc. 1983; 112: 659 - 695.
- Jagoe, C. H., T. A. Haines, D. R. Buckler. Abnormal gill development in Atlantic salmon (Salmo salar) fry exposed to aluminium at low pH. Annal. Soc. R. Zool. Belg.1987; 117 (suppl.1): 375 – 87.
- Johnson, D. and D.Webster. Avoidance of low pH in selection of spawning sites by brook trout Salvelinus fontinalis. Journal of the Fisheries Research Board of Canada. 1977; 34: 2215 – 2218.
- Johnson, D. W. Pesticides and fishes - A review of selected literature. Trans. Am. Fish. Soc. 1968; 97: 398 – 424.
- Kendall, M. and J. Dale. Scanning and transmission electron microscopic observations of rainbow trout (Salmo gairdneri) gill. J. Fisheries Research Board of Canada. 1979; 36: 1072 - 1079.
- Lacorix, G. L. Ecological and physiological response of Atlantic salmon in acidic organic rivers of Nova scotia, Canada. Water Air Soil Pollut.1989; 48: 375 - 386.
- Lacorix, G. L. Effects of low environmental pH on survival growth and ionic composition of post emergent Atlantic salmon. Can. J. fish. Aquat. Sci. 1985; 43: 768 – 75.
- Lacorix, G. L., D. J. Gordon and D. L. Johnson. Effects of low pH on the survival, growth and ionic composition of post-emergent Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1987; 42: 768 – 775.
- Laurnet, P. and S. Dunnel. Morphology of gill epithelia in fish. American Journal of Physiology.1980; 238: R 147 - R 159.
- Leino, R. and Mc Cormick. Morphological and morphometrical changes in chloride cells of the gill of Pimephates promeals after chronic exposure to acid waters. Cell Tissue Res. 1984; 236 : 121 - 128.
- Lewis, S. and I. Potter. A scanning electron microscope study of gills of lamprey Lampetra fluvitalis (L). Micron. 1976; 7 : 205 - 211.
- Madaswamy, S. Ecophysiology of a chosen freshwater fish Aplocheilus lineatum (Valenciennes) Ph. D. thesis. 2001. M. S. University, India.
- Mc Kee, M. J., C. O. Knowles and D. R. Buckler. Effects of Al on biochemical composition of Atlantic salmon. Arch. Environ. Contam. Toxicol.1989; 19: 101 – 118.
- Mc Kim, J. and Benoit. Effects of long-term exposures to copper on survival growth and reproduction of brook trout (Salvelinus fontinalis). Journal of the Fisheries Research Board of Canada.1971; 28: 655 – 662.
- Mills, K. H., S. M. Chalanchok, L. C. Mohr and I. J. Davies. Responses of fish populations in lake 223 to and years of experimental acidification. Can. J. Fish. Aquat. Sci. 1987; 44 : 114 – 125
- Moitra, A. and R. Lal. Effect of sublethal doses of malathion and BHC on the intestine of the fish Puntius sarana. Environ. Ecol., 1989; 7(2) : 412 - 414.
- Mount, D. I. Chronic effect of low pH of fathead minnow survival, growth and reproduction. Water Res. 1973; 7: 987 – 93.
- Mount, D. R. and M. J. Swanson. Responses of brook trout (Salvelinus fontinalis) fry to fluctuating acid, aluminium and low calcium exposure. Can. J. Fish. Aquat. Science. 1990; 47: 1623 – 1630.
- Muller, D. A., D. A. Sanchez and H. L. Bergman and D. G. Mc Donald, R. G. Rhem and C. M. Wood. Nature and time course of acclimation to aluminium in juvenile brook trout (Salvelinus fontinalis). Gill Histology Can J. Fish. Aquat. Sci. 1991; 48 : 2016 - 2027.
- 49.Muniz, I. P. & Leivestad, H. Acidification - effects on freshwater fish. Proceedings of the International Conference on the Ecological Impacts of Acid Precipitation, 1980.84–92, eds Drabløs, D. & Tollan, A. Oslo. Norway: SNSF project.
- Murugesan. A. G. Toxicity of textile mills effluent to an air breathing fish. Ph.D. Thesis.1988.Madurai Kamaraj University. India
- Naidu, Akkilandar, K., K. Abhinender Naidu and R. Ramamurthi. Histopathological studies in liver and intestine tissue of Sarotheordan mossambicus in response to mercury toxicity. Proc. An India Symp. on Physiol. Response of animal to pollution 1982.
- Narayanan, K. R. Toxicity studies on the estuarine portunid crab Scylla serrata (Forskal); Ph. D. Thesis, Annamalai University. 1989;155.
- Nevile,C. M. Sub lethal effects of environmental acidification on rainbow trout (Salmo gairdneri) J. Fish. Res. Board. Can., 1979; 36: 84 - 87.
- Olson,R. and P. Fromm. A scanning electron microscope study of secondary lamellae and chloride cells of rainbow trout (Salmo gairdneri). Zeitschrift for Zeller for schung and Mikroskopische Anatomie. 1973; 143 : 439 - 449.
- Ortiz-Delgado JB, Segner H, Arellano JM, Sarasquete C. Histopathological alterations, EROD activity, CYP1A protein and biliary metabolites in gilthead seabream Sparus aurata exposed to Benzo (a) pyrene. Histology and Histopathology. 2007; 22(4): 417-432.
- Packer,R. and W. Dunson. Anoxia and sodium loss associated with the death of brook trout at low pH. Comp. Biochem. Physiol. 1972; 41A : 17 - 26.
- Peterson,R. H., P. G. Daye and J. L. Metcalfe. Inhibition of Atlantic salmon (Salmo salar) hatching at low pH. Can. J. Fisheries and Aquat. Sci. 1980; 37 : 770 – 774.
- Rasmussen, G. and Geertz-Hansen. Influence of ohre and acidication on the survival and hatching of brown trout eggs (Salmo trutta). In : Sublethal and chronic effects of pollutants in fresh water fish (Muller, R. and Lyod, R. eds.) 1994; 196 - 210. Oxford. Black well.
- Roseland, B. Physiological responses to acid water in fish. Drablos and Tollan. 1980; 348 - 349.
- Runn, P. N., Johansson and G. Milbrink. Some effects of low pH on the hatchability of eggs of Perch Perca fluviatilis L. Zoon. 1977; 5: 115 – 125.
- Salate Rengithabai,U. Effect of low pH on the feeding energetics and respiratory metabolism of Sarotheordran mossambicus M.Phil. Thesis, 1987. Madurai Kamarajar University, India.
- Schaperclaus. Fish disease, 1991, published by Gulab Primalani Oxonian Press Pvt. Ltd. New Delhi.
- Schwaiger J, Wanke R, Adam S, Pawert M, Honnen W, Triebskorn R. The use of histopathological indicators to evaluate contaminant-related stress in fish. Journal of Aquatic Ecosystem Stress and Recovery. 1997; 6(1):75- 86.
- Sharma, Aravind and M. S. Sharma. Acute and chronic toxicity of zinc and cadmium to some developmental stages of Lebistes reticulates (Peters). Poll. Res. 1995; 14 (1) : 83 – 92.
- Smith, R.and Terry A. Haines. Mortality, growth, swimming activity and gill morphology of brook trout Salvelinus fontinalis and Atlantic salmon (Salmo salar) exposed to low pH with and without aluminium. Environmental Pollution.1995; 90(1):33 - 40.
- Tietge, J. E., R. D. Johnson and H. L. Bergman.Morphometric changes in gill secondary lamellae of brook trout (Salvelinus fontinalis) after long term exposure to acid and aluminium. Can. J. Fish. Aquat. Sci. 1988; 45 : 1643 - 1648.
- Townsend,L. D. and H. Cheyne. The influence of hydrogen ion concentrations on the minimum dissolved oxygen toleration of silver salmon Oncorhynchus kisutch. Ecology. 1944; 25: 461 – 466.
- Trojnar, J. R. Egg and larval survival of white sucker (Catostomus commersoni) at low pH. J. Fish. Res. Board. Can. 1977; 34 : 262 – 266.
- Tucker, R. K. and J. S. Leitzke. Comparative toxicology of insecticides for vertebrate wild life and fish. J. Pharmacol. Ther.1979; 6: 167 – 220.
- Ultsch, G. and Gros. Mucus as a diffusion barrier to oxygen: possible role in O2 up take at low pH in carp (Cyprinus carpio) gills. Comparative Biochemistry and physiology.1979; 62 : 685 - 689.
- Vaala, S. S. and R. B. Mitchell. Blood oxygen tension changes in acid expressed brown trout. Penn. Aqua. Sci. 1970; 41 - 44.
- Van der Oost R, Beyer J, Vermeulen N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003; 13:57-149.
- Watt, W. D. A summary of the effects of acid rain on Atlantic salmon (Salmo salar) in Canada. Water Air Soil Pollut.1987; 35 : 27 – 35.
- Weesner. Frances .M. General zoological microtechniques; 1960, Huntington Publisher, N.Y. USA
- Westfall, B. Coagulation film anoxia in fishes. Ecology. 1945; 26: 283 - 287.
- Wiebe, A. H., A. M. Mc Gavrock, A. C. Fuller and H. C. Markus. The ability of fish to extract oxygen at different hydrogen ion concentrations. Physiol. Zool. 1934; 7: 435 – 488.
- Winkaler EU, Silva AG, Galindo HC, Martinez CBR. Biomarcadores histológicos e fisiológicos para o monitoramento da saúde de peixes de ribeirões de Londrina, Estado do Paraná. Acta Scientiarum. 2001; 23(2):507-514
- Wood, C. M.The physiological problems of fish in acid waters. In Acid toxicity and Aquatic animals (Morns, R., Taylor, E. W., Brown, D. J. A. and Brown J. A) Cambridge university press. 1989. 125 - 152.
- Youson, J. and C. Neville. Deposition of aluminium in gill epithelium of rainbow trout (Salmo gairdneri Richardson) subjected to sublethal concentrations of heavy metal. Can. J. Zool. 1987; 65: 647 - 656.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
