Global Journal of Rare Diseases
Compensatory alterations in dermal innervations in patients with congenital insensitivity to pain
Jan Minde1,2, Olle Svensson2, Göran Toolanen2 and Sol-Britt Lonne-Rahm3*
2Department of Surgery and Perioperative Science, Division of Orthopaedics, Umea University Hospital, Umea, SE-901 85, Sweden
3Department of Rheumatology and Dermatology, Malarsjukhuset, Hospital in Eskilstuna, Karolinska Institute, Sweden
Cite this as
Minde J, Svensson O, Toolanen G, Lonne-Rahm SB (2019) Compensatory alterations in dermal innervations in patients with congenital insensitivity to pain. Glob J Rare Dis 4(1): 022-026. DOI: 10.17352/2640-7876.000018Context: The purpose of this study was to determine whether the expression of sensory neuropeptides, NK1, 5-HT1A receptors, as well as mast cells in the skin of patients with hereditary neuropathy and sensory and autonomic deficits (HSAN type 5) was elevated. Such increase might reflect an attempt to compensate for nerve loss.
Materials and methods: Six patients with HSAN type 5, three of which were heterozygous and three were homozygous and examined whether there were any compensatory mechanisms in the skin cells to prevent tissue damage.
We compared the innervation of nerve fibres and mast cells in skin biopsies from the arms and legs of these patients and compared these to biopsies from healthy control individuals.
Results: Both types of patient groups showed reduced cutaneous nerve fibres and the existence of the sensory neuropeptide substance P in the skin. In the homozygous patients, the Neurokinin 1 (NK1) Receptor(R) was present at a higher level in the dermis of the legs than in both the heterozygous patients and in the healthy controls.
In addition, cells staining positively for serotonin (5-HT; 5-hydroxytryptamin) as well as 1AR and tryptase positive cells (mast cells) were more frequent in the patients compared to controls.
Conclusion: Thus, increase in the number of cells that express the receptors for NK1 and 5-HT1A, along with tryptase positive cells, may be associated with the severely reduced number of nerve fibres observed in our patients.
Introduction
Pain is an unpleasant sensory experience that serves the purpose of triggering avoidance and thereby protecting the body from damage. In the skin we have c-fibers and alpha delta fibers which via chemical messenger signal pain in skin damage. The experience of pain is a result of actual or potential tissue damage. In the absence of nerve fibers in the skin, can bodily injury lead to ulcerations, large tissue damage, infections and autonomic dysfunctions such as anhidrosis.
The heterogeneous group of rare Hereditary Neuropathies with Sensory and Autonomic Deficits (HSAN) are traditionally classified into 5 groups based on age at the time of onset, pattern of inheritance and clinical presentation [1-3]. To date, five genes have been linked to autosomal dominant and seven to recessive forms of HSAN [4]. The patients usually exhibit pronounced loss of distal sensation, including pain and temperature sensitivity which can lead to ulceration, self-mutilation, infections and autonomic dysfunctions such as anhidrosis [5].
HSAN 1, the most common form of sensory and autonomic deficit, is autosomal dominant and involves loss of all modalities of sensation as well as foot ulcers. In the case of the autosomal recessive HSAN 2, the patient exhibit chronic ulcerations in both the arms and the legs. HSAN 3 is characterized by widespread autonomic dysfunction in combination with loss of the sensations of pain and temperature. HSAN 4 is a rare autosomal recessive disorder with anhidrosis [6,7]. Finally, HSAN 5 is associated with selective loss of feelings of pain and temperature, caused by mutations in the genes encoding Neurotrophic Tyrosine Kinase (NKT) Receptor (R) and Nerve Growth Factor β (NGFβ), but few other autonomic deficits [8-10]. They have R221W mutation in the NGF β gene.
Previously, we characterized patients in northern Sweden who carry a mutation in the gene encoding NGF β [9]. Homozygous individuals exhibit clinical symptoms, painless fractures and progressive joint destruction that debut in childhood, with normal sweating and mental capacity [11], while heterozygous patients develop joint disorders later in life [12]. Indeed, two other families recently described as afflicted by HSAN 5 also carry a mutation in the NGF β gene [13,14].
The sensory and autonomic deficits in the skin of such patients are due to loss of both myelinated and unmyelinated nerve fibres among the rich sensory network that reacts to painful stimuli. Neuropeptides are involved in a variety of cutaneous functions and Calcitonin Gene-Related Peptide (CGRP) and substance P are expressed in C-fibres. Substance P binds to its NK1R, which plays an important role in pain and hyperalgesia [15].
Mast cells stimulate cutaneous nerve fibres to secrete neuropeptides [16] and may contribute to the development of pain and hyperalgesia, possibly also by exerting direct effects on nerves, as proposed in the case of endometriosis [17].
In addition, serotonin (5-hydroxytryptamin; 5-HT) 1ARs are often co localized with NK1R in the brain and are essential for neuronal survival dendricity.
Patients and Methods
Patients
We chose six patients with attenuated sensitivity to deep pain and temperature that had normal mental development and sweating.
Three homozygous patients for this mutation, two men and one woman (age 20,27 and 45) are all insensitive to deep pain and have experienced painless fractures and progressive joint destruction since childhood.
Previous studies of these patients have shown normal velocity of nerve conduction and Electromyography (EMG) patterns, but Sympatic Skin Response (SSR) was absent in two. Their sural nerve biopsies exhibited a moderate loss of thin myelinated fibres (Aδ) and severe loss of unmyelinated (C) fibres [1].
Three heterozygous patients two women and one man (range 50,55 and 78 years), the oldest suffered from polyneuropathy, with Charcot joints in the knees and ankles, while the other two were clinically normal except for pronounced Carpal Tunnel Syndrome (CTS). All three had undergone sural nerve biopsy, which revealed moderate loss of both thin myelinated (Aδ) and unmyelinated fibres (C) [2].
The three control subjects without the NGFB R221W mutation or other neurological disease were two men and one woman with a mean age of 69 years.
All subjects gave their informed written consent to participate and preapproval by the local ethics committee of Umeå university was obtained (04-058M).
Table 1 documents the clinical features and neurological findings for all 6 patients and control subjects.
Processing of skin biopsies
Skin biopsies of 4mm were taken from the lateral upper arm, the thigh (behind the trochanter major) and the calf (20cm below the tibial tubercle) of each subject under local anesthesia. These biopsies were immediately fixed in phosphate-buffered 10% formalin (v/v) containing 0.4% picric acid (w/v) at 4°C for 2h and then rinsed in cold 0.1mol/l Sörensen’s phosphate buffer containing 10% sucrose for at least 48h, snap frozen and stored at -70°C until analysis.
Sections (14µm) of the skin biopsies were prepared on a Microm cryostat (Heidelberg, Germany), mounted on Super Frost Plus glass slides (Menzel-Gläser, Freiburg, Germany) and stored at -70°C until being subjected to immunohistochemistrical analysis. Sections at three levels encompassing the entire biopsy were performed.
Immunohistochemistry
A blocking solution containing 1% bovine serum albumin (w/v), 0.3% Triton X-100, and 0.1% sodium azide in phosphate-buffered saline (PBS) was placed gently on top of each section, followed by incubation for 1h at room temperature. The sections were then rinsed again in PBS and thereafter incubated overnight (4°C) in a humid chamber with one of the antibody preparations described in Table 2. The 5-HT1AR antibody is directed against amino acid residues 170-186 in the second extracellular loop of the rat protein [18]. No staining specific for autonomic fibres was performed.
On the following day the sections were rinsed again in PBS and then incubated either with a biotinylated goat anti-rabbit (for polyclonal primary antibodies) (BA-1000) or a biotinylated horse anti-mouse (monoclonal) (BA-2000) secondary antibody (both diluted 1:200, Vector, Burlingame, CA, USA) for 40min. The primary antibodies were visualized by incubation with the fluorochrome Cy2-labelled streptavidin (PA42001; diluted 1:2 000; Amersham Pharmacia Biotech, Uppsala, Sweden) and the sections then rinsed in PBS and mounted with Kaisers glycerol gelatin (Merck, Darmstadt, Germany) before being covered with glass slips.
Microscopy
The stained sections were examined under a Nikon epifluorescence microscope (Eclipse E800, Yokohama, Japan) with Cy 2 and FITC fluorescence being visualized following excitation at 465-495nm and Texas Red fluorescence following excitation at 540-580. Digital photographs (Nikon DXM 1200) of the stained slides were then coded and examined blindly by one of the authors (SLR).
Statistical methods and data management
Multiple comparisons of continuous data were performed by analysis of variance, ANOVA. In the case of a statistically significant result in the ANOVA, statistical comparisons were made by use of the post-hoc test proposed by Fisher to control for multiplicity [18,19]. In addition to that descriptive statistics was used to characterize the data. All analyses were carried out by use of statistical software (The SAS system for Windows 9.3. SAS Institute Inc. Cary, NC, USA.). A p-value of <0.05 was considered as significant and in the case of a statistically significant result the probability value (p-value) has been given.
Results
The control individuals exhibited the highest densities of nerve fibres in the papillary dermis of the upper arm and thigh.
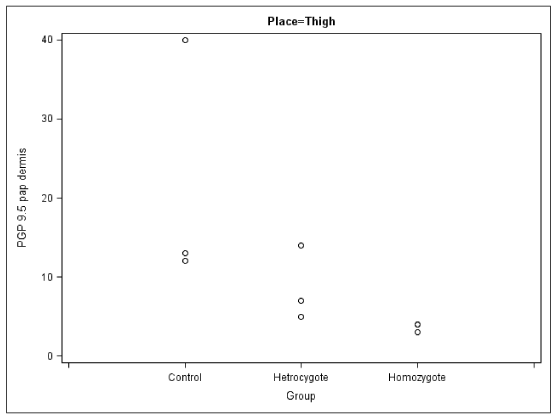
All patients both homozygous and heterozygous had significantly reduced density of peripheral nerve fibres expressing PGP 9.5 in all sites where the biopsies were taken. In the biopsies from the homozygous patients the most pronounced differences were observed in the thigh and calf, the largest difference being observed in the upper arm in epidermis and dermis in crus. All biopsies from the heterozygous patients also demonstrated a moderate decrease of nerve fibre density (Figure 1).
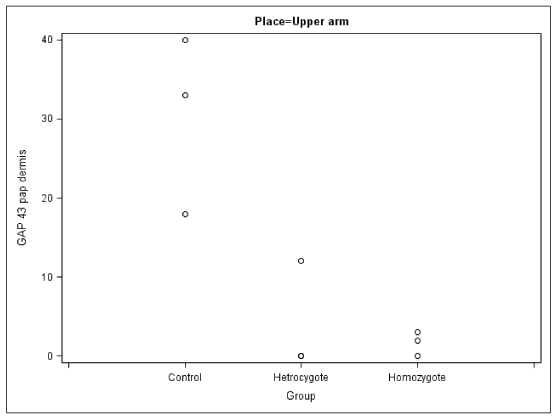
No axon outgrowth (i.e., positive staining for GAP 43) was observed in the homozygous patients, and this outgrowth was also attenuated in the heterozygous individuals.
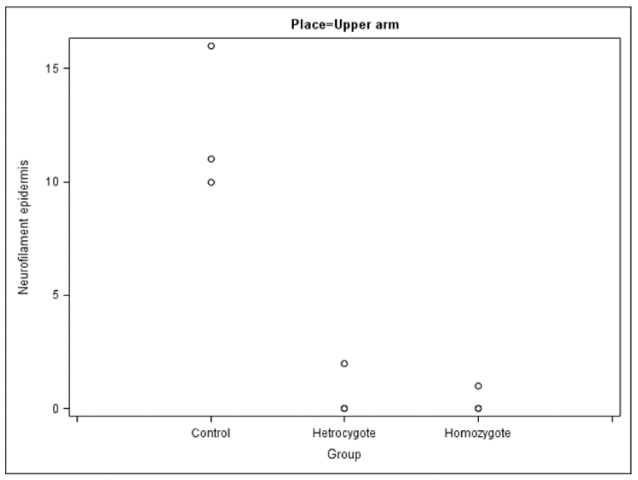
The number of myelinated nerve fibers (i.e., expressing NF200) was low in both groups of patients, and in the homozygous ones the number of fibers in biopsies from the calf were extremely low.
Moreover, in the homozygous patients sensory nerve fibers staining for substance P and in particular for CGRP were absent from the epidermis and severely reduced in number in the papillary dermis of the upper arm.
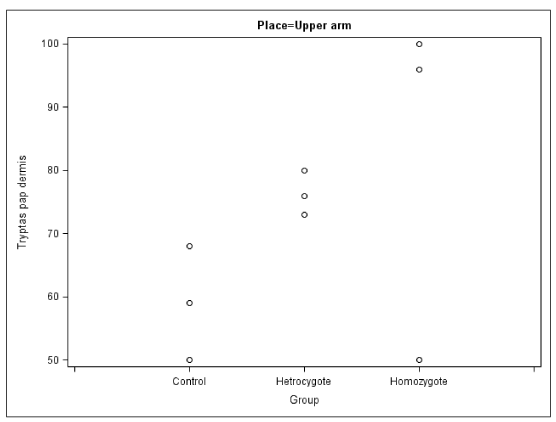
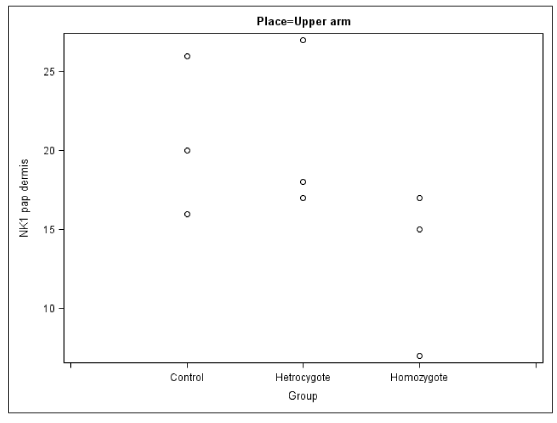
In the papillary dermis of the arms and legs of the homozygous patients the number of sensory nerve fibers positively staining for NK1R were similar to that of the corresponding values in the control group, but this number was elevated in their calves. These homozygous patients also exhibited elevated numbers of cells expressing 5-HT1AR and tryptase (mast cells) (Figures 2,3).
Discussion
In the present study we have found reductions in the number of thin nerve fibres from our HSAN type 5 patients. There was also an increased number of NK1R and 5-HTA receptors positive cells in dermis as well as tryptase positive cells (Figures 4,5).
Alterations in the number of cells expressing the NK1 and 5-HT1A receptors, as well as that of mast cells, may be associated with the severe nerve loss, and may thus be involved in the underlying pathogenic mechanism [20]. Moreover, the reductions in intraepidermal nerve fibres, as well as in the expression of the sensory neuropeptides substance P and CGRP at all locations examined in these patients is consistent with the severe loss of unmyelinated nerve fibres (C) observed previously in sural nerve biopsies from patients homozygous for the HSAN type 5 patients [10].
The level of substance P in the synovial fluid of HSAN type 4 patients has been reported to be reduced [21] and it has been proposed that the high incidence of bone fractures in these patients can be explained by their lower number of substance P positive nociceptive fibres. This may also be the case for the patients studied here, whose more advanced neural deficiency in the lower extremities may be the cause of their high incidence of arthropathy.
Furthermore, both cutaneous sensory nerve fibres and epidermal and dermal cells are capable of producing a variety of neuromediators, including the sensory neuropeptides substance P and CGRP. Substance P is widely expressed in the peripheral nervous system and exerts a wide range of peripheral and central activities, including transmission of pain [16].
Finally, the elevated numbers of NK1 and 5-HT1A receptor positive cells observed in this study may represent protective compensation by skin containing a reduced number of sensory nerves. Serotonin is essential for numerous basic cellular functions including proliferation, differentiation, maturation and migration [22]. Agonists to its well- characterized 5-HT1AR provide protection against apoptosis and, moreover, this receptor is neuroprotective in animal models of brain ischemia (Figure 6) [23].
Nerve conduction and electromyography characterize primarily the function of large myelinated nerve fibres and, at present, only a few methods for the visualization of small fibre neuropathy are available. In this context skin biopsies are less invasive than sural nerve biopsies. Moreover, the former is useful for characterizing neuropathies involving sensory small fibres and can be taken repeatedly to monitor the progression of a neuropathy [24].
We are grateful to Ms Anna-Lena Kastman and Ms Christelle Jakobsson for technical assistance. Financial support was provided by the Norrbotten Research Institute (FOU), the Committee for Cooperation among Northern County Councils (Visare Norr), and the Welander/Finsen foundation.
- Dyck PJ, Mellinger JF, Reagan TJ, Horowitz SJ, McDonald JW, et al. (1983) Not `indifference to pain´ but varieties of hereditary sensory and autonomic neuropathy. Brain 106: 373-390. Link: http://bit.ly/2tpithO
- Axelrod FB, Gold-von Simson G (2007) Hereditary sensory and autonomic neuropathies: types II, III, and IV. Orphanet J Rare Dis 2: 39. Link: http://bit.ly/34vQVV4
- Nagasako EM, Oaklander AL, Dworkin RH (2003) Congenital insensitivity to pain: an update. Pain 101: 213-219. Link: http://bit.ly/2Eq7A1E
- Davidson G, Murphy S, Polke J, Laura M, Salih M, et al. (2012) Frequency of mutations in the genes associated with hereditary sensory and autonomic neuropathy in a UK cohort. J Neurol 259: 1673-1685. Link: http://bit.ly/38Tst3o
- Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, et al. (1996) Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet 13: 485-488. Link: http://bit.ly/38OHaof
- Nolano M, Crisci C, Santoro L, Barbieri F, Casale R, et al. (2000) Absent innervations of skin and sweat glands in congenital insensitivity to pain with anhidrosis. Clin Neurophysiol 111: 1596-1601. Link: http://bit.ly/2PRTMlO
- Capsoni S, Covaceuszach S, Marinelli S, Ceci M, Bernardo A, et al. (2011) Taking pain out of NGF: A "painless" NGF mutant, linked to hereditary sensory autonomic neuropathy type V, with full neurotrophic activity. PLoS One 6: E17321. Link: http://bit.ly/2qZuRUS
- Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, et al. (2004) A mutation in the nerve growth factor-beta gene (NGFβ) causes loss of pain perception. Hum Mol Genet 15: 799-805. Link: http://bit.ly/36WFkjx
- Houlden H, King RH, Hashemi-Nejad A, Wood NW, Mathias CJ, et al. (2001) A novel TRK A (NTRK1) mutation associated with hereditary sensory and autonomic neuropathy type V. Ann Neurol 49: 521-525. Link: http://bit.ly/2Eq8gEe
- Minde J, Toolanen G, Andersson T, Nennesmo I, Nilsson-Remahl I, et al. (2004) Familial insensitivity to pain with neurogenic arthropathy but without anhidrosis (HSAN V). A neurophysiological and neuropathological studyin. Muscle Nerve 30: 752-760. Link: http://bit.ly/2Q2SQv8
- Minde J, Andersson T, Fulford M, Aguierre M, Nennesmo I, et al. (2009) A novel NGFB point mutation: a phenotype study of heterozygous patients. J Neurol Neurosurg Psychiatry 80: 188-195. Link: http://bit.ly/36WFCXF
- Carvalho OP, Thornton GK, Hertecant J, Houlden H, Nicholas AK, et al. (2011) A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet 48: 131-135. Link: http://bit.ly/2syTxEd
- Covaceuszach S, Capsoni S, Marinelli S, Pavone F, Ceci M, et al. (2010) In vitro receptor binding properties of a "painless" NGF mutation, linked to hereditary sensory autonomic neuropathy type V. Biochem Biophys Res Commun 391: 824-829. Link: http://bit.ly/2tsev89
- Laird JM, Roza C, De Felipe C, Hunt SP, Cervero F (2001) Role of central and peripheral tachykinin NK1 receptors in capsaicin-induced pain and hyperalgesia in mice. Pain 90: 97-103. Link: http://bit.ly/36G1HJE
- Puxeddu I, Piliponsky AM, Bachelet I, Levi-Schaffer F (2003) Mast cells in allergy and beyond. Int J Biochem Cell Biol 35: 1601-1607. Link: http://bit.ly/2Z1dMX6
- Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, et al. (2006) Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril 86: 1336-1343. Link: http://bit.ly/34xXiqA
- Azmitia EC, Yu I, Akbari HM, Kheck N, Whitaker-Azmitia PM, et al. (1992) Antipeptide antibodies against the 5-HT1A receptor J Chem Neuroanat 5: 289-298. Link: http://bit.ly/2rX63gQ
- Daniel Wayne W (1995) Biostatistics: A foundation for analysis in the health sciences. 6 edn John Wiley and Sons New York.
- Montgomery Douglas C (1991) Design and analysis of experiments. 3 edn John Wiley and Sons New York.
- Azmitia EC, Rubinstein VJ, Strafaci JA, Rios JC, Whitaker-Azmitia PM (1995) 5-HT1A agonist and dexamethasone reversal of para-chloroamphetamine induced loss of MAP-2 and synaptophysin immunoreactivity in adult rat brain. Brain Res 677: 181-192. Link: http://bit.ly/2PPZYuo
- Derwin KA, Glover RA, Wojtys EM (1994) Nociceptive role of substance-P in the knee joint of a patient with congenital insensitivity to pain. J Pediatr Orthop 14: 258-262. Link: http://bit.ly/2M82GKZ
- Slominski A, Wortsman J, Tobin DJ (2005) The cutaneous serotonergic/malatonergic system: securing a place under the sun. FASEB J 19: 176-194. Link: http://bit.ly/38OpyZB
- Bielenberg GW, Burkhart M (1990) 5-Hydroxytryptamine 1A agonists. A new therapeutic principle for treatment. Stroke 21: 161-163. Link: http://bit.ly/36Lxhpp
- Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC (1999) Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology 53: 1634-1640. Link: http://bit.ly/36OEKE2

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
