Global Journal of Rare Diseases
A Rare Cause of Oligohydramnios: A Case Report
Nadia Ben Jamaa1, Radhouane Achour1*, Imen KSIBI1, Mohamed Tahar Yacoubi2,3, Sihem Hmissa2,3 and Moncef Mokni2,3
2Research unit 03-08/21, Faculty of medicine of Sousse, University of Sousse, Tunisia
3anatomopathology Department Farhat Hached university hospital, Sousse, Tunisia
Cite this as
Jamaa NB, Achour R, KSIBI I, Yacoubi MT, Hmissa S, et al. (2016) A Rare Cause of Oligohydramnios: A Case Report. Glob J Rare Dis 1(1): 007-009. DOI: 10.17352/2640-7876.000003Anamnios is due to extrinsic and intrinsic conditions. Intrinsic causes include maternal and fetal abnormalities mostly due to cystic renal changes or absence of kidney. Tubular dysgenesis characterized by a lack of proximal tubules should be considered.
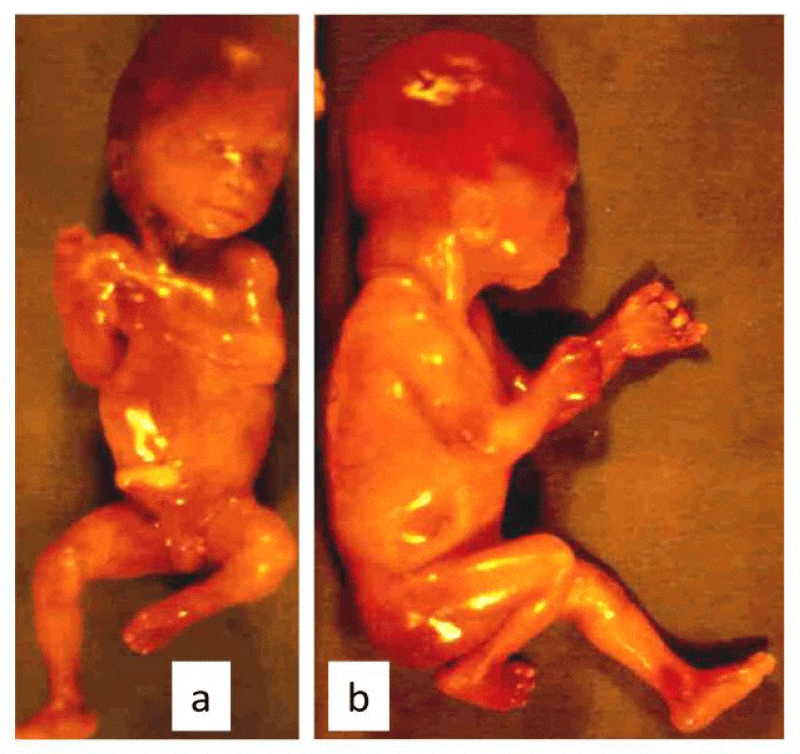
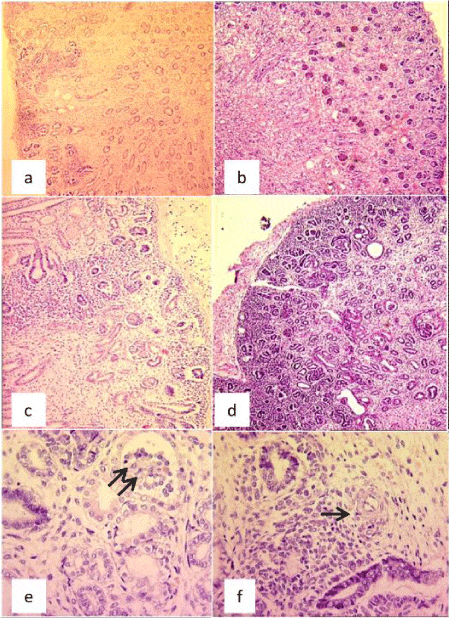
We report a case of tubular dysgenesis associated with lack of vault ossification in the fetus of a 23 year-old woman, prim pare, having a second degree of consanguinity. Ultrasonographic examination performed at 20 weeks of pregnancy identified severe anamnios. Few days later, intrauterine fetal death occurred. The complete autopsy with fetal radiography was performed. The external examination shows a hypotrophic male fetus with oligoamnios sequence deformation and a loss of vault ossification in the skeletal radiography. At dissection, we noticed a pulmonary hypoplasia with small kidneys, having normal architecture at cutting. The histological examination of the samples taken from the kidney shows a hypoplastic feature with a preserved architecture, a conserved cortico-medullary differentiation and the presence of rows of immature nephron at the edges; a very prominent reduction of proximal tubules is noticed. The immunohistochemistry showed a positivity of the tubules with EMA. However, there was lack of positivity with liposomes and alpha anti-trypsine. The diagnosis of tubular dysgenesis associated with lack of vault ossification was retained. Even the kidneys grossly seem to be normal, microscopic study should be meticulous in order to detect segmental nephronic anomalies as tubular dysgenesis. The examination of the bone mineralization and other organs as the liver can sometimes classify this kidney dysgenesis in rare syndromic anomalies.
Introduction
Anamnios is due to extrinsic and intrinsic conditions. Intrinsic causes include maternal and fetal abnormalities mostly due to cystic renal changes or absence of kidney. The renal tubular dysgenesis (RTD) is a recessive autosomal family disorder which leads to absence or severe hypoplasia of proximal tubes [1]. We report a case of anamnios associated with RTD and lack of vault ossification.
Case Report
In a 23 year old patient, prim pare, showing a second degree of consanguinity (her father is the stepfather’s cousin) was detected an anamnios at 20 weeks of pregnancy by ultrasonographic examination. Intrauterine fetal death occurs few days later. No similar case was reported in this family.
The external examination showed a hypotrophic male fetus with oligoamnios sequence deformation: Potter face, comptodactily, clubbed feet and a loss of vault ossification in the skeletal radiography (Figure 1).
At dissection we noticed pulmonary hypoplasia with small kidneys weighting 1g together: inferior to 5th percentile and having normal architecture at cutting.
Histological examination of the samples taken from the kidney shows a hypoplastic feature with a preserved architecture, a conserved cortico-medullary differentiation and the presence of rows of immature nephron at the edges; a very important reduction of proximal tubes is noticed. The interstitial tissue was abundant especially at the medullar level with the presence of vascular structure with thick walls (Figure 2).
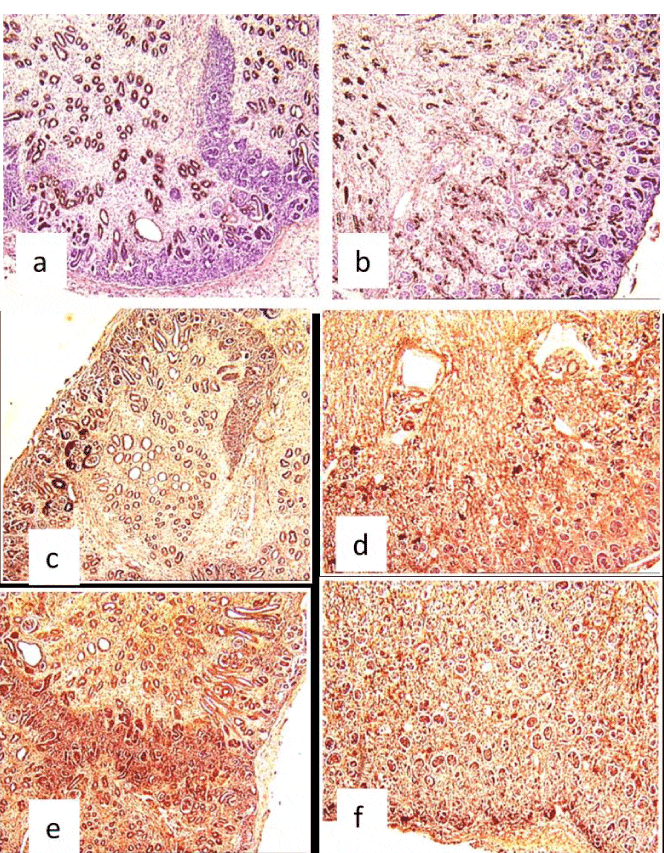
The immunohistochemistry showed a positivity of the tubules with the anti–epithelial membrane antigen (anti-EMA) antibody; however, there was lack of positivity with liposome and alpha anti-trypsine labeling (Figure 3).
The diagnosis of tubular dysgenesis associated with lack of vault ossification was retained.
The lungs were of an immature aspect and the rest of the organs showed no histological anomalies.
Discussion
The renal tubular dysgenesis (RTD) was first described by Allanson [2]. Since then some sixty cases have been reported [3].
It’s an anomaly of renal development accruing at the end of the first trimester of intra uterine life. It’s histological characterized by the absence or rarefaction of distorted proximal tubes. Clinically, it is notable by a precocious and persistent oligoamnios responsible for fetal and prenatal death [3-5].
Renal tubular dysgenesis is a recessive autosomal family disorder which leads to absence or severe hypoplasia of proximal tubules [3]. The RTD is noted with equal frequency in both sexes. Clinically, oligoamnios a constant sign, constitutes the revealing symptom usually detected between 18 and 26 weeks of gestation [6].
The antenatal ultrasound exam does not demonstrate anomalies or obvious causes of oligoamnios, because both kidneys are generally of normal volume and homogeneous echostructure, the urinary cavities are never dilated.
Severe and persistent oligoamnios is a serious complication of RTD, responsible for prenatal fetal death by acute respiratory distress associated with pulmonary hypoplasia. Diagnosis of RTD is always histological and is observed on autopsy examination. The elementary lesion is a global decrease in the number of normally differentiated proximal tubes identifiable by morphological criteria (brush like edges) and immunohistochemistry associated with an abnormal increase in interstitial tissue an important and diffused thickening of the muscular walls of the interlobular and preglomerular arteries; a moderate retraction of glomeruli is also seen [3].
The etiopathogenesis of RTD is a controversial subject.
Some authors advocate an anomaly of the development of the renine-angiotensine system [3,7]. It was shown recently that autosomal recessive renal tubular dysgenesis is genetically heterogeneous and linked to mutations in the genes that encode components of the renin-angiotensin system. Lacoste et coll. recently confirmed the involvement of the RAS by showing that mutations in the genes that encoding renin, angiotensinogen, ACE, and AngII receptor type 1 (AT1) are associated with autosomal recessive RTD. The study of the protein of these systems demonstrated a considerable increase in the distribution of renine in the juxta glomerular system, but also in the mesangium and the smooth muscular cells at the totality of the renal arterial structures. This will be responsible for a renal hypoperfusion.
Some authors proposed within a primitive deficit of the development of proximal tubes due to an anomaly of the growth factor implicated in the differentiation of these nephritic segments [7].
RTD was described in certain fetuses in association with other malformation and/or in metabolic anomalies such as a defect of mineralization of calvaria , resulting in wide cranial sutures and large fontanels, a hemochromatosis [3,4,8,9].The genetic cause of this group of lesion could not be confirmed. Some authors suggest that these lesions are related rather to an antenatal hypoxy (ischemic lesions) [3].
The combination of renal tubular dysgenesis (RTD) and meconium ileus was described for the first time by Swinford and coll [6].
Other authors described a case of congenital hypernephronic nephromegaly with tubular dysgenesis [10]. Light microscopy demonstrates increased numbers of glomeruli, undifferentiated tubules, and interstitial fibrosis. The recurrence of this anomaly in the male children of a consanguineous couple suggests an X-linked recessive mode of inheritance, although an autosomal-recessive mode of inheritance cannot be ruled out.
Daïkha-Dahmane and coll. report renal lesions observed in a fetus exposed throughout pregnancy to angiotensin II type I (AT 1) receptor antagonists. They suggest that the use of AT 1 receptor antagonists during pregnancy may have a severe deleterious effect on kidney development in the fetus with renal tubular dysgenesis [11]. Kato and coll. report another case of secondary drug-induced RTD du to administration of an angiotensin II receptor antagonist (ARB) during the second trimester of pregnancy [5].
RTD was established by the detection of a monomorphous undifferentiated population of tubules, absence of proximal tubules, and dense epithelial membrane antigen immunoreactivity of all tubules. The lack of alpha1-antitrypsin and lysozyme-immunoreactive tubules was first revealed in the kidney with RTD [12,13]. Swinford and coll. noted that most of the tubules in sections of cortex had the lectin-binding and immunohistochemical characteristics of collecting ducts; proximal tubules were not identified by lectin-binding. Electron-microscopic examination showed a general absence of differentiated characteristics in cortical tubular epithelium, except that rare tubules contained rudimentary proximal tubular brush borders [6].
In conclusion, even the kidneys grossly seem to be normal in fetuses with anuria, fetopathological examination with microscopic study should be meticulous in order to detect segmental nephronic anomalies as tubular dysgenesis. The examination of the bone mineralization and other organs as the liver can sometimes classify this kidney dysgenesis in rare syndromic anomalies. The identification of the disease on the basis of precise histologic analysis and the research of the genetic defect allow genetic counseling [14] and early prenatal diagnosis.
This work was supported by the Ministry of public Health in Tunisia and the Research Unit of fetopathology UR 03/08/21.
- Gribouval O1, Gonzales M, Neuhaus T, Aziza J, Bieth E, et al. (2005) Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Gen 37: 964–968. Link: https://goo.gl/Irhy1b
- Allanson JE, Pantzar JT, MacLeod PM (1983) Possible new autosomal recessive syndrome with unusual renal histopathological changes. Am J Med Genet 16: 57-60. Link: https://goo.gl/HLc3eh
- Lacoste M, Cai Y, Guicharnaud L, Mounier F, Dumez Y, et al. (2006) Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: Role of the Renin-Angiotensin system. J Am Soc Nephrol 17: 2253-2263. Link: https://goo.gl/v3sOH6
- Moldavsky M (2010) Non-specific histopathological changes in kidney with renal tubular dysgenesis. Pathol Res Pract 206: 14-18. Link: https://goo.gl/xTkpGV
- Kato K, Okuda M, Ishikawa H, Takahashi T, Hirahara F (2008) Oligohydramnios and pulmonary hypoplasia: a case in which involvement of an angiotensin II receptor antagonist was suspected. J Obstet Gynaecol Res 34: 242-246. Link: https://goo.gl/HnIOLh
- Swinford AE, Bernstein J, Toriello HV, Higgins JV (1989) Renal tubular dysgenesis: delayed onset of oligohydramnios. Am J Med Genet 32: 127-132. Link: https://goo.gl/NKHBdx
- Gubler MC, Lacote M, Guicharnaud L, Mounir F (1995) Dysgénésie tubulaire rénale et système rénine-angiotensine. Ann Pediat 42: 608-611
- Jain V, Beneck D (2003) Renal tubular dysgenesis in an hydropic fetus with trisomy 21: a case report with literature review. Pediatr Dev Pathol 6: 568-572. Link: https://goo.gl/hbnv9W
- Johal JS, Thorp JW, Oyer CE (1988) Neonatal hemochromatosis, renal tubular dysgenesis and hypocalvaria in neonate. Ped Dev Pathol 1: 433-437. Link: https://goo.gl/fpP3Za
- Voland JR, Hawkins EP, Wells TR, Saunders B, Jones M, et al. (1985) Congenital hypernephronic nephromegaly with tubular dysgenesis: a distinctive inherited renal anomaly. Pediatr Pathol 4: 231-245. Link: https://goo.gl/jXeTXg
- Daïkha-Dahmane F, Levy-Beff E, Jugie M, Lenclen R (2006) Foetal kidney maldevelopment in maternal use of angiotensin II type I receptor antagonists. Pediatr Nephrol 21: 729-732. Link: https://goo.gl/K41ZHE
- Moldavsky M (2000) Lysozyme immunostaining in renal tubular dysgenesis. Pediatr Dev Pathol 3: 200-201. Link: https://goo.gl/Y6a0j8
- Moldavsky M, Shahin A, Turani H (1994) Renal tubular dysgenesis present in a newborn with meconium ileus. Pediatr Pathol 14: 245-251. Link: https://goo.gl/JmXcNf
- Gubler MC (2014) Renal tubular dysgenesis. Pediatr Nephrol 29: 51-59. Link: https://goo.gl/pU23i3

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
