Global Journal of Obesity, Diabetes and Metabolic Syndrome
Exercise targeted at the level of maximal lipid oxidation (LIPOXmax) improves weight loss, decreases orexigenic pulsions and increases satiety after sleeve gastrectomy
Jean-Frédéric Brun1*, Valentin Lasteyrie1, Lylia Hammoudi1,4, David Nocca2, Edouard Ghanassia3, Philippe Noirez4, Constance Chevalier1, Jacques Mercier1 and Eric Raynaud de Mauverger1
2Visceral Surgery Department, CHU Montpellier, France
3St.Therese Clinic, Sète, France
4UFR Staps-Irmes, Ea7329, University Paris Descartes, Paris, France
Cite this as
Brun JF, Lasteyrie V, Hammoudi L, Nocca D, Ghanassia E, et al. (2019) Exercise targeted at the level of maximal lipid oxidation (LIPOXmax) improves weight loss, decreases orexigenic pulsions and increases satiety after sleeve gastrectomy. Glob J Obes Diabetes Metab Syndr 6(2): 017-021. DOI: 10.17352/2455-8583.000037Background and Purpose: Sleeve gastrectomy is a major therapy of morbid obesity, but recent reports suggest that its effects on weight loss are improved when patients increase their muscular activity, while a weight regain may occur in more than 30% of patients after 40 months. Exercise is an effective mean of preventing weight regain. In a recent preliminary study, we reported that exercise training targeted at the level of maximal lipid oxidation (LIPOXmax) improved weight loss and weight stabilization at 30 months. This study extends this previous one and aims at describing the kinetics of weight loss on 60 months after sleeve in people performing or not a LIPOXmax training and to see whether this variety of training exerts a satietogenic effect in sleeve patientsas has been evidenced in nonoperated obese subjects.
Methods: We present a longitudinal study of sleeve associated or not with regular exercise prescribed and followed over 60 months. Two groups of obese patients matched for baseline BMI and age were compared. The first group was treated by sleeve gastrectomy alone and patients did not increase their muscular activity. The second group practiced 3x45 min/week endurance exercise targeted at the level of maximal lipid oxidation (LIPOXmax) after sleeve gastrectomy.
Findings: In a series of 43 patients (splitted into two matched subgroups, 20 trained, and 23 untrained) we confirm that LIPOXmax training associated to sleeve induces a greater weight loss than sleeve alone (p=0.001). In a subset of 10 subjects (6 women and 4 men) we show that the first sessions of of training decrease all items related to hunger (p<0,05) and increase satiety (p<0,05). However, the intensity of these effects is lower than previously observed in nonoperated obese subjects.
Conclusion: Therefore, the long lasting, moderate, and slightly progressive weight-reducing action of LIPOXmax endurance training is observed after sleeve gastrectomy and synergistically adds its effect to that of surgery. This effect is associated with a decrease in orexigenic drive and an improvement of satiety.
Introduction
Sleeve gastrectomy (SG) is a major therapy for morbid dietary-resistant obesity [1,2]. This operation leads to considerable weight loss accompanied by important morphological and psychological changes [3], but on the long term there is a considerable number of patients who regain weight [4]. Reported rates of weight regain range from 5.7 % at 2 years to 75.6 % at 6 years. Proposed causes of this weight regain included initial sleeve size, sleeve dilation, increased ghrelin levels, inadequate follow-up support and maladaptive lifestyle behaviours [5]. Recent literature emphasizes the importance of regular physical exercise as a prevention for this weight gain [6-8]. We recently reported that low intensity training targeted at the maximal level of lipid oxidation, ie, a very easy to apply and well-tolerated exercise protocol [9,10], is a very efficient strategy for this purpose [11].
However, the reasons for the efficiency of such a soft exercise protocol after sleeve gastrectomy is not clear. According to the classical paradigm [12] exercise induces weight loss because it induces an energy deficit. Clearly, a volume of 3x45 min of LIPOXmax training results in very little energy deficit (less than 200 kCal per session), and this mechanism cannot explain the results. In fact, current research on exercise and obesity has led to the conclusion that exercise makes something more than an energy deficit [13,14]. Most of its weight-lowering action is explained by epigenetic alterations modifying energy metabolism [15] and by changes in eating behavior [16]. Exercise targeted on lipid oxidation decreases hunger, nibbling and calorie intake [17], while exercise bouts at higher intensities are associated with a waste of carbohydrates which is orexigenic and may induce hyperphagia and weight gain [18].
Whether this latter mechanism (alterations in the orexigenic drive) may account in the explanation of the synergy between sleeve and LIPOXmax training is not clear, because the efficacy of sleeve gastrectomy is already explained to a large extent by profound changes in the neuroendocrine regulation of eating behavior [19]. Does low intensity exercise exert an additional effect on appetite and satiety in this very particular situation? This is largely unknown.
The goal of the current study is thus: 1) to confirm on a longer period of follow-up our previous preliminary findings of an efficacy of LIPOXmax training for improving the effects of sleeve gastrectomy on weight loss; 2) to investigate the effects of LIPOXmax training sessions on orexigenic drive.
Materials and Methods
Subjects
Patients’ characteristics of the subjects included in the first study (follow-up over 60 months) are shown on table 1. Controls were subjects that did not start any change in their diet or exercise habits despite repeated advice, but still attended regularly outpatient consultations. The 2 cohorts were matched for weight and body mass index. Anthropometric characteristics of the subjects of study N°2 (effects of training sessions on eating behavior) are shown on table 2.
Bioelectrical impedance measurements
Prior to the exercise-test, subjects’ body composition was assessed with bioimpedance analysis with a six terminal impedance plethismograph BIACORPUS RX 4000 Biacorpus RX4000, (SoAGIL DEVELOPPEMENT, 8 avenue Jean-Jaurès F-92130 Issy-les-Moulineaux, France) with data analysis with the software BodyComp 8.4. This device measures total resistance of the body to an alternative electric current of 50 kHz [20,21]. Body fat mass and fat-free mass were calculated in each segment of the body according to manufacturer’s database-derived disclosed equations, and total water with published equations using the classical cylindric model and Hanai’s mixture theory [22].
Exercise calorimetry
Exercise Calorimetry was performed in all patients before targeting exercise on the zone where lipid oxidation is maximal [23]. All subjects were asked to come and perform test in the morning after an overnight fast (12 hours). The test consisted of five six minute steady-state workloads theoretically set at 20, 30, 40, 50, and 60% of Pmax. However these intensity levels can be modified during the test according to the evolution of the respiratory exchange ratio (RER=VCO2/VO2) in order to obtain values of RER below and above 0.9 which is the level of the Crossover Point (COP). The subjects performed the test on an electromagnetically braked cycle ergometer (Ergoline Bosch 500). Heart rate and electrocardiographic parameters were monitored continuously throughout the test by standard 12-lead procedures. Metabolic and ventilatory responses were assessed using a digital computer based breath to breath exercise analyzing system (COSMED Quark CPET). Thus, we could measure VO2, VCO2 (in ml/min) and calculate the non-protein RER. Lipid oxidation (Lipox) and carbohydrate utilization (Glucox) rates were calculated by indirect calorimetry from gas exchange measurements according to the non-protein respiratory quotient technique as previously reported [24]. This technique provided carbohydrate and lipid oxidation rates at different exercise intensities. Additionally, after smoothing the curves, we calculated the two parameters quantifying the balance between carbohydrates and lipids induced by increasing exercise intensity: the maximal lipid oxidation point (LIPOXmax) and the Crossover Point (COP). The LIPOXmax is the exercise intensity at which lipid oxidation reaches its maximal level before decreasing while carbohydrate utilization further increases. It is calculated as previously reported after smoothing of the curve plotting lipid oxidation as a function of power [24].
Coaching and follow-up of patients
Each subject included in the exercise group of the 1st study or in the 2nd study was enrolled in eight exercise sessions of 45 min at the LIPOXmax determined with the exercise test in order to include in his/her everyday life at least 3 similar bouts of low intensity endurance exercise per week. Subjects were then followed monthly in outpatient unit for the first year and then every 3 or 4 months [9].
Evaluation of training-induced changes in hunger and satiety
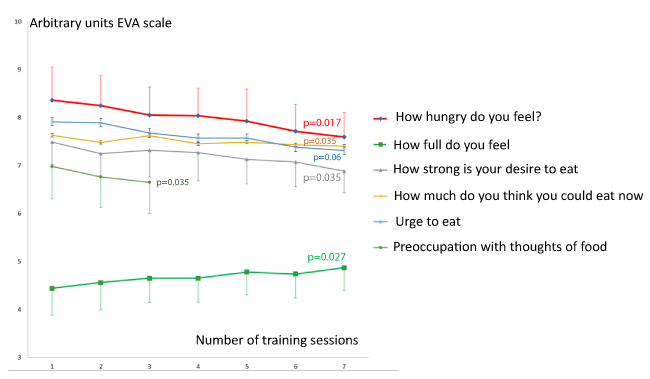
As usual in this kind of studies we used a French adaptation of Hill & Blundell’ scale [25-27]. The original version of this questionnaire contained six questions: `How hungry do you feel?’ (not at all hungry/as hungry as I’ve ever felt); `How full do you feel?’ (not at all full/as full as I have ever felt); `How strong is your desire to eat?’ (very weak/very strong); `How much do you think you could eat now?’ (nothing at all/a large amount); `Urge to eat’ (no urge to eat/strong, want to eat now, waiting is very uncomfortable); `Preoccupation with thoughts of food’ (no thoughts of food/very preoccupied dificult to concentrate on other things). All items were quoted on an analogic numeric scale.
Statistical analysis
Descriptive statistics were performed. A two-way analysis of variance (ANOVA) was used to determine whether there was an effect of training vs non training over time and whether it was different between the two groups (study one), and whether there was an acute effect of a LIPOXmax training session over Hill’s scale and whether this effect changed after a period of training (study two). ANOVA was performed with Sigmastat (Jandel Scientific). A p-value less than 0.05 was used to assess statistical significance.
Results
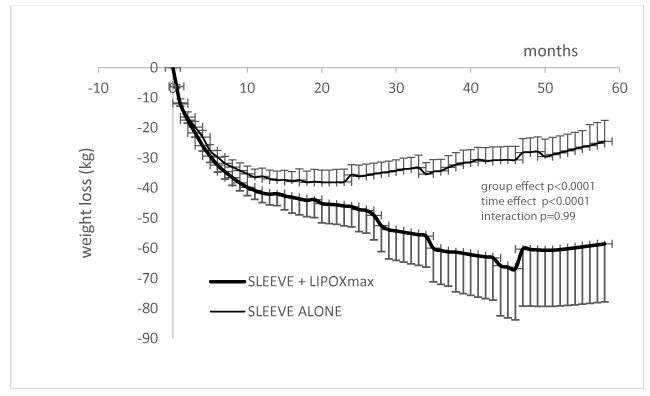
Figure 1 shows the average decrease in body weight over 5 years. There is a highly significant difference between sleeve only and sleeve+lipoxmax (p=3.1 x 10-55). Curves become significantly different after 30 months (p=0,047). At 60 months the trained group has lost on the average 57.92±19.4 kg vs only 23.25±6,76 in the group of patients who did not benefit of training.
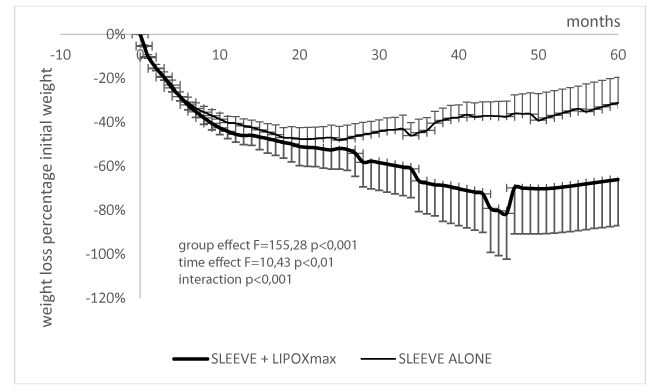
Figure 2 shows weight loss in the two groups expressed in percentage of initial weight. There is a highly significant difference between sleeve only and sleeve+lipoxmax (F=155.8 p=8.5 x 10-34). At 60 months the trained group has lost on the average 66.05 ±20.94 vs -31.15 ± 11.53 % of initial body wieigt in the group of patients who did not benefit of training.
Figure 3 and table 3 show the evolution of the ratings of eating behavior on Hill’s scale in study 2. All items related to hunger and the drive to eat more (with the exception of `Urge to eat’) significantly decreased after one session and the baseline value also decreased after 9 sessions of training. The item `How full do you feel?’ which represents satiety was also increased at baseline after several training session but the tendency to increase after a single session did not reach significance (p=0.062).
Discussion
This study confirms our preceding reports of a strong efficacy of LIPOXmax training in patients undergoing sleeve gastrectomy. On the whole, trained patients have lost 35% more weight than untrained patients after 60 months. In addition, it shows that LIPOXmax training sessions in patients in whom a sleeve gastrectomy has been performed induce significant changes in satiety and orexigenic drive leading to a tendency to eat less and to be more rapidly satiated.
Therefore, this work confirms over a period of 5 years (60 months) that low intensity exercise training targeted at the LIPOXmax has an additional efficiency on weight loss in patients treated by sleeve gastrectomy. While patients treated by sleeve alone have lost on the average 31 kg and exhibit a trend to weight regain after 4 years, patients that regularly exercise (3x45 min per week at the level of the LIPOXmax) lose significantly more weight (66 kg) and maintain their weight loss over this period. In our preceding paper on this topic [11] a similar tendency was evidenced by there was a shorter follow-up (30 months). Clearly, a soft and easy to perform training procedure such as LIPOXmax training, which is easily acceptable by these patients, has a prolonged and marked efficacy after sleeve, resulting in a more important weight loss and a better stabilization. What will occur with this procedure over a longer period of time is unknown.
Previous studies have also shown that LIPOXmax training is efficient by its own for losing weight [28,29] and maintaining this weight loss over several years [30]. This finding has been explained by the fact that this type of exercise makes more than an energy deficit [14], ie, it induces profound changes in eating behavior and in spontaneous physical activity [31].
Our current findings show that the same modifications occur after sleeve, despite the major changes in regulation of eating behavior induced by this surgery. Exercising at the level of lipid oxidation improves satiety and decreases ratings of hunger. In fact, when comparing these results to those obtained during a similar experiment in obese who have not been operated with a sleeve gastrectomy [31], we can observe that the magnitude of the effect is less important. The effect of training on Hill’s questionnaire items is evidenced but is quite moderate.
Low intensity endurance exercise, which promotes lipid oxidation, has been proposed since almost twenty years as a promising strategy against obesity [23,32-37]. When more precisely targeted with exercise calorimetry at levels where lipid oxidation is maximal (LIPOXmax), it has been shown to improve mitochondrial respiration [38], blood glucose control and blood lipids, low grade inflammation and body composition, even at low weekly volume [28]. Surprisingly, this approach generated less literature, perhaps because its weight-reducing effects over the short term, although significant, were not spectacular.
However, its prolonged use has been shown to result in a sustained weight loss [39], which contrasts with the quite constant weight regain observed in almost all classical strategies [40].
The main limit of this study is obviously that this is an observational study, without initial randomization. By contrast it represents situations of ‘true life’. A randomized control trial of three similar groups will be useful to do, but such a study will probably be extremely expensive and difficult to manage.
In conclusion, our study clearly demonstrates that regular exercise training targeted at the LIPOXmax, which is a simple and easily acceptable procedure in such patients, is a powerful complement to sleeve gastrectomy and improves its weight lowering effect over more than 60 months, preventing weight regain over this period. The mechanism of this fair effect of a very soft procedure in not clear and may involve epigenetic reprogramming of various metabolic functions, but we show here that this exercise training has favorable effects on eating behavior, similar to those previously observed in non-operated obese subjects.
- Gagner M, Garcia-Ruiz A, Arca MJ, Heniford TB (1999) Laparoscopic isolated gastric bypass for morbid obesity. Surg Endosc 13: S19.
- Nocca D, Krawczykowsky D, Bomans B, Noël P, Picot MC, et al. (2008) A Prospective Multicenter Study of 163 Sleeve Gastrectomies: Results at 1 and 2 Years. Obesity Surgery 18: 560–565. Link: https://bit.ly/31nzFRe
- Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, et al. (2004) Bariatric Surgery: A Systematic Review and Meta-analysis. JAMA 292: 1724–1737. Link: https://bit.ly/2wIwIwF
- Velapati SR, Shah M, Kuchkuntla AR, Abu-Dayyeh B, Grothe K, et al. (2018) Weight Regain After Bariatric Surgery: Prevalence, Etiology, and Treatment. Curr Nutr Rep 7: 329-334. Link: https://bit.ly/2WoRuvL
- Lauti M, Kularatna M, Hill AG, MacCormick AD (2016) Weight Regain Following Sleeve Gastrectomy-a Systematic Review. Obes Surg 26: 1326-1334. Link: https://bit.ly/2ZhhlY6
- Keren D, Matter I, Lavy A (2014) Lifestyle modification parallels to sleeve success. Obes Surg 24: 735-740. Link: https://bit.ly/2EZlQiq
- Filou V, Richou M, Bughin F, Fédou C, Mauverger E, et al. (2018) Complementarity of bariatric surgery and physical activity. Science & Sports 33: 65-72.
- Coen PM, Carnero EA, Goodpaster BH (2018) Exercise and Bariatric Surgery: An Effective Therapeutic Strategy. Exerc Sport Sci Rev 46: 262-270. Link: https://bit.ly/2WBwRBx
- Brun JF, Varlet-Marie E, Romain AJ, Mercier J (2011) Measurement and physiological relevance of the maximal lipid oxidation rate during exercise (LIPOXmax). Sports Medicine and Sports Injuries. Link: https://bit.ly/31iDwPE
- Brun JF, Malatesta D, Sartorio A (2012) Maximal lipid oxidation during exercise: A target for individualizing endurance training in obesity and diabetes? J Endocrinol Invest 35: 686-691. Link: https://bit.ly/2hBhjXd
- Drapier E, Fédou C, Ghanassia E, Nocca D, Mercier J, et al. (2016) L’exercice en endurance à faible intensité renforce-t-il l’efficacité amaigrissante d’une gastrectomie en manchon ? Sci Sports 31: 162-165. Link: https://bit.ly/2Wvfpd7
- Tremblay A, Després J-P, Leblanc C, Craig CL, Ferris B, et al. (1990) Effect of intensity of physical activity on body fatness and fat distribution. Am J Clin Nutr 51: 153-157. Link: https://bit.ly/2I4SBg1
- Johnson J, Slentz C, Houmard JA, Samsa GP, Duscha BD, et al. (2007) Exercise training amount and intensity effects on metabolic syndrome: STRRIDE. Am J Cardiol 100: 1759-1767. Link: https://bit.ly/2KdvbHV
- Brun JF (2015) Exercise Makes More than an Energy Deficit: Toward Improved Protocols for the Management of Obesity? EBioMedicine 18: 1862-1863. Link: https://bit.ly/2K6xLz0
- Huffman KM, Koves TR, Hubal MJ, Abouassi H, Beri N, et al. (2014) Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 57: 2282-2295. Link: https://bit.ly/2ZeLmIe
- Guiraudou M, Romain AJ, Mawunu M, Bedjih K, Fédou C, et al. (2016) Effets chroniques de l'exercice ciblé au niveau d'oxydation maximale des lipides (LIPOXmax) sur le comportement alimentaire de sujets obèses sédentaires. Science & Sports 31: 13-18. Link: https://bit.ly/2Ze5udr
- Guiraudou M, Chérif A, Richou M, Fidani T, Romain AJ, et al. (2018) Effects Over 24Hr of Exercise Targeted on Lipid versus Carbohydrate Oxidation on Eating Behaviour in Normal Weight Volunteers. Int J Sports Sci Med 2: 031-035. Link: https://bit.ly/2WY2SD9
- Brun JF, Romain AJ, Sferlazza A, Fédou C, Raynaud de Mauverger E, et al. (2016) Which individuals become fatter when they practice exercise? Science & Sports 31: 214-218. Link: https://bit.ly/2I5yIW4
- Mans E, Serra-Prat M, Palomera E, Suñol X, Clavé P (2015) Sleeve gastrectomy effects on hunger, satiation, and gastrointestinal hormone and motility responses after a liquid meal test. Am J Clin Nutr 102: 540-547. Link: https://bit.ly/2K2TibW
- Brun JF, Guiraudou M, Mardemootoo C, Traoré A, Raingeard I, et al. (2013) Validation de la mesure segmentaire de la composition corporelle en comparaison avec la DEXA: intérêt de la mesure de la masse grasse tronculaire. Science & Sports 28: 158-162. Link: https://bit.ly/31nAx8I
- Guiraudou M, Maimoun L, Dumas J-M, Julia M, Raingeard I, et al. (2015) Composition corporelle mesurée par impédancemétrie segmentaire (BIAS) et performance de sprint chez les rugbymen / Body composition measured by bioimpedance segmental (BIAS) analysis and sprint performance in rugby players. Science & Sports 30: 298-302. Link: https://bit.ly/2ZeMyeG
- Jaffrin MY, Morel H (2008) Body fluid volumes measurements by impedance: A review of bioimpedance spectroscopy (BIS) and bioimpedance analysis (BIA) methods. Medical Engineering & Physics 30: 1257-1269. Link: https://bit.ly/2MDSpsv
- Perez-Martin A, Mercier J (2001) Stress tests and exercise training program for diabetics - Initial metabolic evaluation, Ann Endocrinol 62: 291-293.
- Brun JF, Romain AJ, Mercier J (2011) Maximal lipid oxidation during exercise (Lipoxmax): From physiological measurements to clinical applications. Facts and uncertainties. Science & Sports 26: 57-71. Link: https://bit.ly/2Zj5o4b
- Hill AJ, Blundell JE (1983) Nutrients and behaviour: research strategies for the investigation of taste characteristics, food preferences, hunger sensations and eating patterns in man. J Psychiatr Res 17: 203-212. Link: https://bit.ly/2KEWMkv
- Hill AJ, Blundell JE (1990) Sensitivity of the appetite control system in obese subjects to nutritional and serotoninergic challenges. International Journal of Obesity 14: 219-233. Link: https://bit.ly/2K6XgAo
- Hill AJ, Rogers PJ, Blundell JE (1995) Techniques for the experimental measurement of human eating behaviour and food intake: a practical guide. International Journal of Obesity 19: 361-375. Link: https://bit.ly/2XvV7kR
- Besnier F, Lenclume V, Gérardin P, Fianu A, Martinez J, et al. (2015) Individualized exercise training at maximal fat oxidation combined with fruit and vegetable-rich diet in overweight or obese women: The LIPOXmax-Réunion randomized controlled trial. PLoS One 10: e0139246. Link: https://bit.ly/2R3Rn7U
- Romain AJ, Carayol M, Desplan M, Fedou C, Ninot G, et al. (2012) Physical activity targeted at maximal lipid oxidation: a meta-analysis. J Nutr Metab 285-395. Link: https://bit.ly/2WEmVaw
- Drapier E, Cherif A, Richou M, Bughin F, Fedou C, et al. (2018) Long term (3 years) weight loss after low intensity endurance training targeted at the level of maximal muscular lipid oxidation. Integr Obesity Diabetes 4. Link: https://bit.ly/31kYxJv
- Javernaud E, Brun JF, Richou M, Bughin F, Mercier J (2017) Les effets du réentrainement ciblé au LIPOXmax sur le comportement alimentaire et la composition corporelle sont précoces et se prolongent au moins 4 ans. Nutrition Clinique et Métabolisme 32: 260. Link: https://bit.ly/2QZzCXe
- Dériaz O, Dumont M, Bergeron N, Després JP, Brochu M, et al. (2001) Skeletal muscle low attenuation area and maximal fat oxidation rate during submaximal exercise in male obese individuals. Int J Obes Relat Metab Disord 25: 1579-1584. Link: https://bit.ly/2F0LhjD
- Pérez-Martin A, Dumortier M, Raynaud E, Brun JF, Fédou C, et al., Balance of substrate oxidation during submaximal exercise in lean and obese people. Diabetes Metab 27: 466-474. Link: https://bit.ly/2KD2wer
- Achten J, Gleeson M, Jeukendrup AE (2002) Determination of the exercise intensity that elicits maximal fat oxidation, Med Sci Sports Exerc 34: 92-97. Link: https://bit.ly/2WvgyBr
- Achten J, Jeukendrup AE (2004) Optimizing fat oxidation through exercise and diet, Nutrition 20: 716-727. Link: https://bit.ly/2JalDwX
- Brandou F, Dumortier M, Garandeau P, Mercier J, Brun JF (2003) Effects of a two-months rehabilitation program on substrate utilization during exercise in obese adolescents. Diabetes Metab 29: 20-27. Link: https://bit.ly/2IuNwwK
- Venables MC, Jeukendrup AE (2008) Endurance training and obesity: effect on substrate metabolism and insulin sensitivity. Med Sci Sports Exerc 40: 495-502. Link: https://bit.ly/2R2tfCh
- Bordenave S, Metz L, Flavier S, Lambert K, Ghanassia E, et al. (2008) Training-induced improvement in lipid oxidation in type 2 diabetes mellitus is related to alterations in muscle mitochondrial activity. Effect of endurance training in type 2 diabetes. Diabetes Metab 34: 162-168. Link: https://bit.ly/2K8BBaW
- Guiraudou M, Fédou C, Romain AJ, Sferlazza A, Calas E, et al. (2015) Effects over one year of low-intensity endurance exercise targeted at the level of maximal lipid oxidation. Science & Sports 30: e127-e131. Link: https://bit.ly/2WSGZoP
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, et al. (2008) Comparison of Strategies for Sustaining Weight Loss: The Weight Loss Maintenance Randomized Controlled Trial. JAMA 299: 1139-1148. Link: https://bit.ly/2I6dEPr
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
