Global Journal of Obesity, Diabetes and Metabolic Syndrome
Obesity, Diabetes and Gastrointestinal Malignancy: The role of Metformin and other Anti-diabetic Therapy
Michael Ashamalla1, Irini Youssef2, Mena Yacoub3, Apoorva Jayarangaiah4, Nikita Gupta2, Justina Ray2, Sadat Iqbal2, Regina Miller2 Joie Singh2 and Samy I McFarlane2*
2Department of Radiation Oncology, Department of Medicine, Division of Endocrinology, SUNY-Downstate, Brooklyn, NY 11203, USA
3Northside Hospital, St. Petersburg, Florida, 33709, USA
4Department of Internal Medicine, Wake Forest University, Baptist Health System, Winston-Salem, N.C, USA
Cite this as
Ashamalla M, Youssef I, Yacoub M, Jayarangaiah A, Gupta N, et al. (2018) Obesity, Diabetes and Gastrointestinal Malignancy: The role of Metformin and other Anti-diabetic Therapy. Glob J Obes Diabetes Metab Syndr 5(2): 008-014. DOI: 10.17352/2455-8583.000032The association between Diabetes and cancer has been known for decades with obesity and insulin resistance being postulated as the main underlying risk factors for both disorders. With rise of the epidemic of obesity in the USA and around the globe, there has been a rise in diabetes that is currently reaching epidemic proportions. Diabetes is known to be associated with increased risk of several types of malignancy including breast, cervical, pancreatic and colon cancer. In this review, we discuss the epidemic of obesity and its consequential epidemic of diabetes highlighting the pathophysiologic mechanisms of increased cancer in the diabetic population. We will then discuss the role of insulin therapy as well as other antidiabetic medications, particularly metformin that has been to be associated with lower risk as well as better survival with GI malignancies based on several studies including a study that was recently published by our group.
Review Article
Obesity and diabetes are becoming increasingly prevalent globally. The incidence of cancer is also predicted to increase by over 20% by 2020 [1].
Worldwide, cancer is the second leading cause of death, and diabetes is the 12th [2]. The relationship between Diabetes and cancer has been well established for decades. Indeed, a plethora of epidemiological studies have shown type 2 Diabetes Mellitus to be an independent risk factor for cancer. Obesity and secondary insulin resistance are implicated underlying risk factors in both diabetes and cancer development [3]. Concurrent with the rise in the obesity epidemic worldwide is a rise in diabetes. The mechanistic role of diabetes in carcinogenesis is robust and clear for several malignancies. In this review, we discuss the epidemic of obesity, its consequential rise in diabetes, highlighting the pathophysiological mechanisms of increased cancer in the diabetic population. Finally, we will discuss the role of insulin therapy and metformin therapy in cancer risk and mortality.
Epidemiology of obesity in the United States and globally
Obesity is a rapidly increasing chronic disease both in the United States and globally. Estimates of obesity prevalence in the United States have increased almost unmitigated over the past 50 years, increasing from 22.9% to 30.5% to 39.6 % (NHANES). These values are projected to rise to 51% of the population, a 130% increase, by 2030. From 2015 to 2016, the prevalence of obesity in men was 37.9%, 41.1% in women (NHANES, [4,5]) and 18% in children [6]. The prevalence was higher in the Midwest and the South and lower in the Northeast and the West coast of the USA (NHANES,[4,5]). The mean BMI is also increasing worldwide with obesity rates nearly tripling since 1975. In 2016, more than 1.9 billion adults greater than 18 years were overweight (WHO). Although the USA boasts the largest absolute value of overweight and obese adults, other nations exceed this value in both prevalence and growth rates. To name a few, the prevalence of overweight and obese adults in Mexico is estimated to be 71.3%, an increase of 15% since 2000 placing Mexico as the fastest accelerator in obesity, while in China, obesity has nearly quadrupled in men and doubled in women and children [6].
Relationship between obesity and cancer
Excess weight is associated with an increased risk of multiple types of cancer. Overweight and obesity were estimated to cause 40% of all cancers in the United States in 2014 [7]. The Million Women Study has revealed that almost 50% of cancers in post-menopausal women can be attributed to obesity [3]. More notably, a retrospective study of 2,347 subjects found that the risk of cancer increased by 9% per SD increase in childhood BMI [8], while excess weight in teenagers was linking to a 200% increase in colon cancer risk during adulthood [9]. Overweight- and obesity-related cancer incidence rates were higher among older persons (ages≥50 years), females, and among non-Hispanic black and non-Hispanic white adults compared with other groups [7]. Additionally, obesity and overweight may increase the likelihood of dying from cancer. Abdominal obesity has been shown to increase the risk of cancer mortality by 24%[10]. Higher severity of obesity has also been linked to higher tumor histologic grade, mitotic cell count and larger tumor size compared to lower BMI quartiles [11]. Cancers commonly found in obese people include endometrial, esophageal adenocarcinoma, colorectal, postmenopausal breast, prostate and renal [3]. Biological mechanisms linking obesity to cancer incidence include caloric excess promoting cancer cell proliferation, increased circulating insulin and IGF-1 activating cellular proliferation pathways, increasing insulin resistance induced insulin elevations (as will be discussed later), increased circulating sex steroids secondary to increased aromatization in lipids, and oversecretion of pro-inflammatory cytokines including IL-6, TNF-a, and leptin, molecules implicated in cancer development and progression [3].
Relation between obesity and diabetes
It is exceedingly well established that obesity is the number one risk factor for type 2 diabetes. Nationally, increases in obesity prevalence have paralleled those of diabetes. Studies have reported that women with a BMI of 30 kg/m2 have 28 times a greater risk of diabetes, and 93 times if BMI is 35 kg/m2, compared to women of normal weight [12]; in men, the rate of incident diabetes was 11.4 per 1000 person-years among obese subjects versus 1.6 among normal-weight subjects [13]. Even more, type 2 DM has emerged as a pediatric dilemma: the prevalence of diabetes has increased by 35% since 2001 amongst youth ages 10-19 [14]. The mechanism whereby obesity causes insulin resistance is becoming increasingly clear. Adipose tissue distribution is an important determinant in diabetes development, with the highest degrees of insulin resistance occurring in subjects with central or abdominal obesity. Increased pro-inflammatory cytokines and hormones released predominantly by intra-abdominal adipose tissue are involved in insulin resistance [15]. B-cell dysfunction is the next step required for diabetes to develop. As B-cell dysfunction causes inadequate insulin secretion, hyperglycemia pursues. Further increases in blood glucose propagate insulin resistance through the glucotoxic effects on pancreatic B-cells. Non-esterified fatty acids secreted by central adipose tissue may play a role in deregulation of glucose-stimulated insulin secretion pathways, insulin biosynthesis, and subsequent B-cell dysfunction [16]. There are a several other molecular and biochemical mechanisms proposed for the role of obesity on insulin resistance and diabetes. However, they are beyond the scope of this review.
Diabetes and Gastrointestinal malignancies: Epidemiology and mechanistic insight
Patients with diabetes experience a roughly 20-25% higher cancer incidence compared to individuals without diabetes [17]. Moreover, patients with diabetes who further develop cancer have increased early and late mortality compared to cancer patients without diabetes [17]. The following is a brief overview of the relationship between diabetes and varying cancers.
Liver cancer: A meta-analysis by Wang et al. demonstrated that both men and women with Type 2 DM had a 2-fold increased risk of developing hepatocellular carcinomas (HCC), compared with non-diabetics [18]. In a meta-analysis by Dyal et al. it was shown that type 2 DM was associated with increased risk of HCC, independent of factors including age, sex, and obesity. Even more, obesity and hepatic steatosis were independent predictors of HCC [19]. This suggests that metabolic derangements such as obesity, in addition to diabetes, work synergistically to increase HCC risk. The mechanisms involves exposure of the liver to high levels of endogenously produced insulin through the portal vein. These elevated insulin levels subsequently increase production of IGF-1 levels, which thereafter increase cellular proliferation and inhibit apoptosis in the liver [20]. A study by Jee et al. has shown a dose relationship between fasting serum glucose and cancer mortality rates, in addition to liver cancer rates [21]. Additionally, Insulin resistance induced release of pro-inflammatory cytokines promote hepatic steatosis and inflammation. As such, liver steatosis, hepatitis, and cirrhosis are more frequent among diabetic patients and are well known risk factors for HCC [22].
Colorectal cancer: The relationship between type 2 diabetes and colorectal cancer incidence has been shown in several epidemiological studies. A meta-analysis study by Jiang et al. showed that patients with diabetes were at a 27% increased risk of colorectal cancer compared to non-diabetics [23]. Additionally, Peeters et al. demonstrated a 1.3-fold increase in risk of colorectal cancer amongst diabetic populations, along with a trend of increased risk for longer duration of obesity [24]. Potential mechanisms include slower peristalsis and constipation in diabetic patients, causing increased exposure to bowel toxins including fecal bile acids and potential carcinogens [25,26]. Hyperinsulinemia and increased IGF-1 levels also contribute to adenocarcinomas through increased cellular proliferation [22].
Pancreatic cancer: The greatest association between diabetes and GI malignancy has been demonstrated in pancreatic cancer. Chari et al showed that subjects with new-onset DM had a RR of pancreatic cancer of 7.94 compared to non-diabetics [27]. It may be unclear whether diabetes contributes to pancreatic cancer or precancerous pancreatic cells are incapable of producing insulin (reverse causality). A meta-analysis by Everhart found that in patients who have had diabetes for at least 5 years, the relative risk of pancreatic cancer was 2.0 [95% CI, 1.2-3.2] [28]. However, Huxley et al found that individuals with diabetes for less than 4 years had a 50% increased risk of pancreatic cancer than did patients with diabetes for greater than 5 years [29]. Another study showed that risk estimates decrease as the number of years with diabetes increased [30]. Presently, it is hard to differentiate between which came first. Insulin resistance and subsequent hyperinsulinemia and IGF-1 are the most hypothesized underlying mechanisms for cancer in diabetics, including pancreatic cancer, and expose pancreatic tissue to higher insulin levels than seen in circulation. In addition, Islet cell turnover is implicated in pancreatic cell carcinogenesis. In hamster cells, destruction of Islet cells by streptozotocin or alloxan inhibited pancreatic cancer induction [31].
Gastric cancer: The impact of diabetes on gastric cancer incidence has shown differing results in studies. A meta-analysis by Ge et al. on studies between 1966 and 2011, found a similar risk of gastric cancer between diabetics and non-diabetics. However, a subgroup analysis showed that diabetic women had an 18% increased risk, although not the case for diabetic men [32]. However, another meta-analysis by Yoon et al, including studies from any date, found a significantly increased relative risk (1.19; 95%CI [1.08-1.31] after adjusting for well known risk factors including smoking and H-pylori infection. The authors similarly found that female subgroups were at a higher risk [33]. Shimoyama et al. also demonstrated an increased relative risk of gastric cancer amongst diabetic patients (RR of 1.41, [95%CI: 1.10-1.81]). This study found that both sexes had a significant association, although greater in women. Some studies have shown that diabetics have a higher rate of H. Pylori infection, a well-known risk factor for gastric cancer, due to impaired immune function [34]. Respectively, concomitant diabetes and H. Pylori infection are associated with a dramatically increased risk of gastric cancer [35].
Diabetes and Cancer: Mechanism
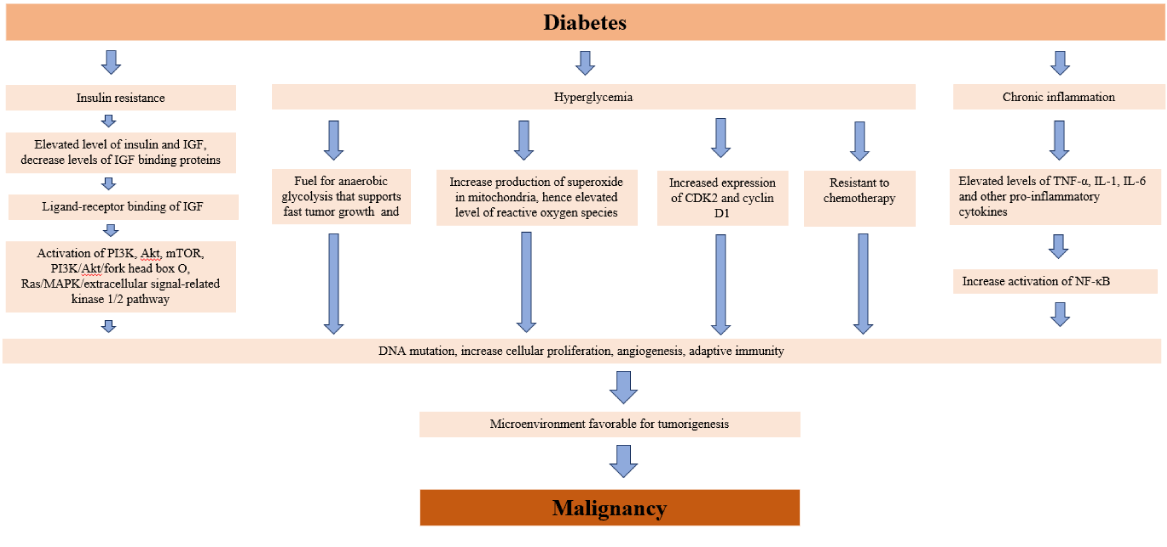
Several mechanisms have been described linking diabetes to increased carcinogenesis. Insulin resistance is known to increase circulating levels of insulin and IGF. Ligand binding of these increased circulating factors to insulin receptors expressed on cancer cells exert a pro-growth mitogenic effect, stimulating tumor cell growth. This observation is further confirmed by decreased tumor growth in xenografts with reduced expressed of insulin receptors. Downstream signaling after ligand-receptor interaction activates the phosphoinositide 3-kinase (PI3K), protein kinase B (Akt), mammalian target of rapamycin (mTOR), PI3K/Akt/forkhead box O, and Ras/MAPK/extracellular signal-related kinase 1/2 pathway, which plays important roles in cancer cell growth and carcinogenesis [22].
Hyperglycemia increases cancer risk both directly and indirectly. Directly, glucose provides the fuel source for the increased aerobic glycolysis observed in rapidly proliferating cancer cells, typically known as the Warburg effect. Indirectly, in vitro studies with cancer cell lines have shown enhanced expression of genes associated with cell proliferation, invasion, and migration, including epidermal growth factor (EGF) in pancreatic cancer cell lines, which subsequently activates the epidermal growth factor receptor (EGFR), a well-studied oncogenic pathway [36]. The exposure of susceptible cells to hyperglycemia results in increased production of superoxide by mitochondrial electron transport chains. These elevated levels of reactive oxygen species lead to cellular DNA instability and mutations [37]. In cancer cell lines, high glucose stimulated increased expression in cdk2 and cyclin D1, cell cycle checkpoint proteins, causing increased cell proliferation [37]. In-vitro studies have also shown overexpression of GLUT-transporters in cancerous cell lines, allowing for increased utilization of glucose and a switch from aerobic to anaerobic glycolysis, as first described by Warburg. Increased lactate production and a decrease in mitochondrial respiration cause acidification of tumor environment, as tumor cells become resistant to the toxic effects of acidosis through increased H+ transported activity, and decreased apoptosis, a phenomenon which naturally occurs in non-malignant cells in acidic environments [37]. Hyperglycemia also contributes to cancer metastasis through increased epithelial-mesenchymal transition (EMT) [38]. Hyperglycemia has also been shown to confer resistance to chemotherapy, while causing increased toxicity in breast cancer cell lines [39].
Additionally, chronic inflammation has been associated with increased carcinogenesis. The deregulated metabolism associated with poorly controlled diabetes increases both oxidative stress and upregulation of pro-inflammatory cytokines, including IL-6, TNF-a, and C-reactive protein. Increased oxidative stress causes damage to DNA, fatty acids, and amino acids, all involved in cell transformation to malignancy. TNF-a also causes increased activation of nuclear factor-kappa B (NF-KB) which induces downstream signaling of cell proliferation, angiogenesis, survival, subversion of adaptive immunity, and response to hormone and chemotherapeutic agents [22]. These mechanisms have been summarized in figure 1.
Insulin therapy and its role in carcinogenesis
Three large randomized trials have compared insulin therapy with other treatments for an assessment of cancer risk. The UK Prospective Diabetes Study (UKPDS) reported a cumulative incidence of cancer-related death of 4.9% for insulin therapy versus 5 % for other treatments, over the course of 11 years [40]. The Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial randomized 12, 537 patients with diabetes to either glargine insulin or oral therapy [41]. The study found a similar overall incidence of cancer between the two groups (HR 1.00, CI [.88-1.13]), and cancer related mortality (HR 0.94 [0.77–1.15]) [41]. Conversely, the extended follow-up of the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI-1) trial reported a significantly higher cumulative cancer-related mortality (4.4% versus 2.0%) in the chronic insulin treatment group [42]. It is worthwhile to note that none of these trials were specifically designed to assess cancer risk associated with insulin therapy. Additionally, in the aforementioned trials, insulin was a rescue therapy for patients in the control group, potentially attenuating differences in outcomes between the two groups.
In vitro studies have shown that glargine has an affinity for IGF-1 receptors much higher than human insulin [43]. Glargine also stimulates the growth of breast cancer cells in vitro. However, the concentrations of insulin used in in-vitro experiments are often much higher than those reached during insulin treatment for type 2 diabetes, and interestingly, the products of glargine metabolism have a low affinity for the IGF-1 receptor [43]. A prospective study by Hemkens et al. on 127,031 patients with diabetes treated with human insulin, aspart, lispro or glargine found that after adjusting for dose, a statistically significant dose-dependent increase in cancer risk was found for treatment with glargine compared with human insulin [44]. Other epidemiologic studies have shown differing results. Jonasson and colleagues noted that women on glargine alone had a higher risk of breast cancer than woman on other types of insulin, but a reduced risk of all-cause mortality [45]. Whereas a study in Scotland found no increased risk of cancer with overall glargine use (albeit six events of cancer occurred in exclusive glargine users who were more likely to be older, women, and have higher BMI) [46]. Finally, a study by Currie et al of 62, 809 patients comparing insulin to other therapies found that insulin therapy of any type increased the risk of solid tumors [47].
Currently, there is inconclusive evidence that exogenous insulin is associated with increased risk of cancer. The prior mentioned randomized control trials have failed to show increased cancer incidence, were not designed to do so, and have inherent biases and limitations. Additionally, in vitro studies, while helpful, have disadvantages when translating their results to human subjects. Clearly, more randomized control trials are required before the true impact of exogenous insulin therapy on cancer incidence and risk is known.
Metformin and reduction in cancer risk
Several epidemiological studies have confirmed an association between metformin use and reduction in cancer risk. In a meta-analysis by Soranna et al, the use of metformin was associated with a significantly reduced relative risk of all cancers (RR 0.61, 95% confidence interval [CI] 0.54-0.70) [48]. Similarly, another meta-analysis of 12 RCTs and 41 observational studies found a reduction in cancer death by 35%, and cancer risk, by 27%, with the use of metformin. Particularly, the authors found that metformin was associated with reduction in GI malignancies only [49]. A plethora of other observational studies have noted the same impact [50]. A retrospective study by Tseng et al. also reported a significantly lower risk of gastric cancer in metformin users, especially after a 2 year use duration (HR .448, 95% CI [0.359-0.558]) [51]. In another large prospective study of 480,984 patients by Lee and colleagues, the use of metformin in diabetics was associated with a reduction in total, colorectal, pancreatic, and liver carcinomas [52]. Interestingly, the use of metformin has been shown to improve chemotherapeutic response rates, and decrease locoregional recurrence after radiation therapy, in esophageal cancer patients [53].
Mechanistic insight into metformin and reduced cancer risk
A mechanism currently proposed for the anticancer action of metformin includes the inhibition of the mammalian target of rapamycin complex 1 (mTORC1) pathway, which plays an important role in metabolism, growth and proliferation of cancer cells [54]. Research by Mohammed et al. showed reduction in cancer spread in pancreatic tissue of mice treated with metformin, along with significant inhibition of the mTOR pathway [55]. The mTOR pathway is activated through IGF-1 and Insulin ligand binding to their respective receptors, which causes the insulin receptor substrate (IRS) signal to transmit to phosphoinositide 3-kinase (PI3K), and Akt/protein kinase B (PKB), which indirectly activate mTORC1 [50] . Thus, another mechanism includes reduction in circulating insulin and IGF-1 levels through increased insulin sensitivity induced by the drug, as also supported by in-vitro studies [56]. In breast cancer cells, metformin decreased levels of epidermal growth factor receptor 2 (HER2) through inhibition of the mTOR effector, p70S6K, a molecule responsible for phosphorylation of S6 ribosomal protein and thereby, protein synthesis [57]. In gastrointestinal tumors, metformin has been shown to inhibit epithelial to mesenchymal transition, a phenomenon implicated in cancer metastasis [58]. Additional molecules involved in the inhibition of cancer growth by metformin have been studied and discussed in detail, but are beyond the scope of this review article [50].
Survival with metformin therapy
In addition to improving cancer incidence, the evidence today shows that metformin additionally has overall survival benefits. The landmark UK Prospective Diabetes Study (UKPDS) showed that metformin significantly reduced diabetes-related mortality by 42%, all-cause mortality by 36%, myocardial infarction by 39%, and any diabetes related endpoint by 32% [40]. In a prospective study performed by our authors, the use of metformin showed significant risk reduction in mortality, and improved overall survival, in patients with colorectal carcinoma [59]. Additionally, a study of 30,493 patients with colorectal cancer, of which 3,391 were diabetic, and 1962 were treated with metformin, found an increased HR for all-cause mortality in the diabetes group, and a reduction in mortality by 15% in the metformin treated group [60]. A meta-analysis by Li et al. on the effect of metformin on survival of patients with pancreatic cancer found a significant improvement in survival (HR 0.86, 95% CI ] 0.76–0.97] in patients on metformin compared with control [61]. The long term use of metformin is also associated with a lower risk of gastric cancers, and in gastric cancer patients treated with gastrectomy, a reduction in disease recurrence, all-cause and cancer-specific mortality [62]. Beyond gastrointestinal cancers, metformin use has also been shown to improve overall survival and cancer specific survival in patients with prostate cancer [63]. In patients with non-resectable hepatocellular carcinoma, the use of metformin has been associated with improved overall survival after radiotherapy [64]. In-vitro and In-vivo, esophageal cancer cell viability has been found to be reduced with metformin treatment through cell-cycle arrest, apoptosis induction, and IGF-1 downstream signaling inhibition, along with tumor burden reductions in mice [65].
Diet, exercise and their role in cancer
Although obesity rates continue to grow, efforts to mitigate its impact on cancer incidence are underway. Studies have shown that moderate intensity activity is associated with a reduction in risk of breast, colon and endometrial cancer [66]. Findings have indicated that women with breast cancer who engage in 2-3 hours of break walking a week had a significantly lower risk of recurrence and mortality from breast cancer. This outcome is related to reduction in circulating sex hormone levels through reductions in body fat [66]. Physical activity is also known to improve insulin resistance and reduce risk of diabetes through increased muscle insulin sensitivity. Prospective studies have also shown improved disease-free survival in colon cancer patients who engaged in physical activity [67], likely through increased colon motility and reduced carcinogen exposure in the colon. Physical activity also has beneficial effects on DNA oxidative damage repair [68]. Even more noteworthy is the effect of diet on cancer mortality. A meta-analysis by Schwedhelm et al. demonstrated that a higher intake of vegetables and fish were inversely associated with overall mortality in cancer survivors, while a western diet e.g (red and processed meats, nitrite/nitrate containing) was associated with increased risk of overall cancer mortality, and mortality in colorectal cancer survivors [69].
Conclusion and Future Direction
As the obesity epidemic continues to rise, its repercussions continue to be felt by populations globally. The evidence for the impact of obesity on diabetes, and its role in carcinogenesis and cancer mortality is becoming increasingly robust. Obesity is not only required for the development of type 2 DM, but both obesity and diabetes are independent risk factors for tumor development and progression, and are associated with worse survival outcomes and decreased response to therapy in cancer patients. Although seemingly unyielding in its impact, obesity and diabetes are both preventable and controllable diseases, and several studies have shown the impact of proper diet and physical activity on cancer mortality. Future directions in mitigating the brunt of obesity and diabetes on carcinogenesis and mortality require randomized control trials. Currently, there are ongoing trials studying the impact of diabetes, weight loss, and therapy including but not limited to the impact of weight loss on breast cancer recurrence (NCT02750826), repurposing metformin as an anti-cancer drug (NCT03137186), and the feasibility of diet and exercise in non-invasive bladder cancer (NCT03137186).
- Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S (2015) The past, present, and future of cancer incidence in the United States: 1975 through 2020. Cancer 121: 1827-1837. Link: https://tinyurl.com/yd62sbk3
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367: 1747-1757. Link: https://tinyurl.com/ybw4ad85
- De Pergola G, Silvestris F (2013) Obesity as a major risk factor for cancer. J Obes 2013: 291546. Link: https://tinyurl.com/yb3k37qd
- (2017) Collaboration, N.C.D.R.F. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 390: 2627-2642. Link: https://tinyurl.com/ya7nywjn
- Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, et al. (2009) Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr 90: 1314-1320. Link: https://tinyurl.com/ybmm2sbj
- Hruby A, Hu FB (2015) The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 33: 673-689. Link: https://tinyurl.com/ych4cgyl
- Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, et al. (2017) Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep 66: 1052-1058. Link: https://tinyurl.com/y8o52tlq
- Jeffreys M, Smith GD, Martin RM, Frankel S, Gunnell D (2004) Childhood body mass index and later cancer risk: a 50-year follow-up of the Boyd Orr study. Int J Cancer 112: 348-351. Link: https://tinyurl.com/y8o3we2x
- Bjørge T, Engeland A, Tverdal A, Smith GD (2008) Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol 168: 30-37. Link: https://tinyurl.com/ycyokucg
- Jaggers JR, Sui X, Hooker SP, LaMonte MJ, Matthews CE, et al. (2009) Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer 45: 1831-1838. Link: https://goo.gl/mqR3cb
- Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, et al. (2001) Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer 92: 720-729. Link: https://goo.gl/qFMPKz
- Barnes AS (2011) The epidemic of obesity and diabetes: trends and treatments. Tex Heart Inst J 38: 142-144. Link: https://goo.gl/vpVjwu
- Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, et al., (2004) Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess 8: 1-182. Link: https://goo.gl/eg9sFK
- Bhupathiraju SN, Hu FB (2016) Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res 118: 1723-1735. Link: https://goo.gl/QDysLz
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1994) Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17: 961-969. Link: https://goo.gl/PrwECz
- Al-Goblan AS, Al-Alfi MA, Khan MZ (2014) Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes 7: 587-591. Link: https://goo.gl/k6QqSZ
- Scappaticcio L, Maiorino MI, Bellastella G, Giugliano D, Esposito K (2017) Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine 56: 231-239. Link: https://goo.gl/LFY9ZP
- Wang C, Wang X, Gong G, Ben Q, Qiu W,et al. (2012) Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer 130: 1639-1648. Link: https://goo.gl/pMpwCM
- Dyal HK, Aguilar M, Bartos G, Holt EW, Bhuket T, et al. (2016) Diabetes Mellitus Increases Risk of Hepatocellular Carcinoma in Chronic Hepatitis C Virus Patients: A Systematic Review. Dig Dis Sci 61: 636-645. Link: https://goo.gl/3DV8m6
- Weng CJ, Hsieh YH, Tsai CM, Chu YH, Ueng KC, et al. (2010) Relationship of insulin-like growth factors system gene polymorphisms with the susceptibility and pathological development of hepatocellular carcinoma. Ann Surg Oncol 17: 1808-1815. Link: https://goo.gl/hJ54PN
- Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, et al. (2005) Fasting serum glucose level and cancer risk in Korean men and women. JAMA 293: 194-202. Link: https://goo.gl/RLt9K4
- Xu CX, Zhu HH, Zhu YM (2014) Diabetes and cancer: Associations, mechanisms, and implications for medical practice. World J Diabetes 5: 372-380. Link: https://goo.gl/v2Pi7j
- Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, et al. (2011) Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol 26: 863-876. Link: https://goo.gl/asXL1D
- Peeters PJ, Bazelier MT, Leufkens HG, de Vries F, De Bruin ML (2015) The risk of colorectal cancer in patients with type 2 diabetes: associations with treatment stage and obesity. Diabetes Care 38: 495-502. Link: https://goo.gl/TsNm4t
- Ren X, Zhag X, Gu W, Chen K, Le Y, et al. (2009) Type 2 diabetes mellitus associated with increased risk for colorectal cancer: evidence from an international ecological study and population-based risk analysis in China. Public Health 123: 540-544. Link: https://goo.gl/q8DSoV
- Kajiura K, Ohkusa T, Okayasu I (1998) Relationship between Fecal Bile Acids and the Occurrence of Colorectal Neoplasia in Experimental Murine Ulcerative Colitis. Digestion 59: 69-72. Link: https://goo.gl/KoCB2R
- Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, et al. (2005) Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 129: 504-511. Link: https://goo.gl/we3BZM
- Everhart J, Wright D (1995) Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 273: 1605-1609. Link: https://goo.gl/Kvrg1J
- Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M (2005) Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 92: 2076-2083. Link: https://goo.gl/P6cMRj
- Li D, Tang H, Hassan MM, Holly EA, Bracci PM, et al. (2011) Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control 22: 189-197. Link: https://goo.gl/ypVsmt
- Li, D (2012) Diabetes and pancreatic cancer. Mol Carcinog 51: 64-74. Link: https://goo.gl/ixJWkE
- Ge Z, Ben Q, Qian J, Wang Y, Li Y (2011) Diabetes mellitus and risk of gastric cancer: a systematic review and meta-analysis of observational studies. Eur J Gastroenterol Hepatol 23: 1127-1135. Link: https://goo.gl/PkZKUU
- Yoon JM, Son KY, Eom CS, Durrance D, Park SM (2013) Pre-existing diabetes mellitus increases the risk of gastric cancer: a meta-analysis. World J Gastroenterol 19: 936-945. Link: https://goo.gl/8DyVMn
- Shimoyama S (2013) Diabetes mellitus carries a risk of gastric cancer: a meta-analysis. World J Gastroenterol 19: 6902-6910. Link: https://goo.gl/dKHGvf
- Ikeda F, Doi Y, Yonemoto K, Ninomiya T, Kubo M, et al. (2009) Hyperglycemia increases risk of gastric cancer posed by Helicobacter pylori infection: a population-based cohort study. Gastroenterology 136: 1234-1241. Link: https://goo.gl/oBJeUF
- Ryu TY, Park J, Scherer PE (2014) Hyperglycemia as a risk factor for cancer progression. Diabetes Metab J 38: 330-336. Link: https://goo.gl/hEZShj
- Duan W, Shen X, Lei J, Xu Q, Yu Y, et al. (2014) Hyperglycemia, a neglected factor during cancer progression. Biomed Res Int 2014: 461917. Link: https://goo.gl/UX9njq
- Li W, Zhang, L, Chen X, Jiang Z, Zong L, et al. (2016) Hyperglycemia Promotes the Epithelial-Mesenchymal Transition of Pancreatic Cancer via Hydrogen Peroxide. Oxid Med Cell Longev 2016: 5190314. Link: https://goo.gl/uevvEr
- Zeng L, Biernacka KM, Holly JM, Jarrett C, Morrison AA, et al. (2010) Hyperglycaemia confers resistance to chemotherapy on breast cancer cells: the role of fatty acid synthase. Endocr Relat Cancer 17: 539-551. Link: https://goo.gl/v49Dwo
- (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS). GrouLancet 352: 854-865. Link: https://goo.gl/rYWRZB
- ORIGIN Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Díaz R, et al. (2012) Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 367: 319-328. Link: https://goo.gl/Pe6X8d
- Malmberg K (1997) Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ 314: 1512-1515. Link: https://goo.gl/pU8PY9
- Mannucci E (2012) Insulin Therapy and Cancer in Type 2 Diabetes. ISRN Endocrinology 2012: 12. Link: https://goo.gl/V3QnrW
- Hemkens LG, Grouven U, Bender R, Günster C, Gutschmidt S, et al. (2009) Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009. 52: 1732-1744. Link: https://goo.gl/cfCCvR
- Jonasson JM, Ljung R, Talbäck M, Haglund B, Gudbjörnsdòttir S, et al. (2009) Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in Sweden. Diabetologia 52: 1745-1754. Link: https://goo.gl/QLHChX
- Colhoun HM; SDRN Epidemiology Group (2009) Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia 52: 1755-1765. Link: https://goo.gl/vK6xgp
- Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52: 1766-1777. Link: https://goo.gl/3DzhsV
- Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, et al. (2012) Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist 17: 813-822. Link: https://goo.gl/x1G4uL
- Franciosi M, Lucisano G, Lapice E, Strippoli GF, Pellegrini F, et al. (2013) Metformin therapy and risk of cancer in patients with type 2 diabetes: systematic review. PLoS One 8: e71583. Link: https://goo.gl/XKcxCw
- Kasznicki J, Sliwinska A, Drzewoski J (2014) Metformin in cancer prevention and therapy. Ann Transl Med 2: 57. Link: https://goo.gl/sznZWM
- Tseng CH (2016) Metformin reduces gastric cancer risk in patients with type 2 diabetes mellitus. Aging (Albany NY) 8: 1636-1649. Link: https://goo.gl/p2uHV1
- Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, et al. (2011) Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer 11: 20. Link: https://goo.gl/CR7RY1
- Skinner HD, McCurdy MR, Echeverria AE, Lin SH, Welsh JW, et al. (2013) Metformin use and improved response to therapy in esophageal adenocarcinoma. Acta Oncol 52: 1002-1009. Link: https://goo.gl/LuH99o
- Chiang GG, Abraham RT (2007) Targeting the mTOR signaling network in cancer. Trends Mol Med 13: 433-442. Link: https://goo.gl/85hNxm
- Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, et al. (2013) Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Transl Oncol 6: 649-659. Link: https://goo.gl/grv5fj
- Memmott RM, Mercado JR, Maier CR, Kawabata S, Fox SD, et al. (2010) Metformin prevents tobacco carcinogen--induced lung tumorigenesis. Cancer Prev Res (Phila) 3: 1066-1076. Link: https://goo.gl/Mm7gCA
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA (2009) The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle 8: 88-96. Link: https://goo.gl/VU6Soj
- Valaee S, Yaghoobi MM, Shamsara M (2017) Metformin inhibits gastric cancer cells metastatic traits through suppression of epithelial-mesenchymal transition in a glucose-independent manner. PLoS One 12: e0174486. Link: https://goo.gl/AMHmsR
- Zhu RC, Rattanakorn K, Pham S, Mallam D, McIntyre T, et al. (2017) Survival benefits in colorectal adenocarcinoma with the use of metformin among a black diabetic inner city population. Colorectal Cancer 6: 33-41. Link: https://goo.gl/AejTkh
- Fransgaard T, Thygesen LC, Gögenur I (2016) Metformin Increases Overall Survival in Patients with Diabetes Undergoing Surgery for Colorectal Cancer. Ann Surg Oncol 23: 1569-1575. Link: https://goo.gl/ZTYr6d
- Li X, Li T, Liu Z, Gou S, Wang C (2017) The effect of metformin on survival of patients with pancreatic cancer: a meta-analysis. Sci Rep 7: 5825. Link: https://goo.gl/DHWiUr
- Li P, Zhang C, Gao P, Chen X, Ma B, et al. (2018) Metformin use and its effect on gastric cancer in patients with type 2 diabetes: A systematic review of observational studies. Oncol Lett 15: 1191-1199. Link: https://goo.gl/5mkcpg
- Xiao Y, Zheng L, Mei Z, Xu C, Liu C, et al. (2017) The impact of metformin use on survival in prostate cancer: a systematic review and meta-analysis. Oncotarget 8: 100449-100458. Link: https://goo.gl/5m6MEV
- Jang WI, Kim MS, Lim JS, Yoo HJ, Seo YS, et al. (2015) Survival Advantage Associated with Metformin Usage in Hepatocellular Carcinoma Patients Receiving Radiotherapy: A Propensity Score Matching Analysis. Anticancer Res 35: 5047-5054. Link: https://goo.gl/yJekiX
- Tang JC, An R, Jiang YQ, Yang J (2017) Effects and Mechanisms of Metformin on the Proliferation of Esophageal Cancer Cells In Vitro and In Vivo. Cancer Res Treat 49: 778-789. Link: https://goo.gl/qPJEzX
- McTiernan A (2008) Mechanisms linking physical activity with cancer. Nat Rev Cancer 8: 205-211. Link: https://goo.gl/MHYSiW
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, et al. (2006) Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol 24: 3527-3534. Link: https://goo.gl/LBdpXR
- Martínez ME, Heddens D, Earnest DL, Bogert CL, Roe D, et al. (1999) Physical activity, body mass index, and prostaglandin E2 levels in rectal mucosa. J Natl Cancer Inst 91: 950-953. Link: https://goo.gl/1XDfyN
- Schwedhelm C, Boeing H, Hoffmann G, Aleksandrova K, Schwingshackl L (2016) Effect of diet on mortality and cancer recurrence among cancer survivors: a systematic review and meta-analysis of cohort studies. Nutr Rev 74: 737-748. Link: https://goo.gl/iXZni5
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
