Global Journal of Obesity, Diabetes and Metabolic Syndrome
Fixed Dose Combination of Voglibose & Repaglinide in the Management of Postprandial Hyperglycemia in Indian Subjects
Arif Faruqui*
Cite this as
Faruqui A (2017) Fixed Dose Combination of Voglibose & Repaglinide in the Management of Postprandial Hyperglycemia in Indian Subjects. Glob J Obes Diabetes Metab Syndr 4(3): 063-065. DOI: 10.17352/2455-8583.000026To evaluate the efficacy and tolerability of fixed dose combination of voglibose and repaglinide in postprandial hyperglycemia (PPHG) in Indian subjects. A non-randomized, open labeled, non-comparative, single-centric, study was conducted in total of 20 type 2 diabetes mellitus (T2DM) patients (9 men and 11 women, mean age 69.07 ± 3.495 years). Each patient was administered a fixed dose combination of voglibose 0.3mg and repaglinide 0.5/1mg three times a day, just before each meal for 30 days. Fasting blood glucose (FBG) and postprandial blood glucose (PPBG) was performed at baseline and at the end of study as assessment tools. At the end of study data was extractable only in 15 patients because 5 patient dropped out as they did not turn up for follow up at day 30.Postprandial blood glucose reduced significantly {-61.67mg/dl (95% confidence interval -76.79 to -46.54 p<0.0001)} from baseline at day 30. Also a significant reduction (p<0.0001) was found with FBG {-42.13mg/dl (Confidence interval -61.25 to -23.02 P value <0.0001)} at end of day 30. There were no adverse events reported including hypoglycemia. Voglibose and Repaglinide combination was effective and safe for the management of PPHG in T2DM patients.
Introduction
Diabetes is a complex, chronic illness requiring continuous medical care with multifactorial risk reduction strategies beyond glycemic control [1]. As per International diabetes federation (IDF) data, in 2035 India will stand 2nd in the world with a total of 109.0 million people suffering from diabetes [2].
Type 2 diabetes mellitus (T2DM) is a disorder characterized by insulin resistance and a progressive decline in pancreatic β-cell function associated with increasing hyperglycaemia [3]. The net result is that more glucose (endogenous + ingested) enters the circulation at a faster rate than the body can remove it, resulting in prolonged elevations of plasma glucose [4].
Postprandial hyperglycaemia occurs when the multiple homeostatic mechanisms that minimize glucose fluctuations and restore normal glucose levels following a meal are blunted.
The degree of postprandial hyperglycaemia is primarily due to reduced suppression of hepatic glucose production [5].
The early and late postlunch glucose values are predictive for poor diabetes control, indicating that PPHG is a major determinant in overall glycaemic control [4].
Postprandial hyperglycemia (PPHG) has been linked to both cardiovascular and chronic kidney diseases (CKD) [6,7].
Various oral anti-hyperglycemic agents have been associated with the serious side effects of hypoglycemic events and also are limited for their use in patients suffering with various stages of CKD.
Hypoglycemia has not been demonstrated to increase substantially with progressive falls in GFR when treated with Repaglinide (non-sulfonylurea insulin secretagogues) [8]. It can be used in renal failure without dose adjustments [9].
Voglibose, an alpha glucosidase inhibitor (AGI) provides another therapeutic option for patients with Type 2 diabetes mellitus in which glycaemic control is inadequate, despite diet alone or with pharmacological therapy with sulfonylurea and Biguanide. It is useful in elderly patients and in those with mild to moderate renal function impairments when other anti-diabetic agents are contraindicated [10].
Methods and Materials
Design and participants
This was a non-randomized, open labeled, non-comparative, single-centric study to determine the effectiveness and safety of the fixed dose combination of voglibose 0.3mg and repaglinide 0.5/1mg thrice daily for 30 days.
A total of 20 T2DM patients (9 men and 11 women, mean age: 69.07 ± 3.495 years reporting to Endocrinologist, were screened for FBG and PPBG at baseline and those fulfilling the criteria of FBG ≥126 mg/dL and/or PPBG ≥200 mg/dL were enrolled in the study.
The study was conducted, under the supervision of Dr. Pratap Narayan Mishra (M.D.) at Ganga Shree Narayan Memorial Diabetes Clinic & Research Centre, Patna (Bihar, India). Blood samples collected were sent for investigations of FBG and PPBG.
Patient characteristics
Inclusion and Exclusion criteria: 20 patients with T2DM who gave their informed consent in the vernacular language were included in the study. Eligible subjects were in the age range of 32-75 years and were diagnosed to have diabetes (T2DM) assessed as per FBG and PPBG.
The exclusion criteria included patients with any of the several conditions listed as follows: Subjects with planned surgery during the treatment course or undergone surgery prior to 3 months of enrollment.
Patients with Type-1 diabetes, female type-2 diabetes patients who were pregnant or planning to conceive and lactating mothers were excluded from the study.
Results
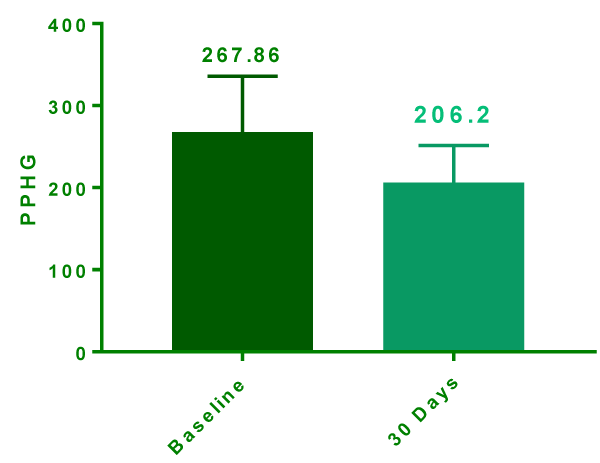
At the end of day 30, data was extractable in only 15 patients due to 5 drop outs as they did not turn up to follow up. At day 30 postprandial glycemic level were (206.2 ± 11.71 mg/dl) significantly lower compared to baseline (267.9 ± 17.57 mg/dl) (Figure 1). The mean reduction was -61.67 ± 21.12 mg/dl (95% confidence interval -76.79 to -46.54 & P Value <0.0001). Similar trend was also seen with FBG which was significantly reduced from baseline 182.3 ± 17.51 mg/dl to 140.2 ± 9.683 mg/dl (Figure 2) at day 30 with mean reduction of -42.13mg/dl (Confidence interval -61.25 to -23.02 P value <0.0001).
There was insignificant (p 0.8857) change in weight of patients compared to baseline at day 30. No adverse events including hypoglycemia were reported in the study.
Discussion
This is the first study in which the effectiveness of fixed dose combination of repaglinide and voglibose was evaluated in Indian population. An analogous type of fixed-dose combination consisting of 10 mg of mitiglinide and 0.2 mg of voglibose is also approved in Japan since 2011 [11].
Growing body of evidence indicates that reducing postprandial glucose excursions is equally or perhaps more important for achieving HbA1c goals. The IDF guidelines on controlling post-meal glucose recommends that postprandial hyperglycemia is harmful and should be lowered by incorporating a variety of both nonpharmacologic and pharmacologic therapies [12].
The results of our study were consistent with earlier trials such as a recent pilot study published in 2017 also resulted in significant improvement in PPHG & FBG after treatment with fixed-dose combination of mitiglinide 10 mg and voglibose 0.2 mg three times a day for 3 months [13].
Another study with similar combination also concluded that the mitiglinide/voglibose combination significantly reduced postprandial glycemic excursions in T2DM patients [14].
In our study it was observed that both postprandial and fasting blood glucose were significantly (P <0.0001) reduced after 30 days of treatment with fixed dose combination of repaglinide and voglibose compared to baseline. Tolerability was also found to be exceptional as there were no side effects including hypoglycemia reported in the study.
Considering the fact that, postprandial hyperglycemia develops early in the course of type 2 diabetes and is often evident even before fasting plasma glucose (FPG) elevations are seen [15], it may be possible that the fixed dose combination of Repaglinide and Voglibose could be a preferable option in order to improve and prevent future micro- and macrovascular diabetic complications in patients with PPHG. The limitation of the study was small sample size. Further studies are warranted, particularly randomized double blind multicentric studies with large sample size to explore the safety and efficacy of fixed dose combination of repaglinide/voglibose and to avoid any kind of preconceived notion in the study.
Conclusion
The Fixed dose combination of Repaglinide/Voglibose appeared to be effective and safe option for significant reduction in postprandial hyperglycemia. This study offers a new armamentarium in the management of PPHG in Indian patients.
- American Diabetes Association Standards of Medical Care in Diabetes 2017. Diabetes Care 40: S1–S2. Link: https://goo.gl/8jNgYo
- International Diabetes Federation (2015) IDF Diabetes Atlas; 7th Edition. Link: https://goo.gl/isjvDa
- Kumar R, Kerins DM, Walther T (2016) Cardiovascular safety of anti-diabetic drugs. Eur Heart J Cardiovasc Pharmacother 2: 32-43. Link:
- Heine RJ, Balkau B, Ceriello A, Del Prato S, Horton ES, et al. (2004) What does postprandial hyperglycaemia mean? Diabet Med 21: 208–213. Link: https://goo.gl/rL5R7s
- Gerich J (2013) Pathogenesis and management of postprandial hyperglycemia: role of incretin-based therapies. Int J Gen Med 6: 877-895. Link: https://goo.gl/uuFDgA
- Hurel SJ, Mohan V (2006) Clinical decision making: managing postprandial hyperglycemia. J Assoc Physicians India 54: 871-876. Link: https://goo.gl/dxCw4C
- Arnouts P, Bolignano D, Nistor I, Bilo H, Gnudi L, et al. (2014) Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant 29: 1284-1300. Link: https://goo.gl/xDn416
- National Kidney Foundation (2012) KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 update. Am J Kidney Dis 60: 850-886. Link: https://goo.gl/KMtLTg
- Yale JF (2005) Oral antihyperglycemic agents and renal disease: new agents, new concepts. J Am Soc Nephrol 16: S7-10. Link: https://goo.gl/hRENfY
- Dabhi AS, Bhatt NR, Shah MJ (2013) Voglibose: An Alpha Glucosidase Inhibitor. J Clin Diagn Res 7: 3023–3027. Link: https://goo.gl/4z1HsB
- PMDA Products Approved in FY 2011: New Drugs.
- Glycemic Pentad (Consensus Statement) (2007). Journal of the Association of Physicians of India 65: 68-79. Link: https://goo.gl/q6MiWz
- Murakami M, Bouchi R, Ohara N, Fukuda T, Takeuchi T, et al. (2014) Beneficial effect of combination therapy with mitiglinide and voglibose on fasting and postprandial endothelial dysfunction in patients with type2 diabetes: a pilot study. OAT 3: 1-4. Link: https://goo.gl/DYCe2Q
- Ono Y, Kameda H, Cho KY (2013) Mitiglinide/voglibose fixed-dose combination improves postprandial glycemic excursions in Japanese patients with type 2 diabetes mellitus. Expert Opin Pharmacother 14: 361-370. Link: https://goo.gl/1CrVx5
- Avignon A, Radauceanu A, Monnier L (1997) Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care 20: 1822-1826. Link: https://goo.gl/GXddpp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
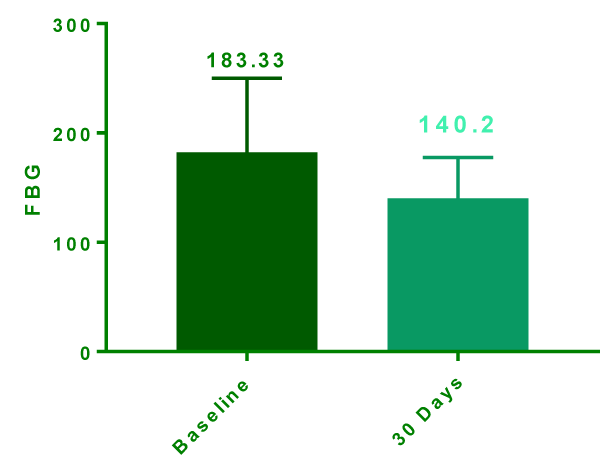

 Save to Mendeley
Save to Mendeley
