Global Journal of Obesity, Diabetes and Metabolic Syndrome
Study of the Effect of Mobile Indirect Calorimeter on Weight Management
Craig Stump1,5*, David Jackemeyer2, Yulia Abidov1, Karen Herbst1, Nongjian Tao2,3 and Erica Forzani2,4
2Center for Bioelectronics and Biosensors, the Biodesign Institute, Arizona State University, USA
3School of Electrical, Computer, and Energy Engineering, Arizona State University, USA
4School for Engineering of Matter, Transport, and Energy, Arizona State University, USA
5Southern Arizona VA Health Care System, Tucson, Arizona, USA
Cite this as
Stump C, Jackemeyer D, Abidov Y, Herbst K, Tao N, et al. (2017) Study of the Effect of Mobile Indirect Calorimeter on Weight Management. Glob J Obes Diabetes Metab Syndr 4(2): 044-050. DOI: 10.17352/2455-8583.000022This study investigates the effect of utilizing a personalized resting metabolic rate (RMR) mobile tracker based on indirect calorimetry during a 6-month pilot weight loss intervention. Volunteer subjects were randomized to an intervention group participating in a weight loss program utilizing the mobile tracker (IG; N=19) or a control group (CG: N=20) who participated in the same weight loss program, but without the RMR mobile tracker. All subjects were overweight or obese with either type 2 diabetes mellitus (T2DM) or high risk for T2DM. The subjects measured their body weight, physical activity, and caloric intake for 6 months attempting to meet a specific caloric intake goal. The total energy expenditure (TEE) of the subjects was defined as follows: For the CG, TEE was calculated based on daily physical activity, and resting energy expenditure estimated by the Harris-Benedict predictive equation. For the IG, TEE was calculated based on daily physical activity and measuring weekly resting energy expenditure with the mobile indirect calorimeter. The calorie intake goal for each subject was defined as a deficit of 500 kCal/day with respect to their TEE. Adherence to the recommended calorie intake goal during the 6-month period was evaluated via the entries in a calorie intake counter application. In addition, changes in weight, body composition, and blood metabolic profile after 6 months was compared to baseline measurements. The results indicated that the use of the mobile indirect calorimeter in the IG had positive effects on weight loss rate (89% in the IG vs. 50% in the CG, p = 0.05), and a 70% higher adherence to calorie tracking than the CG (p = 0.03). Furthermore, the IG showed statistically significant reduction vs. the CG in weight (p=0.03), body mass index (p = 0.03) and percent of weight loss (p=0.01), and an increase in HDL cholesterol vs. CG (p = 0.04).
Introduction
In the “clinical world” doctors, nurses, and other health professionals are devoted to enhancing patient health conditions and managing their disease states with existing resources within the medical practice or group. At the same time, outside the medical community and in the “real” world, there exists a plethora of wireless health monitoring devices and fitness trackers that people can use to collect a wealth of data including personal health (e.g. blood sugar and blood pressure), environment exposure (dietary intake, temperature, altitude), and behavioral (physical and sedentary activities) conditions [1,2]. Connecting data from free-living people in the “real world” to data analysis in the “clinical world” continues to be challenging since there is a requirement for systematic and calibrated data collection, as well as willing and reliable participation from the user. Nevertheless, the advances of personalized technologies with more user-friendly devices, interfaces, and other technologies have demonstrated some success [1-3]. User interest in technology and/or personal health can provide the motivation to collect data, which ultimately brings knowledge of personal health, and education for reinforcement of positive behavioral changes [3].
Obesity and the effects of being overweight are a significant focus in the area of personal health and behavior [4]. The success rate of people experiencing weight loss and weight maintenance is low, while the confusion surrounding therapies that emphasize macronutrient content of diets [5] as opposed to energy balance, resting energy expenditure (known as resting metabolic rate; RMR) and calorie deficit to promote fat loss is widely prevalent [5-7].
The present work investigates the effect of a RMR mobile tracker used by individuals who participated in a weight loss pilot study for 6 months. The study was evaluated for quantifiably measured pre- and post-study changes in physical, metabolic and behavioral metrics. The results of the study revealed the potential benefits of using mobile technologies, and portable indirect calorimetry as a metric for resting energy expenditure, and caloric intake needs.
Material and Methods
Subjects
Forty adult subjects, 9 males and 31 females, were recruited from the diabetes clinics and using local print advertisement to the University of Arizona Collaboratory for Metabolic Disease, Prevention and Treatment. All the subjects had T2DM or were at high risk of developing diabetes (see details below). The study was approved by the Institutional Review Board of Arizona State University (IRB protocol #1012005855), and was carried out between March 2014 and August 2015. All subjects provided written informed consent prior to participation. Initially, 20 subjects were randomly assigned to a control group (CG) and intervention group (IG), respectively (Figure 1). However, one subject from the IG withdrew from the study. The CG consisted of 14 females and 6 males, while the IG had 16 females and 3 males. The average ages and the corresponding standard deviation of the subjects were 54 (7) in the CG, and 56 (13) in the IG.
Study design
All participants were enrolled for a 6 month clinically monitored weight loss program [8]. After consent participants met the research team at the initiation of the weight loss program (Day 1), and at 1, 3 to 5 and 6 months (Figure 1). Physical, metabolic and blood measurements were performed on Day 1 and at 6 months. Through the study, participants electronically recorded weight, caloric intake, and activity on an iPadTM loaded with MyFitnessPalTM and StriivTM applications (apps). MyFitnessPalTM app, primarily a food calorie tracker, was linked to StriivTM, an app that collects step counts and other subject activity. The study participants utilized the group share feature of MyFitnessPalTM to communicate with study peers and researchers. In addition, participants were encouraged to communicate with a study investigator via e-mail during the entirety of the study to resolve technical questions about the apps and data collection.
On Day 1 (see below), the participants were trained in the use of the iPad, and apps. Total energy expenditure (TEE) of each participant was assessed in order to provide the initial caloric intake goal. The criteria to define calorie intake goal was as follows:
Control group (CG): Total energy expenditure (TEE) was estimated from resting energy expenditure (REE), and the assessment of the lifestyle with: TEE = REE *(lifestyle coefficient). For all participants, lifestyle was determined to be sedentary, and therefore, a lifestyle coefficient equal to 1.274 for males, and to 1.179 for females was utilized [9]. For REE, the value was determined based on the Harris-Benedict equation [10] using weight, height, gender and age of the participant. After assessing the TEE, the calorie intake goal was set with a 500 kcal/day deficit.
Intervention group (IG): TEE was estimated from measured REE and the assessment of lifestyle. Similar to the control group, the lifestyle of all participants was determined to be sedentary, and therefore, the above-mentioned coefficients were used in the equation. The main difference between the IG and the CG was the manner in which the calorie intake goal was provided. Participants of the IG were given a mobile indirect calorimeter, Breezing® tracker (www.breezing.com), so that they could self-assess their REE values at home. With the instruction to take one measurement per week (Figure 1), the IG participants were provided with an automatically adjusted daily recommended calorie intake through the app. The participants measured their REE in general at home on a Saturday morning, meeting the REE measurement conditions, which are specified below. After assessing the REE and corresponding TEE, the calorie intake goal was set with a 500 kcal/day deficit.
Assessment methods at the beginning and at the end of 6-month study
All subjects of the study were measured for weight, fat percentage, blood pressure, blood metabolic panel, and REE on Day 1 of the study and after 6 months. All measurements were performed in the morning between 8:00 am and 11:00 am after overnight fasting, abstaining at least 12-hours from strenuous exercise, and no-caffeine ingestion
Weight and fat percentage: A bioelectrical impedance-based body composition analyzer SC-240 from Tanita Co. (https://www.tanita.com) was used to measure weight and body fat percentage. In addition, height and body mass index (BMI = ratio of weight-to-height squared (Kg/(meters)2)) were assessed for each subject.
Blood Pressure: A medical grade blood pressure meter was used to assess the blood pressure of the subjects after resting for at least 10 minutes.
Blood metabolic panel: Blood samples were drawn from the vein, immediately processed for plasma and serum extraction, and then submitted for analysis to Quest Diagnostics (Quest Diagnostics.com).
Resting Energy Expenditure: REE was assessed by the indirect calorimetry method using a mobile metabolic rate tracker called “Breezing” from Breezing Co., AZ (www.breezing.com). The sensing principle of the indirect calorimeter used for REE was validated against the Douglas Bag method [11]. Resting condition of the subjects for assessment of REE included the above-mentioned conditions, and a resting sitting position of 20 minutes before the measurement [12]. After the subjects rested, they were instructed to relax and breathe normally during the measurement.
Assessment methods during 6-month study
The assessment methods used during the 6-month study were as follows:
A- MyFitnessPalTM app measurements: MyFitnessPalTM app was used for tracking calorie intake. Subjects of both groups (CG and IG) set the recommended calorie intake goal as the nutrition goal of the app. The app helped the subjects assess the daily calorie intake, and account for the remaining allowed intake for the day after entering the calories for each meal (breakfast, lunch, snacks, and dinner). In addition, the app accounted for calories recorded for activity energy expenditure (see below) to further adjust remaining caloric intake. It is important to mention that no special diet was prescribed. The app was used as an educational tool to learn about food calorie content and how to replace items in the diet for foods with a lower calorie density. In addition, each participant manually entered body weight in the app from a standardized floor scale. At the end of the study, all app results were exported using a Google Chrome extension: FoodFastFit.
B- Step counter: Step counting measurements were performed using a commercial counter, StriivTM Play. The step counter app estimated energy expenditure due to activity. As mentioned before, the step counter and activity expenditure records were forwarded from StriivTM to MyFitnessPalTM, where it was integrated into the food diary.
C- REE measurements: As explained before, subjects from IG were provided with a mobile indirect calorimeter, Breezing®, which tracks metabolic rate. Therefore, REE measurements were performed by the IG subjects, using the tracker and the Breezing app under resting conditions that were identical to the conditions applied to all subjects on Day 1 and the last day of the study (see above). The app provided the calorie intake goal to the users after each measurement. All measurement, and goal/target information was exported from the app via e-mail in a CSV file format, and e-mailed to study investigators at the given measurement point in time.
Data and statistical analysis
Averaged data pre- and post-study were collected as mean ± standard deviation (SD) or mean ± standard error of the mean (SEM), and changes in the parameters were evaluated using paired t-tests [13]. Statistically significant differences were assessed to compare the study effect between CG and IG, and were defined statistically significant at p ≤ 0.05.
Results
Subject characteristics
Subjects were randomized to the CG and IG groups for 6 months. At the onset of the study there were no statistically significant differences (p>0.05) between the groups for age, weight, height, waist to hip ratio, % body fat or blood pressure (Table 1). Likewise, no significant differences between groups were evident for REE, fasting glucose, HbA1c or lipids (Table 1). There was also no difference between the groups for the “undiagnosed diabetes high risk percentage” (UHRP) a measure of the percentage of subjects in a given group that were diagnosed with diabetes or pre-diabetes who did not know they were at risk for the condition using the American Diabetes Association diagnosis criteria (5.7% < HbA1c < 6.5%, and fasting blood glucose > 100 mg/dL) [14].
Changes after 6 months
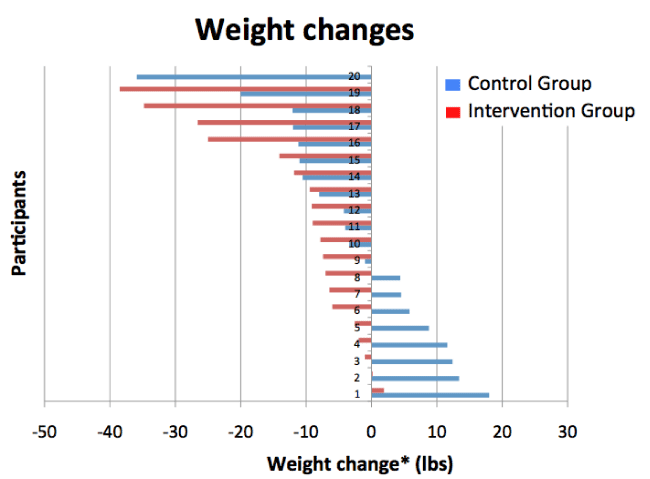
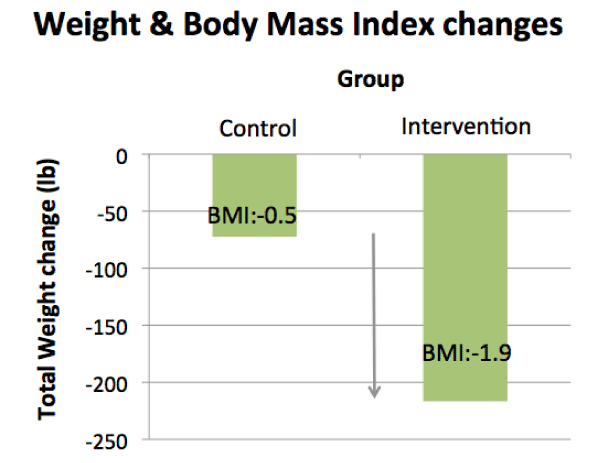
Six months after group assignment, IG and CG were re-evaluated. At this time the IG experienced a significantly (p<0.05) greater decrease in weight (-5.2+ 1.2 kg) and BMI (-1.9+0.4 kg/m2) compared to the CG (-1.2+1.4 kg and -0.5+0.4 kg/m2), respectively. The distribution of individual weight changes is displayed in figure 2, while mean changes in BMI for IG and CG are displayed in figure 3. Percentage body fat did not change in the IG group (0.0+0.6%) but increased slightly in the control group (0.4+0.8%). Diastolic (DBP) but not systolic (SBP) blood pressure was significantly decreased in the IG (-6.4+4.5 mmHg) compared to CG (2.6+2.2 mmHg) after 6 months (Table 2).
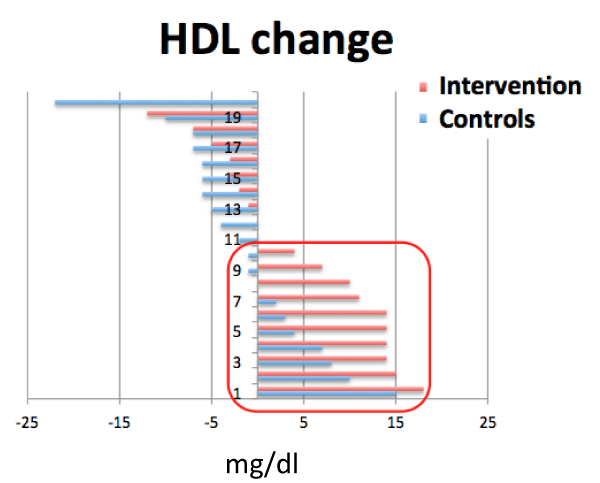
There were no significant changes in REE, fasting glucose and HbA1c between CG and IG groups during the 6 month trial (Table 2). Changes in lipid profiles are shown in table 2. There were no statistical changes in TG level for either group. While LDL and total cholesterol levels were lower in both groups after 6 months the changes did not reach statistical significance (p>0.05). However, HDL cholesterol increased more in the IG than CG (p<0.05). Distribution of individual changes in HDL are displayed in figure 4.
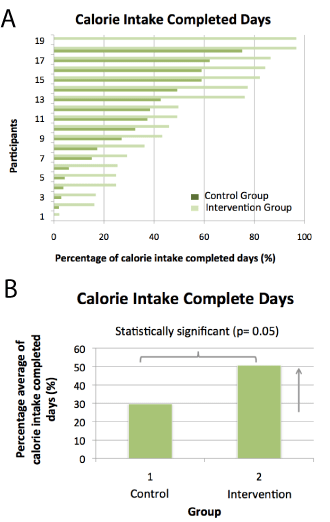
Subject adherence to recording caloric intake is displayed in figure 5a,b. The distribution of individual adherence days for each subject is shown in figure 5a. Adherence was determined from a complete analysis of calorie intake downloaded from MyFitnessPalTM for each participant on each day of the study. A completed calorie intake day was counted if the participant counted a total daily calorie intake equal or 25% higher than the calorie intake recommended goal for a given day. Figure 5b shows the mean percentage of calorie intake completed days for each group considering 100% the total days during the 6-month study. The corresponding absolute mean (± SEM) of the calorie intake completed days is 30 (6) and 51 (7) for the CG and IG, respectively with a p-value from paired t-test comparing CG vs. IG of 0.03, which indicated a statistically significant difference.
Regarding the effects of the 6-month study on HbA1c (glycated hemoglobin) levels of the participants with T2DM and high risk for T2 diabetes, the pre- and post-study glycated hemoglobin level mean (SEM) were 6.8 (0.3) and 5.9 (0.3), respectively for CG, and 6.6 (0.3) and 5.9 (0.3) for IG with p values < 0.05 from paired t-tests for both cases, which indicated statistically significant difference for intra-group changes along the 6-month study.
Discussion
Evaluation of the subjects’ post-study
In recent years, a multitude of health and fitness monitoring devices have been introduced to assist people in their attempts to lose weight, and improve health and physical fitness [1-3,15]. The utility of these devices in clinical practice remains to be defined, validated and effectively implemented. Here we describe the use of a hand held RMR tracking device, Breezing®, designed to assess RMR in a population of overweight or obese individuals with T2DM and/or at risk for diabetes. The RMR measurement is recommended by Academy of Nutrition and Dietetics as first tool for weight management [16]. The participants were randomized to device assisted monitoring and adjustment of diet and caloric expenditure targets, compared to a usual standard of care approach for determining caloric intake targets. Physical, metabolic and behavioral differences were evaluated over 6 months of assignment to intervention or control conditions.
The primary finding was that by tracking RMR and adjusting caloric balance targets during the study the IG lost significantly (p<0.05) more absolute weight (-5.2+1.2 kg vs. -1.2+1.4 kg) and percentage body weight (-5.5+1.2 % vs. -0.8+1.1 %) than the control group (Table 2). Indeed, 17 of the 19 participants in the IG lost weight (maximum 17.5 kg) while only one subject gained weight (0.9 kg). In the CG, 11 of the 20 participants lost weight and 8 gained weight (> 1 kg). If we consider weight loss greater than 3 kg, 68% of the IG participants (13/19) reached this target, while 40% of the CG group (8/20) did so.
Other cardiovascular and metabolic disease risk factors were also evaluated and reported for this study. HDL cholesterol (5+2 mg/dL vs -1+2 mg/dL) was significantly increased (p<0.05) in the IG vs CG, respectively (Table 2 and Figure 4). However, no significant differences were detected in triglycerides, total cholesterol, LDL cholesterol or HDL/LDL ratio between the IG and CG. Moreover, while mean systolic and diastolic blood pressures decreased in the IG during the study while CG blood pressures did not, these differences did not reach statistical significance (p >0.05, Table 2). Likewise, the greater decreases in fasting glucose and HbA1c observed in the IG did not reach statistical significance possibly due to small sample sizes. Nevertheless, HbA1c decreased pre- vs. post-study for both groups, likely due to the fact that both groups lost weight. It is well known that weight loss is associated with HbA1c reductions in diabetic patients, and therefore is among the first recommendations given to newly diagnosed T2DM patients [17]. Interestingly, while the study survey identified 40% of the volunteers having a known diagnosis of diabetes, initial blood tests revealed many others at “high diabetes risk” with HbA1c levels > 5.7%. Indeed, 54% and 58% of the study participants in the CG and IG respectively were referred to primary care physicians for further diabetes risk assessment (UHRP, Table 1).
Adherence to caloric and physical activity monitoring
Figure 5a,b shows the percentage of days each participant in the respective groups documented a complete record of caloric intake. While there was a large variability in adherence in both groups from <20% to >75% of total days, the IG documented 70% more complete days than CG (51+7 vs 30+6 % of days) which was statistically significant (p<0.05). It is known that complete patient recording of caloric intake in ambulatory settings is notoriously difficult and it is only achievable if tracking is concentrated on 7 days a week from time to time in a year [18]. However, the adherence to calorie tracking in the study setting was remarkable, and probably due to the use of the iPad in connection with study setting (e.g. investigators weekly observation).
In addition to documenting daily caloric intake, engaged participation was assessed for CG and IG in terms of physical activity data entry using MyFitnessPalTM. This platform includes recording of activity records, and personal notes. The volume of entry was defined by the total number of pages with recorded activity, and notes generated by each participant. The CG averaged 63 (SEM:8) pages of activities and notes entries, and the IG averaged 79 (SEM:3) pages of activities and notes entries, which was 25% greater for the IG vs. CG. In addition, the IG recorded over 200 REE measurements during the 6-month study which was not included in the activity entries (not shown).
The use of the MyFitnessPalTM app educates participants about calories in each food as well as the daily diet. The platform allows for increasing awareness of caloric content of typical food choices and the need to consider other quality choices while balancing quantity. In addition to calorie counting, the app led to the participants sharing diaries, sharing notes, and meal plans. Taken together, important components of weight management, including recording weight, calorie targets, diet tracking, and activity monitoring were integrated in the same mobile application (MyFitnessPalTM) which could in turn be accessed and tracked using social media. It is important to note that both groups benefited from this platform. The difference between the two groups was in the periodic assessment in RMR during the 6-month study which was used to adjust caloric and activity targets going forward. In fact, a comparison of the measured RMR with the calculated RMR in the CG indicated that 45% of the participants received a calorie recommendation from the equation that was 40% to 90% higher than the actual calorie intake need. This higher calorie recommendation may have driven to a higher calorie intake and, therefore, to higher weight gain. In fact, the weight gain in the control group was 40% compared with almost null rate in the intervention group. Furthermore, the weekly adjustment of calorie intake recommendation in the IG led to a personalized calorie intake recommendation that ultimate yielded a higher success in weight loss (89% vs 50%), and almost negligible weight gain (5%).
Study limitations and overall study analysis
The study lasted 6 months and the durability of the weight loss effect remains unknown. Therefore, longer study period of evaluation with and without active intervention would be needed in future studies. In addition, the assessment of total energy expenditure could be improved by assessing more accurate activity energy expenditure. This could be performed by using personalized indirect calorimetry-calibrated activity trackers. Finally, the current study had mostly women consenting for the study. In a future study, efforts to improve the sex distribution and the size of the study population should be made to assess statistically representative differences in outcomes between males and females.
Obesity, and related metabolic and cardiovascular disease represents an enormous health and economic burden on society. Consistently successful and durable interventions have been difficult to develop and often require significant resources to implement. The recent development of personal electronic devices to monitor and encourage healthy behaviors in healthy, high risk and chronic disease populations are becoming readily available to the public. Here, we described a personalized hand held device capable of accurate measurement of RMR by individual patients. Since one of the obstacles of continuing weight loss is the decrease in RMR over time, this device offered the opportunity to inform health professionals and patients of RMR changes such that adjustments in caloric intake and/or physical activity can be adopted to maintain more favorable weight loss trajectories. We observed that the integration of the device use is feasible, and next steps into the practical applications will require a reimbursement strategy in conjunction with the implementation of a remote monitoring site to enable teleconsultation of patients.
Conclusion
We have shown the addition of RMR to a standard caloric restriction and physical activity based weight loss program resulted in greater weight loss, improved HDL cholesterol and greater adherence to health monitoring. Indeed, the use of a personal indirect calorimetry device facilitated a more comprehensive use of the calorie counter app, which included calorie intake tracking, activity tracking, weight tracking and sharing functions. Future, studies in larger groups involving additional biomarkers are indicated. Moreover, the durability (>6 months) of weight loss and health improvements in patients using the Breezing® tracker or similar device during a weight loss intervention needs to be established. The findings in this study further demonstrate the potential of providing personalized, precise, and accurate information to patients for health monitoring and improvement. In this regard, the current study recognizes the importance of implementing the Precision Medicine Initiative [19] with current on-going efforts in large scale populations.
We would like to acknowledge full compliances from all 39 subjects of the study, and financial support of Flinn Foundation (Grant # 1904), and complementary support from OKED, Arizona State University. We would like to especially acknowledge Mr. Bill Read for his encouragement to pursue the study.
Author Contributions
Craig Stump (study design, direction the study logistic and discussions), Yulia Abidov (subject recruitment, blood sample draw and analysis, study participants’ scheduling and data collection), David Jackemeyer (study support, and data collection and analysis), Karen Herbst (study design, discussion on understanding of weight management), Erica Forzani (study design, management, IRB logistics, discussions), Nongjian Tao (support and discussion of data).
- McKinstry B, Hanley J, Wild S, Pagliari C, Paterson M, et al. (2013) Telemonitoring based service redesign for the management of uncontrolled hypertension: multicentre randomised controlled trial. BMJ 346: 1-18. Link: https://goo.gl/gNQgla
- Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, et al. (2011) Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 34: 1934-1942. Link: https://goo.gl/ZJTWrq
- Stephen A, Rob H, Khinlei MU, Joseph K, Kamal J (2013) The Impact of Using Mobile-Enabled Devices on Patient Engagement. J Diabetes Sci Technol 7: 623-629. Link: https://goo.gl/uqUG5i
- Spring B, Duncan JM, Janke EA, Kozak AT, McFadden HG, et al. (2013) Integrating technology into standard weight loss treatment: a randomized controlled trial. JAMA Intern Med, 173: 105-111. Link: https://goo.gl/Ma7LIW
- Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, et al. (2016) Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 104: 324-333. Link: https://goo.gl/obyCWW
- Criscione L, Durr-Gross M (2011) Eating healthy and dying obese. Vitasanas GmbH. Link: https://goo.gl/hyxzQg
- Nestle M, Nesheim M (2012) Why Calories Count: from science to politics. University of California Press. Link: https://goo.gl/W3bm2k
- American Diabetes Association (2017) Standards of Medical Care in Diabetes – 2017. Diabetes Care 40: 1. Link: https://goo.gl/sCjtec
- McArdle WD, Katch FI, Katch VL (2007) Exercise Physiology: Energy, Nutrition, & Human Performance. Lippincott Williams & Wilkins. Link: https://goo.gl/xIqfZJ
- Harris JA, Benedict FG (1918) A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci U S A 4: 370–373. Link: https://goo.gl/sqvbC1
- Xian X, Quach A, Bridgeman D, Tsow F, Forzani E, et al. (2015) Personalized Indirect Calorimeter for Energy Expenditure (EE) Measurement. Glob J Obes Diabetes Metab Syndr 2: 107: 004-008. Link: https://goo.gl/9ttjPn
- Fullmer S, Benson-Davies S, Earthman CP, Frankenfield DC, Gradwell E, et al. (2016) Evidence Analysis Library Review of Best Practices for Performing Indirect Calorimetry in Healthy and None Critically Ill Individuals. J Acad Nutr Diet 115: 1417-1446. Link: https://goo.gl/CSsJL0
- Kaplan LA, Pesce AJ (1984) Clinical Chemistry: theory, analysis, and correlation. Mosby, Co, St Louis, Toronto, Princeton. Link: https://goo.gl/TboFxj
- American Diabetes Association. Diagnosing Diabetes and Learning About Prediabetes. Link: https://goo.gl/jGPy6x
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, et al. (2007) Using Pedometers to Increase Physical Activity and Improve Health – A Systematic Review. JAMA 298: 2296-2304. Link: https://goo.gl/a4zxJg
- Seagle H, Strain GW, Makris A, Reeves RS (2009) Position of the American Dietetic Association: Weight Management. J Am Diet Assoc 109: 330-346. Link: https://goo.gl/tvndsj
- Tayek JA (2002) Is Weight Loss a Cure for Type 2 Diabetes? Diabetes Care 25: 397-398. Link: https://goo.gl/xZO02v
- St. Jeor S (1997) Obesity Assessment: Tools, Methods, Interpretations”. Jones & Bartlett Learning. Link: https://goo.gl/BRyiiQ
- Precision Medicine Initiative (2016). Link: https://goo.gl/LGR6TV
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
