Global Journal of Obesity, Diabetes and Metabolic Syndrome
A rare and challenging case of ROHHAD (Rapid-onset Obesity with Hypoventilation, Hypothalamic Dysfunction, Autonomic Dysregulation) syndrome
Cláudia Correia1*, Inês Cascais1, Rita Gomes1, Helena Ferreira Mansilha1, Lurdes Morais1, Marta Rios1 and Alberto Caldas Afonso1-4
2Institute of Biomedical Sciences Abel Salazar, University of Porto, Porto, Portugal
3EPIUnit - Institute of Public Health, University of Porto, Porto, Portugal
4Laboratory for Integrative and Translational Research in Population Health (ITR), University of Porto, Porto, Portugal
Cite this as
Correia C, Cascais I, Gomes R, Mansilha HF, Morais L, et al. (2023) A rare and challenging case of ROHHAD (Rapid-onset Obesity with Hypoventilation, Hypothalamic Dysfunction, Autonomic Dysregulation) syndrome. Glob J Obes Diabetes Metab Syndr 10(1): 005-008. DOI: 10.17352/2455-8583.000062Copyright License
© 2023 Correia C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Rapid-onset Obesity with Hypoventilation, Hypothalamic Dysfunction and Autonomic Dysregulation is a rare and complex pediatric syndrome with unknown etiology. The disease hallmark is sudden and severe obesity in early childhood, with a later onset of central hypoventilation, responsible for high mortality.
We present the case of a 2.5-year-old boy with sudden weight gain starting after 12 months of age (18 kg in a year) and hypoventilation in the setting of acute nasopharyngitis, requiring Non-Invasive Ventilation (NIV) initiation. Additionally, he presented symptoms and signs of autonomic disturbance, neurodevelopmental delay and behavior disorders. High prolactin, leptin and insulin were also present. Despite periodic adjustment of settings and adherence to NIV and great efforts to control food intake, he remained morbidly obese and died during an infectious intercurrence at 6 years of age.
This case illustrates the challenging diagnosis and treatment/management of this rare syndrome, which can have a variable and not always complete presentation and has no specific diagnostic test available. Identifying hypoventilation and NIV treatment is essential to decrease morbimortality. However, most patients do not live past ten years old.
Introduction
Rapid-onset Obesity with Hypoventilation, Hypothalamic Dysfunction and Autonomic Dysregulation (ROHHAD) is a rare and complex pediatric syndrome, with only about 150 described cases worldwide [1-3].
The clinical timeline of the disease is variable. Usually, it starts as rapid-onset obesity in early childhood (between 18 months and 7 years old), followed by hypothalamic dysfunction, hypoventilation and Autonomic Nervous System Dysregulation (ANSD) [4-6].
Hypothalamic dysfunction can manifest as insipid diabetes, hypothyroidism, hypercortisolism, Growth Hormone (GH) deficiency, precocious puberty, hypogonadotropic hypogonadism and hyperprolactinemia; and ANSD as dysregulation of body temperature, sweating and pain perception, ophthalmologic manifestations, gastrointestinal dysmotility and syncope [4].
The etiopathogenesis is unknown and no specific diagnostic test is currently available, making prompt diagnosis difficult, a cause for concern due to the high morbidity and mortality rates associated with this condition [4].
Case description
We present the case of a 2.5-year-old boy with weight gain starting after he was 12 months old, with dramatic rapid-onset obesity from 18 months of age (gain of 18 Kg in 12 months), and hypoventilation identified in the setting of acute nasopharyngitis.
He was the second son of a 30-year-old morbidly obese mother. His sister was diagnosed with Wolff-Parkinson-White syndrome and his maternal grandparents had metabolic syndrome. Some social risk factors were identified, corresponding to a socio-economic class IV family (Adapted Graffar Scale classification [7]).
He was a term newborn, with 3230g (50th percentile) and 50.5cm (50th-85th percentile). There was no relevant medical history until he was 10 months old, when he had two episodes suggestive of focal seizure, with no evidence of paroxysmal activity on the electroencephalogram.
At 2.5 years of age, he was admitted to the emergency department after presenting two apnea episodes during acute nasopharyngitis. He exhibited type 2 respiratory insufficiency (pH 7.29, PaO2 55 mmHg, PaCO2 64 mmHg, HCO3- 30.7 mmol/L), requiring Non-Invasive Ventilation (NIV) initiation.
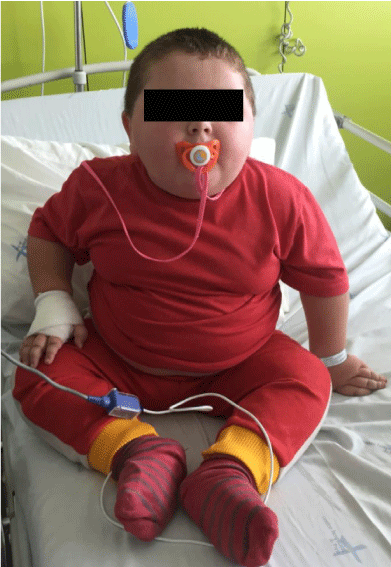
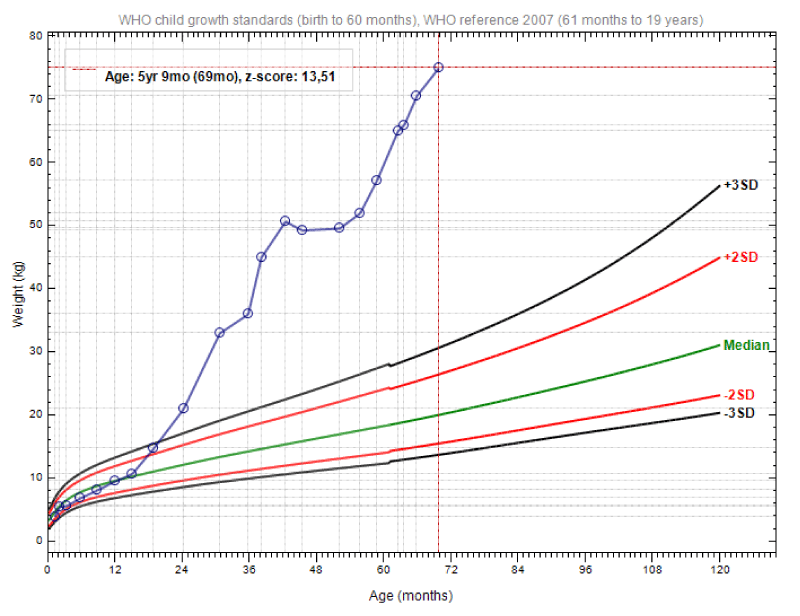
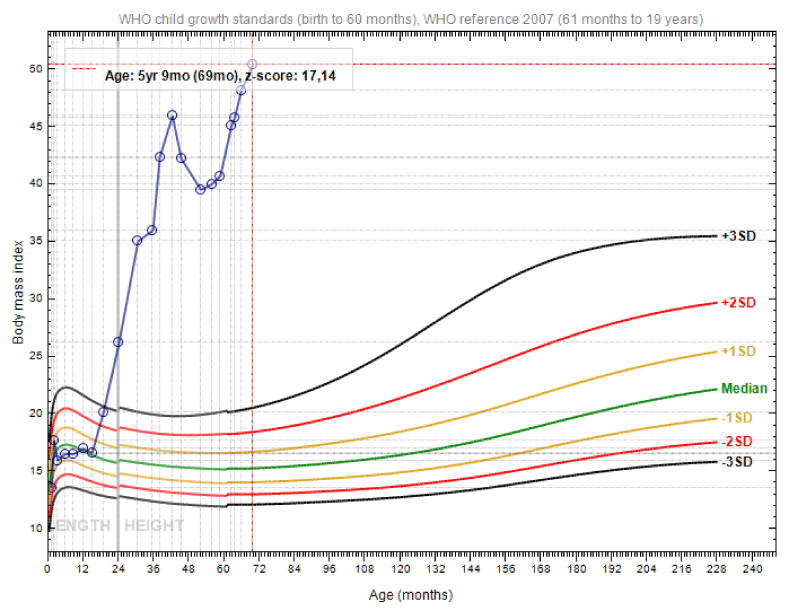
Severe obesity was notorious: weight 33 Kg (z-score +9.37), height 97 cm (z-score +1.33), and body mass index (BMI) 35.07 Kg/m2 (z-score +11.99) (Figures 1,2). There was significant hyperphagia and multiple eating errors.
He also presented snoring, insomnia and symptoms and signs of ANSD (strabismus, excessive sweating, decreased pain sensitivity, cold hands and feet and low heart rate variability during nocturnal oximetry).
Additionally, there was a developmental delay (with language regression), intellectual disability and behavioral changes/disorders, namely frequent irritability, auto and hetero aggression and difficulty walking due to obesity.
The analytical panel showed hyperprolactinemia (26.0 ng/mL), hyperinsulinism (60.8 uIU/mL), and high leptin levels (63.3 ng/mL), with no other endocrinologic or ionic imbalance (e.g., thyroid and adrenal functions were normal, there was no GH deficiency nor hypogonadotropic hypogonadism or dysnatremia was found).
Nocturnal polysomnography was inconclusive due to NIV’s dependence to initiate and maintain sleep (he could not fall asleep without NIV); however, transcutaneous CO2 monitoring was higher than 50 mmHg during awake periods without NIV.
The genetic study conducted was negative for Angelman and Prader-Willi syndromes. The absence of PHOX2B mutation ruled out Late-Onset Congenital Central Hypoventilation Syndrome and the array-based comparative genomic hybridization was normal.
No arrhythmia or atrial-ventricular conduction anomalies were detected in the cardiovascular Holter study and the echocardiogram was normal.
Brain Magnetic Resonance Imaging (MRI) was conducted, and no parenchymal or hypothalamic-hypophysis alterations were evident. Thoracic-abdominal MRI excluded significant anomalies and, specifically, neural crest tumors.
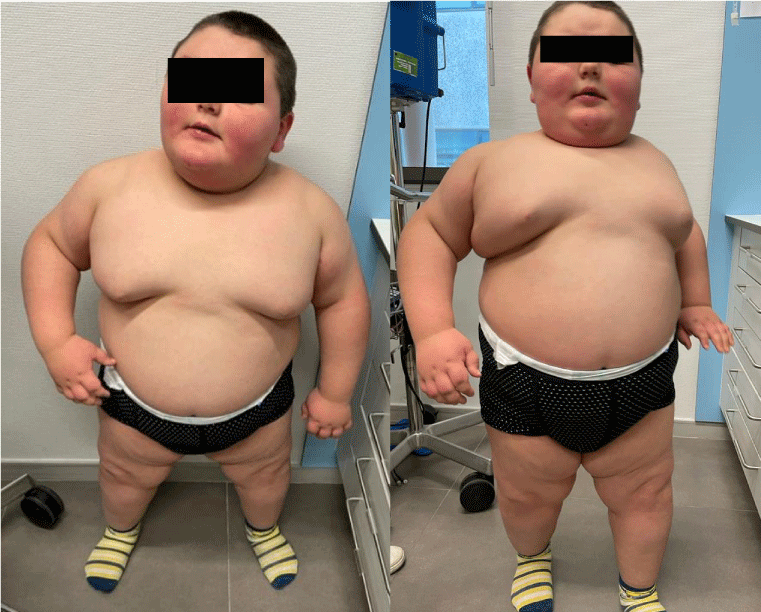
During the following three years, the patient continued severely obese, despite all efforts from the multidisciplinary team (Figures 2,3). Between 42 and 52 months of age, the weight was stabilized (due to better compliance with the prescribed nutritional diet, as he was institutionalized in a continuous care unit) with a concomitant increase in height. At 5 years and 9 months old, he weighed 75 Kg (z-score +13.51), was 122 cm tall (z-score +1.47) and had a BMI of 50.39 Kg/m2 (z-score +17.14) (Figure 4). Ventilation settings were periodically adjusted and there was good adherence to NIV during sleep. Development-enhancing therapies were offered, with some improvements. Endocrine and metabolic disturbances were routinely and periodically investigated.
Ultimately, at 6 years of age, the patient died at home during the night, with NIV, a few hours after two episodes of diarrhea and a fever spike. The medico-legal autopsy ruled the death of natural causes, excluding violent death. However, it was impossible to investigate ionic or endocrine disturbances further.
Discussion
Extreme childhood obesity is a severe global health problem with a rising prevalence [8,9]. This case reflects the importance of valuing cases of syndromic obesity and the clinician’s need to consider secondary causes when evaluating children that present early, severe and sudden obesity that is hard to control. ROHHAD syndrome must come to mind in these situations and the other features described in the acronym should be investigated [10]. The fact that the mother was morbidly obese could be a confounding variable in this case.
The diagnosis is clinical, as there is still no identifiable genetic cause. Nevertheless, excluding other genetic causes that imply morbid/extreme obesity is obligatory [11].
Severe obesity in early childhood and hyperphagia is often the first recognizable sign of the disease. Generally followed by hypothalamic and autonomic dysfunction and typically after that, hypoventilation, seizures and behavioral changes [12].
However, the clinical presentation is heterogeneous and variable in severity and timing. Some cases have marked endocrine involvement, while others exhibit more prominent behavioral disturbances [4], as seen in this patient.
Sleep-Disordered Breathing (SDB) can be seen after the onset of obesity and the obstructive component of SDB due to obesity typically precedes the central one [3,13,14]. In this case, suspending the NIV during the nocturnal polysomnography was impossible and therefore, it was hard to characterize SDB.
Hyperprolactinemia was evident in the presentation of this case. It has been reported as a frequent finding, with a systematic review describing it in 96% of patients with ROHHAD [15]. Therefore, this diagnosis should also be considered in patients with early-onset obesity and hyperprolactinemia without any structural abnormality in neuroimaging.
Insulin and leptin resistance, as seen in this patient, glucose dysregulation with impaired glucose tolerance, diabetes mellitus, hypertriglyceridemia and fatty liver disease have been described in ROHHAD cases [4,10,15,16]. Plasma leptin concentrations were depicted as comparable with those observed in obese children of comparable BMI [11], with the presence of high levels previously reported [10,17].
Other manifestations include behavioral problems, developmental delay, regression and seizures [2,4], which were present in this case. Seizures may be related to episodes of hypoxemia due to inadequate ventilation, but despite what is reported, in this case, the epileptic seizures happened before the rapid weight increase.
In ROHHAD cases, excluding cardiac pathology and Neuroendocrine Tumors (NET), such as neural crest tumors (ganglioneuroblastomas and neuroblastomas), is mandatory. These entities are described in almost 40% - 50% of ROHHAD patients [14,15], justifying the extension of the original acronym to ROHHAD-NET [18]. Nevertheless, there are currently no agreed recommendations regarding the frequency of screening with whole-body MRI scans, plasma metanephrines, and urinary catecholamines [15].
A poor prognosis is associated with this condition, with high morbidity and 50% – 60% mortality [19]. Most patients, as happened in this case, do not live past the age of ten [12], with a few case reports of patients that reached adulthood [10]. Central hypoventilation is responsible for progressive inadequate ventilation, and its combination with dysautonomia carries a high risk of sudden death [14], making NIV essential to prevent morbimortality. A cardiorespiratory arrest is the leading cause of death [19] and infections [10] or ionic imbalances can potentiate it. This reinforces the recommendation to perform a blood gas analysis in the presence of infectious/gastrointestinal complications, as there may be ionic imbalances or carbon dioxide retention without the clinical signs of respiratory distress. Possibly, in this case, the fatal outcome of cardiorespiratory arrest might have been precipitated by ionic imbalances in an infectious context, with fluid loss due to diarrhea.
In conclusion, this case illustrates the challenging diagnosis and management of ROHHAD syndrome, for the variable and not always complete presentation in time. The diagnostic and therapeutic approach was especially difficult in this case due to changes in behavior and social context, expressing the importance of ensuring multidisciplinary management in the long-term follow-up of these patients.
- Ibáñez-Micó S, Marcos Oltra AM, de Murcia Lemauviel S, Ruiz Pruneda R, Martínez Ferrández C, Domingo Jiménez R. Rapid-onset obesity with hypothalamic dysregulation, hypoventilation, and autonomic dysregulation (ROHHAD syndrome): A case report and literature review. Neurologia. 2017 Nov-Dec;32(9):616-622. English, Spanish. doi: 10.1016/j.nrl.2016.04.008. Epub 2016 Jun 21. PMID: 27340018.
- Lee JM, Shin J, Kim S, Gee HY, Lee JS, Cha DH, Rim JH, Park SJ, Kim JH, Uçar A, Kronbichler A, Lee KH, Shin JI. Rapid-Onset Obesity with Hypoventilation, Hypothalamic, Autonomic Dysregulation, and Neuroendocrine Tumors (ROHHADNET) Syndrome: A Systematic Review. Biomed Res Int. 2018 Nov 21;2018:1250721. doi: 10.1155/2018/1250721. PMID: 30584530; PMCID: PMC6280256.
- Selvadurai S, Benzon D, Voutsas G, Hamilton J, Yeh A, Cifra B, Narang I. Sleep-disordered breathing, respiratory patterns during wakefulness and functional capacity in pediatric patients with rapid-onset obesity with hypothalamic dysfunction, hypoventilation and autonomic dysregulation syndrome. Pediatr Pulmonol. 2021 Feb;56(2):479-485. doi: 10.1002/ppul.25199. Epub 2020 Dec 17. PMID: 33270379.
- Lazea C, Sur L, Florea M. ROHHAD (Rapid-onset Obesity with Hypoventilation, Hypothalamic Dysfunction, Autonomic Dysregulation) Syndrome-What Every Pediatrician Should Know About the Etiopathogenesis, Diagnosis and Treatment: A Review. Int J Gen Med. 2021 Jan 29;14:319-326. doi: 10.2147/IJGM.S293377. PMID: 33542648; PMCID: PMC7853626.
- Ceccherini I, Kurek KC, Weese-Mayer DE. Developmental disorders affecting the respiratory system: CCHS and ROHHAD. Handb Clin Neurol. 2022;189:53-91. doi: 10.1016/B978-0-323-91532-8.00005-7. PMID: 36031316.
- Marpuri I, Ra E, Naguib MN, Vidmar AP. Weight management in youth with rapid-onset obesity with hypothalamic dysregulation, hypoventilation, autonomic dysregulation, and neural crest tumor (ROHHAD-NET): literature search and case report. J Pediatr Endocrinol Metab. 2021 Dec 23;35(4):543-548. doi: 10.1515/jpem-2021-0600. PMID: 34954931.
- Fausto A. Adapted Graffar Scale. Costa, Ana Ma Bénard. Functional Resumes. Lisbon: IIE. 1996; II: 1990.
- Chung YL, Rhie YJ. Severe Obesity in Children and Adolescents: Metabolic Effects, Assessment, and Treatment. J Obes Metab Syndr. 2021 Dec 30;30(4):326-335. doi: 10.7570/jomes21063. PMID: 34924365; PMCID: PMC8735819.
- Obesity and overweight. 2023 Mar 6. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- Jalal Eldin AW, Tombayoglu D, Butz L, Affinati A, Meral R, Ontan MS, Walkovich K, Westerhoff M, Innis JW, Parikh ND, Oral EA. Natural history of ROHHAD syndrome: development of severe insulin resistance and fatty liver disease over time. Clin Diabetes Endocrinol. 2019 Jul 9;5:9. doi: 10.1186/s40842-019-0082-y. PMID: 31333877; PMCID: PMC6617654.
- Bougnères P, Pantalone L, Linglart A, Rothenbühler A, Le Stunff C. Endocrine manifestations of the rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neural tumor syndrome in childhood. J Clin Endocrinol Metab. 2008 Oct;93(10):3971-80. doi: 10.1210/jc.2008-0238. Epub 2008 Jul 15. PMID: 18628522.
- Harvengt J, Gernay C, Mastouri M, Farhat N, Lebrethon MC, Seghaye MC, Bours V. ROHHAD(NET) Syndrome: Systematic Review of the Clinical Timeline and Recommendations for Diagnosis and Prognosis. J Clin Endocrinol Metab. 2020 Jul 1;105(7):dgaa247. doi: 10.1210/clinem/dgaa247. PMID: 32407531.
- ROHHAD. About the Disease - Genetic and Rare Diseases Information Center. 2023 Mar 1. https://rarediseases.info.nih.gov/diseases/10407/rohhad
- Özcan G, Özsu E, Şiklar Z, Çobanoğlu N. A Rare Cause of Sleep-Disordered Breathing: ROHHAD Syndrome. Front Pediatr. 2020 Nov 20;8:573227. doi: 10.3389/fped.2020.573227. PMID: 33330273; PMCID: PMC7714909.
- Hawton K, Hilliard T, Langton-Hewer SC, Burren C, Crowne EC, Hamilton-Shield JP, Giri D. Rapid-onset obesity, hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome - neuro-endocrine tumours (ROHHAD-NET): case series and learning points. J Pediatr Endocrinol Metab. 2023 Jan 25;36(4):418-423. doi: 10.1515/jpem-2022-0376. PMID: 36696572.
- Ize-Ludlow D, Gray JA, Sperling MA, Berry-Kravis EM, Milunsky JM, Farooqi IS, Rand CM, Weese-Mayer DE. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics. 2007 Jul;120(1):e179-88. doi: 10.1542/peds.2006-3324. PMID: 17606542.
- Emery E, Patra K, Mains N, Brant RW, Kothari V, Ely B. Rapid-onset Obesity with Hypothalamic Dysregulation, Hypoventilation and Autonomic Dysregulation (ROHHAD) in a 3-year-old boy: A case report. Pediatrics. 2019 Aug 1;144:213–213.
- Gharial J, Ganesh A, Curtis C, Pauranik A, Chan J, Kurek K, Lafay-Cousin L. Neuroimaging and Pathology Findings Associated With Rapid Onset Obesity, Hypothalamic Dysfunction, Hypoventilation, and Autonomic Dysregulation (ROHHAD) Syndrome. J Pediatr Hematol Oncol. 2021 May 1;43(4):e571-e576. doi: 10.1097/MPH.0000000000001927. PMID: 32925400.
- Reppucci D, Hamilton J, Yeh EA, Katz S, Al-Saleh S, Narang I. ROHHAD syndrome and evolution of sleep disordered breathing. Orphanet J Rare Dis. 2016 Jul 30;11(1):106. doi: 10.1186/s13023-016-0484-1. PMID: 27473663; PMCID: PMC4967322.
- Growth reference 5-19 years - Application tools. 2023 Mar 6. https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
