Global Journal of Medical and Clinical Clinical Image
An uncommon “third window” in retrofenestral otosclerosis
Zambonini Giulia1*, Ghiselli Sara1, Di Trapani Giuseppe1, Maroldi Roberto2 and Cuda Domenico1
2DSMC, University of Brescia, Italy
Cite this as
Zambonini G, Ghiselli S, Di Trapani G, Maroldi R, Cuda D (2022) An uncommon “third window” in retrofenestral otosclerosis. Glob J Medical Clin Case Rep 9(4): 063-067. DOI: 10.17352/2455-5282.000162Copyright License
© 2022 Zambonini G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Otosclerosis is an otologic disease characterized by disordered resorption and deposition of the otic capsule bone. It can lead to progressive conductive, mixed or sensorineural Hearing Loss (HL). In rare cases, it manifests itself with a tendency for massive bone resorption with subsequent formation of cavities (“cavitating otosclerosis”). Cavities can sometimes realize communication between the Cerebrospinal Fluid (CSF) at the Internal Auditory Canal (IAC) and the cochlear duct. In these uncommon cases, a “third-window” phenomenon may be established as a concomitant cause of conductive HL. Therefore, the feasibility of stapes surgery should be evaluated, without underestimating the risk of gusher complications.
In this report, we discuss the case of a female patient affected by cavitating otosclerosis realizing a connection between IAC and cochlear duct, with mixed hearing loss.
Abbreviations
HL: Hearing Loss; SNHL: Sensorineural Hearing Loss; IAC: Internal Auditory Canal; OW: Oval Window; RW: Round Windows; ABG: Air-Bone Gap; CSF: CerebroSpinal Fluid; CT: Computed Tomography; cVEMPS: Cervical Vestibular-Evoked Myogenic Potentials Camps; BC: Bone Conduction; CBCT: Cone Beam CT; SSC: Superior Semicircular Canal; MRI: Magnetic Resonance Imaging; SCD: Superior Semicircular Canal Dehiscence
Introduction
Otosclerosis is a disease characterized by progressive hearing loss caused by pathologic remodeling of the bony labyrinth, especially the otic capsule. It can cause conductive or mixed Hearing Loss (HL); more rarely it can evolve as a pure Sensorineural HL (SNHL).
Otosclerosis begins with an “otospongiotic” phase: normal lamellar otic capsule bone around vessels is resorbed, creating perivascular spaces. Then, new woven bone is deposited in the “otosclerotic” phase [1].
Classically, otosclerosis is classified according to the remodeled foci location into fenestral and retrofenestral. Fenestral otosclerosis, most frequent (74%), is associated with an increased bone turnover in the lateral wall of the otic capsule, especially at the fissula ante fenestram, which leads to stapes fixation, resulting in conductive HL [2]. Retrofenestral otosclerosis is located more medially in the otic capsule; it involves the cochlear endosteum causing SNHL or mixed HL [3].
The otosclerotic foci can also involve the anterior wall of the Internal Auditory Canal (IAC) in 19% of cases [4]. Some authors have reported rare cases of advanced otosclerosis extending to the IAC forming a cavity. This specific form of advanced otosclerosis has been named ‘‘cavitating otosclerosis’’ [5].
Within the bony labyrinth, there are incompressible fluids that nevertheless communicate with the middle ear and the cranial cavity through different openings: cochlear aqueduct, vestibular aqueduct and Oval and Round Windows (OW, RW). In particular, RW and OW are mobile windows: upon movement of the ossicular chain and stapedial footplate, a solicitation on the OW is triggered, resulting in a fluid stream to the RW. This low impedance flow produces movement of the basilar membrane and induces a potential difference in the hair cells, generating the sound sensation. The presence of a “third” pathological window induces leakage of sound energy away from the cochlea leading to a conductive or mixed HL. A pathological “third-window” can be observed in case of dehiscence of semicircular canals, large vestibular aqueduct syndrome, dehiscence between the cochlea and carotid canal or between cochlea and IAC, DFN-3 (X-Linked Deafness with Stapes Gusher) [6].
These situations may preclude successful closure of the ABG after stapes surgery [7] or can lead to the complication of “gusher”. This is considered a tragic event during stapes surgery when a sudden and profuse flow of perilymph and CSF occurs immediately upon opening OW. An abnormal communication between the perilymphatic and subarachnoid spaces is the cause of this phenomenon [8]. CSF escape from the labyrinth may lead to severe hearing loss, tinnitus, or vestibular dysfunction [8]. Just 28% of patients preserve preoperative hearing after the gusher while 31% worsen to some extent and 25% experience profound or total HL [8].
The pericochlear otosclerotic cavitation connected to the IAC can also cause a “third window” phenomenon when it communicates with the cochlear duct: sound energy penetrates the inner ear via the ossicular chain and OW, but is carried away from the basilar membrane and is decompressed in this newly formed space; the result of such sound energy dissipation is a conductive HL [5,9].
Computed Tomography (CT) images are crucial in cavitating otosclerosis recognition to prevent CSF leaks and incorrect positioning of cochlear implant electrodes [9].
Case report
A 57-year-old female has referred us for stapes surgery because of a bilateral and progressive HL. She complained of hearing difficulty and mild tinnitus in her right ear. No fullness, dizziness, or vertigo was reported.
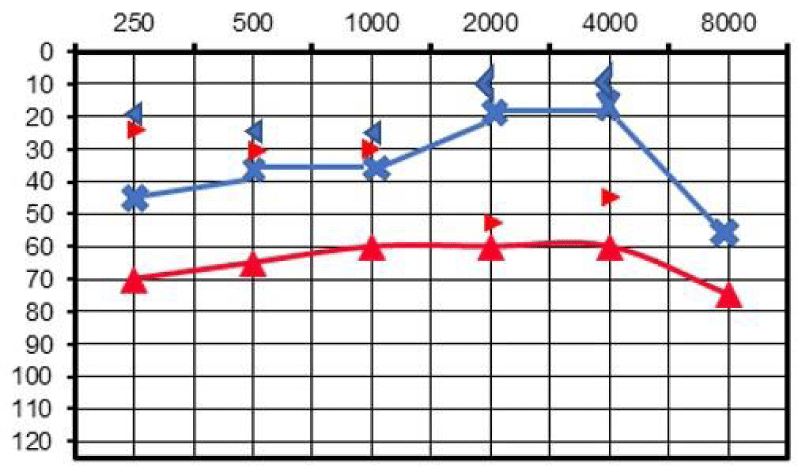
At pure tone audiometry a mixed bilateral HL was found, mild on the left and moderate-to-severe on the right ear (Figure 1). Type-A tympanograms were bilaterally recorded while acoustic reflexes were bilaterally absent.
The patient underwent vestibular evaluation through bedside examination and instrumental exams. At v-HIT (video-Head Impulse Test) no abnormal findings or asymmetry between the two sides were found. Air-conducted cVEMPs (Cervical Vestibular-Evoked Myogenic Potentials) were absent at maximum stimulation intensity.
In consideration of the interaural asymmetric Bone Conduction (BC) threshold a comprehensive radiological evaluation was requested.
At high-resolution cone beam, CT (CBCT, 150-micron slice thickness) in the right ear was observed in an area of bony reabsorption compatible with an otosclerotic focus in the prefenestral site, at the level of the ante-fenestram fissula that reaches the anterior stapes joint. Another area of bony demineralization was detected in the pericochlear area (“double ring” sign). The area of osteodystrophy formed a cavity in continuity with a large diverticulum arising from the anterior wall of the IAC (anteroposterior diameter of 8 mm and transverse diameter of 3 mm). It encircled the basal turn of the cochlea and reached anteriorly the horizontal tract of the carotid canal and posteriorly the niche of the RW. On the left ear, similar features were observed with a large cavity around cochlear turns communicating with a diverticulum of the IAC (anteroposterior diameter of 6 mm and transverse diameter of 3 mm). On this side, focal dehiscence of the superior Semicircular Canal (SSC) was also present (Figures 2,3).
Magnetic resonance imaging (MRI) was then required to verify the content of the pericochlear cavities. A heavily-T2-weighted sequence (MRI cisternography) shows a cavity, filled by hyperintense signal, extending from around the cochlea to the IAC; the cavity communicates with the cochlear lumen. These cavities show the same signal on the MRI cisternography, both contain fluid with the same signal characteristics of the CSF. The cavities communicate with the anterior labyrinth in the region of RW and in some points of the cochlear basal turn.
Figures 4,5 show MR scans in the axial plane, T2-weighted sequences.
Considering the image findings, it was decided not to perform the planned surgery on the right ear and to recommend a hearing aid evaluation. Audiological follow-up was also recommended.
Discussion
The diagnosis of otosclerosis is confirmed histopathologically or after stapedotomy or stapedectomy. From a practical point of view, however, the diagnosis is based on coherent features of clinical history, physical examination, pure tone, and impedance audiometry. The systematic use of preoperative CT is recommended [10], but it is still controversial. The sensitivity of CT in diagnosing otosclerosis, in fact, varies widely, ranging from 34% to 95% [10].
Typical findings on CT include hypodensity within the otic capsule and demineralization of the area outlining the cochlea (“double ring” sign). However, in our patient, the retrofenestral otosclerosis has created not only more than a rarefied bone but a really large space containing CSF. This area communicates with IAC via a diverticulum and constitutes cavitating otosclerosis. However, the special aspect of this case is that the cavity communicated with the cochlear duct as clearly evidenced by MRI.
The changes induced by this type of otosclerosis may account for the mixed-type HL on the right ear. The SNHL component is probably related to the contact of the osteosclerotic reaction with the cochlear endosteum: collagen deposition is established across the spiral ligament (hyalinization) resulting in atrophy of the adjacent vascular stria and disturbance of the potassium ion recycling mechanism and alteration of endo-cochlear potential [1]. Instead, the conductive component of HL can be caused by a stapes joint alteration or imbalance for an osteosclerotic focus in the area of the fissula ante fenestram at least on the right side, but also by the “third window” phenomenon.
The presence of a pathologic “third window” should be considered in the differential diagnosis of conductive HL in an apparently healthy middle ear. This condition shows up with a low-frequency air-bone gap (ABG) with supranormal BC thresholds. These features suggest a middle ear disease such as otosclerosis. Alerting against this hypothesis, however, is the frequent presence of stapedial reflexes and VEMPs. Imaging studies are crucial to verify alternative diagnoses [6].
The peculiarity of the case described here is that both conditions were simultaneously present i.e., fenestral otosclerosis and the “third window” associated with the cavitary component of retrofenestral localizations of this disease.
Probably, the placement of a stapedial prosthesis would have partially closed the ABG: only the stapedial fixation would be solved. Instead, a significant gap would have remained because of the “third window” persistence.
Shim Ye, et al. conducted a study to investigate the prognostic effects of stapes surgery in cavitating otosclerosis (described as a focal hypodense notch connecting to the anterior wall of the IAC). Audiological outcomes were compared between patients with IAC diverticulum (IAC group) and patients without it (non-IAC group). The principal findings of this study were as follows: “the IAC group showed significantly poorer postoperative audiological outcome with regard to air and BC thresholds as well as air-bone gap closure compared to the non-IAC group” [7]. According to the Authors, the cause lies in the “third window” effect, as reported also by Makarem, et al. [5].
None of Shim Ye’s patients had gusher intraoperatively [7] but the Authors only dealt with patients with small diverticula, without true communication with the cochlear lumen.
Instead, the most relevant aspect in our case would have been the risk of gusher associated with the surgery due to the abnormal continuity between the CSF and the perilymphatic compartment.
In support of this hypothesis, two cases are reported in the medical literature: both showed on CT scans area of bone dehiscence between the bottom of the IAC and the basal turn of the cochlea; both of them had an important gusher at the time of stapedectomy or cochleostomy [11]. This report can be conceptually assimilated to the cavitating otosclerosis present in the patient described here.
On the left ear of our patient, furthermore, a conductive HL can be also supported by superior semicircular canal dehiscence (SCD). SCD is the most frequent and most studied cause of the “third window” phenomenon; it is defined as Minor’s syndrome [12]. It is characterized by potential communication between the lumen of the SSC with the cranial cavity due to the lack of a bony roof of the canal. Symptoms are conductive HL associated or not with vertigo. Generally, pure tone audiometry shows an ABG for low and medium frequencies (≤ 2000 Hz) [6]. The otosclerotic radiological images of the left ear are comparable to those of the right, although the audiometric thresholds are very different. In fact, the left ear shows a better BC threshold and a smaller ABG. SCD probably has minimal influence on the audiometric examination and fenestral otosclerotic damage is instead the cause of HL. The left ear will probably develop mixed HL because of the similarity of the radiological images between the two sides.
Our patient was also tested for cVEMPs, but no responses were bilaterally detected at the highest stimulation intensity. This test records on sternocleidomastoid muscle a vestibular and myogenic response induced by sounds and mainly reflects the function of the saccule and its afferents.
This finding can be related to otosclerotic damage on the saccular cells because of their proximity to the areas most often affected by bone remodelling [13]; some authors have also hypothesized in degeneration of vestibular nerve fibers or even modifications of the biochemical composition of the endolymph by the release of proteolytic enzymes from the osteosclerotic process [14,15]. All these alterations may therefore explain the absence of a saccular response to the test.
Conclusion
Retrofenestral otosclerosis can cause localized or diffuse bone rarefaction; only in rare cases, it can form real cavities. When these cavities make a clear connection between the cochlear duct and CSF, they can lead to a “third window” effect and can cause gusher complications during stapes surgery for the communication between subarachnoid and perilymphatic spaces.
In patients with a suspected clinical diagnosis of otosclerosis, but with impaired BC, CT and MRI should be considered in the preoperative evaluation to prevent surgical failure or serious complications.
- Quesnel AM, Ishai R, McKenna MJ. Otosclerosis: Temporal Bone Pathology. Otolaryngol Clin North Am. 2018 Apr;51(2):291-303. doi: 10.1016/j.otc.2017.11.001. Epub 2018 Feb 3. PMID: 29397947.
- Schuknecht HF, Barber W. Histologic variants in otosclerosis. Laryngoscope. 1985 Nov;95(11):1307-17. doi: 10.1288/00005537-198511000-00003. PMID: 4058207.
- Cureoglu S, Baylan MY, Paparella MM. Cochlear otosclerosis. Curr Opin Otolaryngol Head Neck Surg. 2010 Oct;18(5):357-62. doi: 10.1097/MOO.0b013e32833d11d9. PMID: 20693902; PMCID: PMC3075959.
- Rudic M, Keogh I, Wagner R, Wilkinson E, Kiros N, Ferrary E, Sterkers O, Bozorg Grayeli A, Zarkovic K, Zarkovic N. The pathophysiology of otosclerosis: Review of current research. Hear Res. 2015 Dec;330(Pt A):51-6. doi: 10.1016/j.heares.2015.07.014. Epub 2015 Aug 12. PMID: 26276418.
- Makarem AO, Hoang TA, Lo WW, Linthicum FH Jr, Fayad JN. Cavitating otosclerosis: clinical, radiologic, and histopathologic correlations. Otol Neurotol. 2010 Apr;31(3):381-4. doi: 10.1097/MAO.0b013e3181d275e8. PMID: 20195188; PMCID: PMC2880664.
- Merchant SN, Rosowski JJ. Conductive hearing loss caused by third-window lesions of the inner ear. Otol Neurotol. 2008 Apr;29(3):282-9. doi: 10.1097/mao.0b013e318161ab24. PMID: 18223508; PMCID: PMC2577191.
- Shim YJ, Bae YJ, An GS, Lee K, Kim Y, Lee SY, Choi BY, Choi BS, Kim JH, Koo JW, Song JJ. Involvement of the Internal Auditory Canal in Subjects With Cochlear Otosclerosis: A Less Acknowledged Third Window That Affects Surgical Outcome. Otol Neurotol. 2019 Mar;40(3):e186-e190. doi: 10.1097/MAO.0000000000002144. PMID: 30741893.
- Alicandri-Ciufelli M, Molinari G, Rosa MS, Monzani D, Presutti L. Gusher in stapes surgery: a systematic review. Eur Arch Otorhinolaryngol. 2019 Sep;276(9):2363-2376. doi: 10.1007/s00405-019-05538-x. Epub 2019 Jul 4. PMID: 31273448.
- Bou-Assaly W, Mukherji S, Srinivasan A. Bilateral cavitary otosclerosis: a rare presentation of otosclerosis and cause of hearing loss. Clin Imaging. 2013 Nov-Dec;37(6):1116-8. doi: 10.1016/j.clinimag.2013.07.007. Epub 2013 Sep 17. PMID: 24050941.
- Virk JS, Singh A, Lingam RK. The role of imaging in the diagnosis and management of otosclerosis. Otol Neurotol. 2013 Sep;34(7):e55-60. doi: 10.1097/MAO.0b013e318298ac96. PMID: 23921926.
- Varadarajan VV, DeJesus RO, Antonelli PJ. Novel Computed Tomography Findings Suggestive of Perilymph Gusher. Otol Neurotol. 2018 Sep;39(8):1066-1069. doi: 10.1097/MAO.0000000000001916. PMID: 30113567.
- Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998 Mar;124(3):249-58. doi: 10.1001/archotol.124.3.249. PMID: 9525507.
- Amali A, Mahdi P, Karimi Yazdi A, Khorsandi Ashtiyani MT, Yazdani N, Vakili V, Pourbakht A. Saccular function in otosclerosis patients: bone conducted-vestibular evoked myogenic potential analysis. Acta Med Iran. 2014;52(2):111-5. PMID: 24659067.
- Pauw BK, Pollak AM, Fisch U. Utricle, saccule, and cochlear duct in relation to stapedotomy. A histologic human temporal bone study. Ann Otol Rhinol Laryngol. 1991 Dec;100(12):966-70. doi: 10.1177/000348949110001203. PMID: 1746843.
- Tramontani O, Gkoritsa E, Ferekidis E, Korres SG. Contribution of Vestibular-Evoked Myogenic Potential (VEMP) testing in the assessment and the differential diagnosis of otosclerosis. Med Sci Monit. 2014 Feb 7;20:205-13. doi: 10.12659/MSM.889753. PMID: 24509900; PMCID: PMC3930677.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.





 Save to Mendeley
Save to Mendeley
